Oral and Subcutaneous Immunization with a Plant-Produced Mouse-Specific Zona Pellucida 3 Peptide Presented on Hepatitis B Core Antigen Virus-like Particles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Construction of HBcAg-mZP3 Expression Vector
2.2. Expression of HBcAg-mZP3 in N. benthamiana Leaves
2.3. Protein Extraction
2.4. Purification of Virus-like Particles (VLPs)
2.5. SDS-PAGE and Western Blot Analysis
2.6. ELISA
2.7. Transmission Electron Microscopy (TEM)
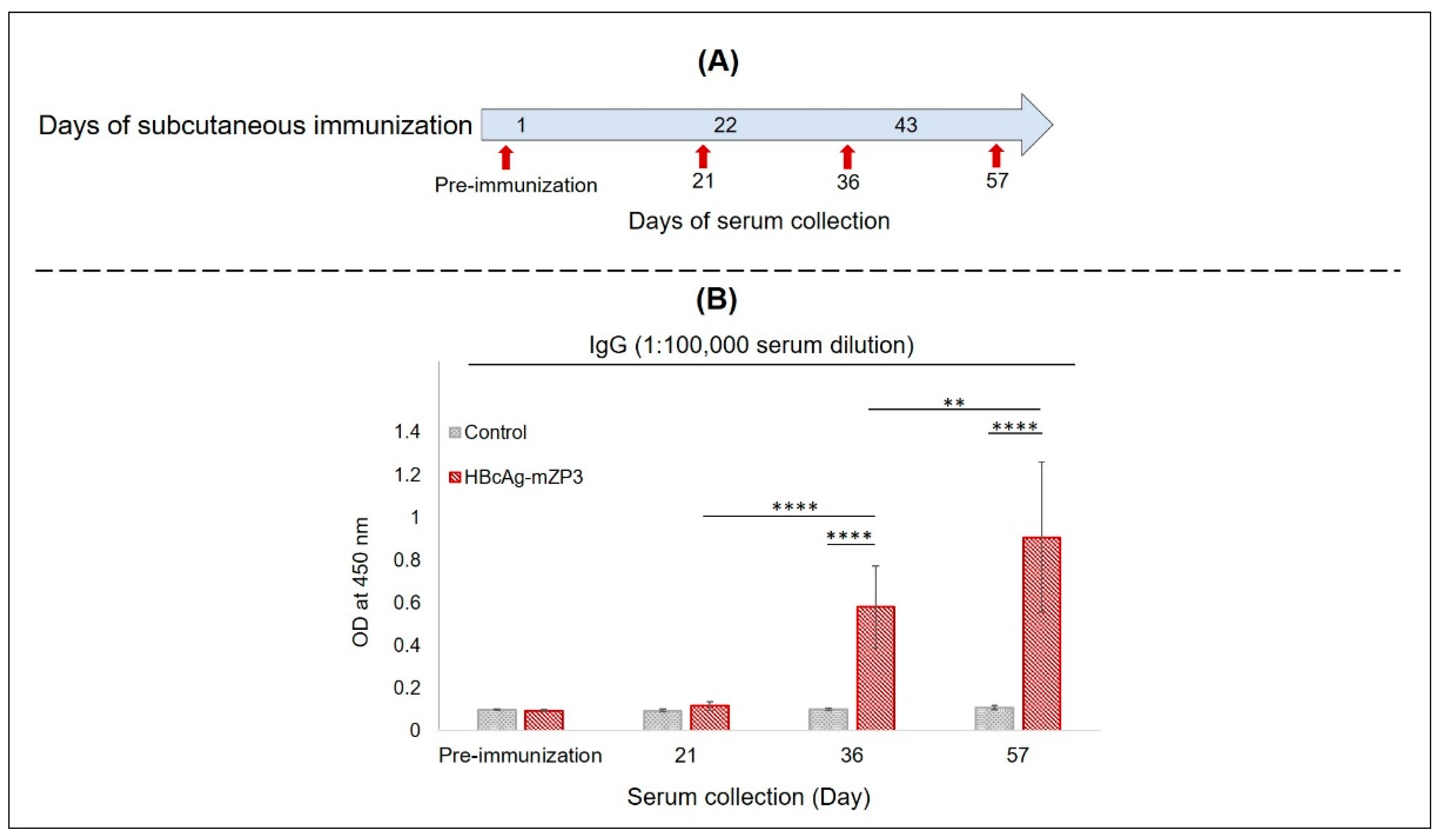
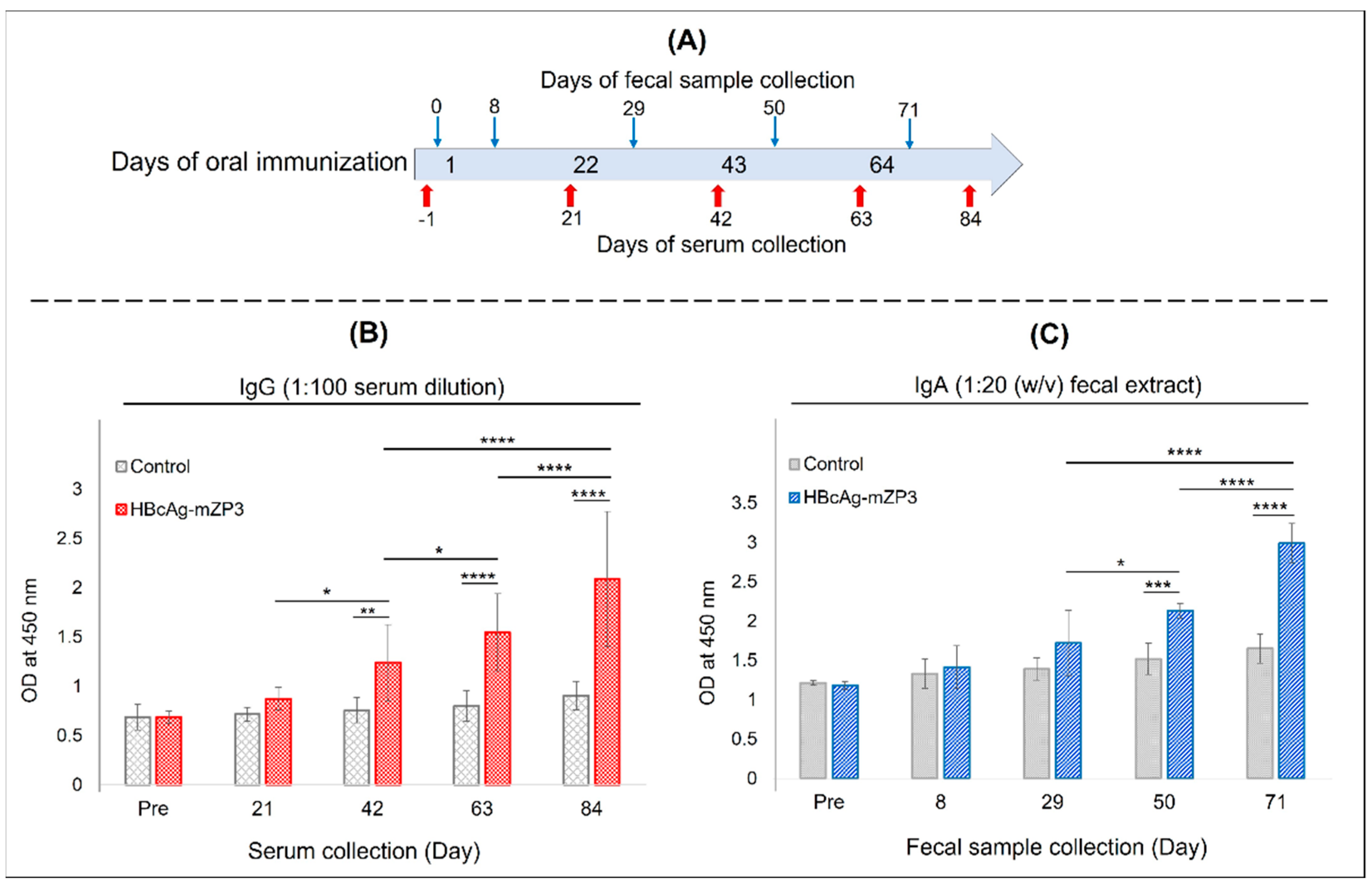
2.8. Mouse Immunization and Specific Serum IgG and Fecal IgA Measurement
2.9. Indirect Immunofluorescence
2.10. Statistical Analysis
3. Results
3.1. Design and Cloning of the HBcAg-mZP3 Expression Vector
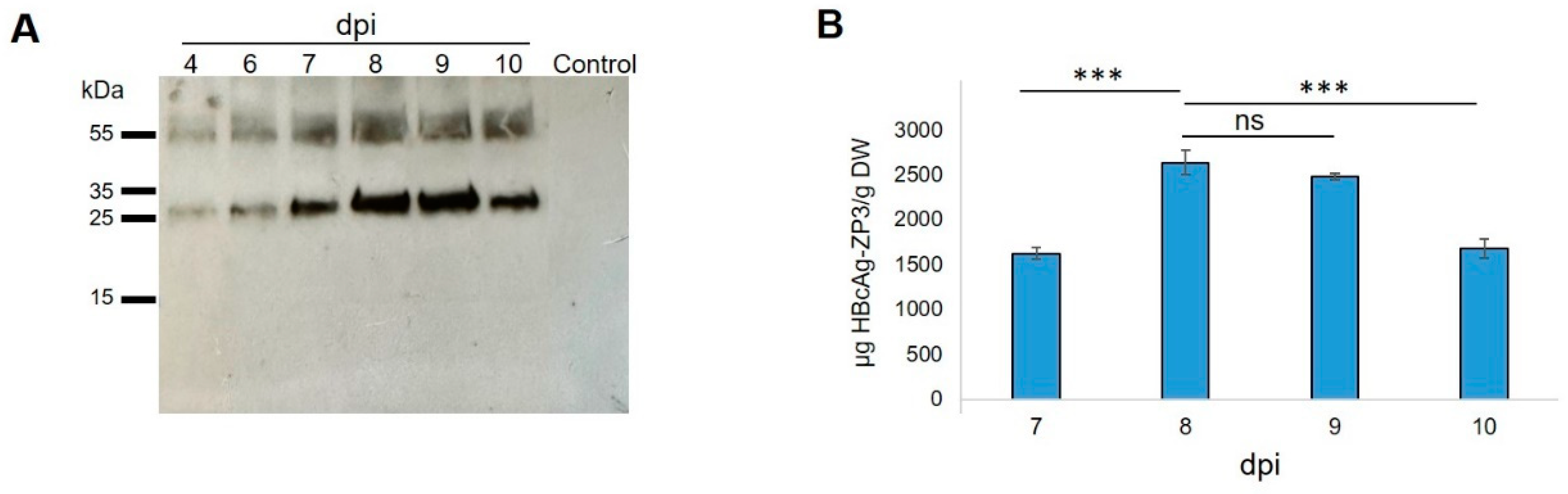
3.2. Transient Expression of HBcAg-mZP3 in N. benthamiana Plants
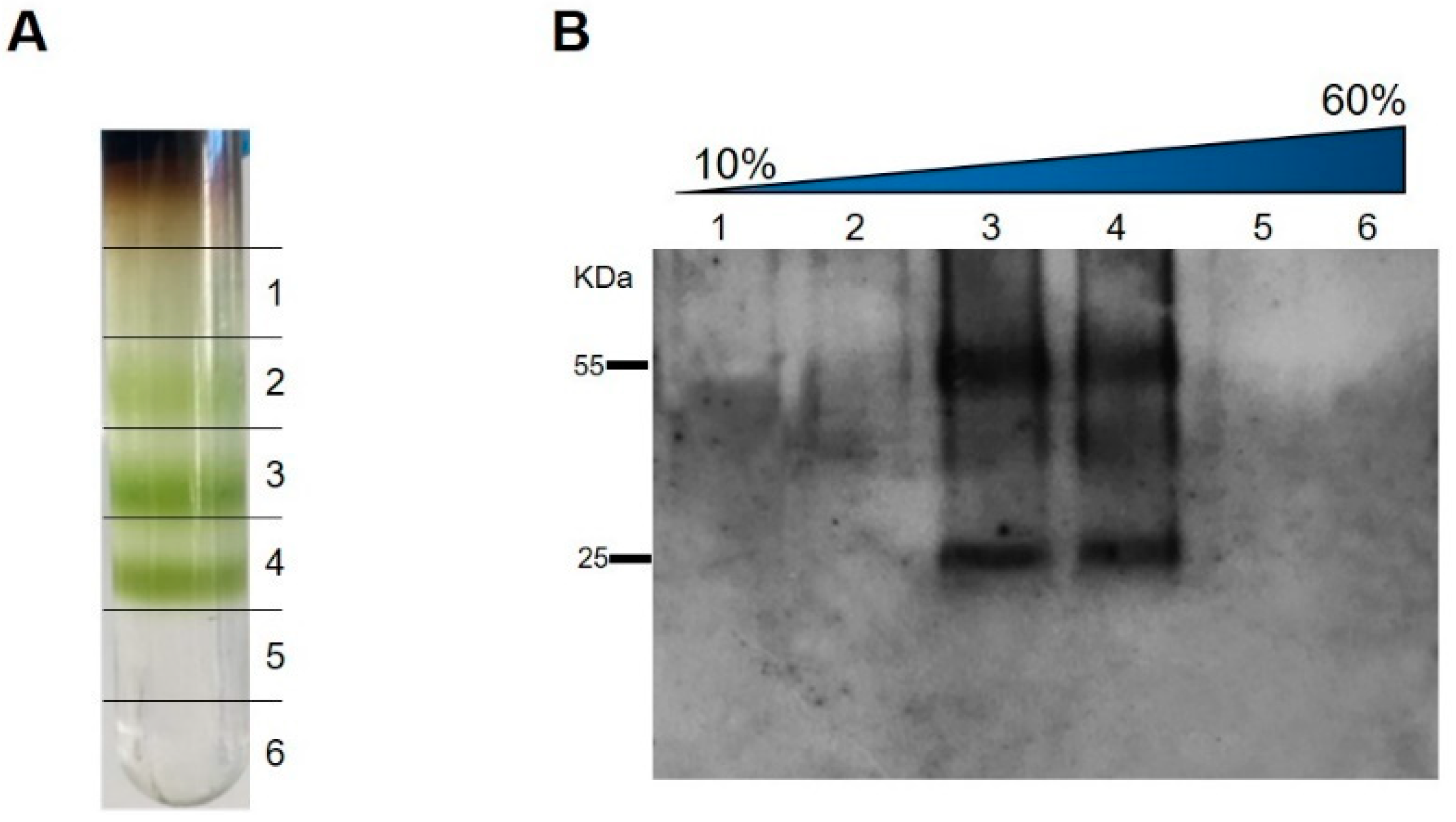
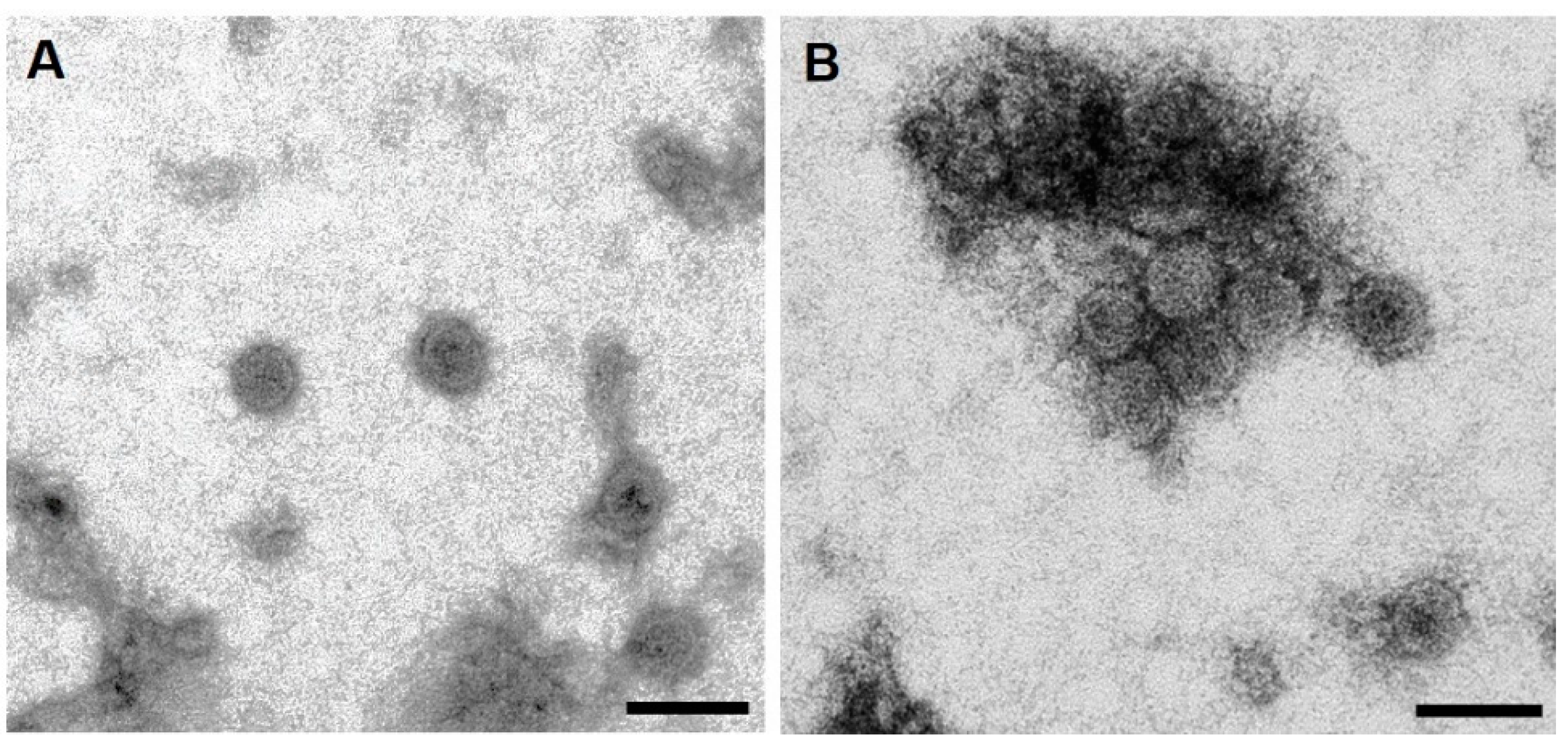
3.3. Purification, Characterization, and Detection of Assembled HBcAg-mZP3 VLPs
3.4. Systemic Immunogenicity of Plant-Produced HBcAg-mZP3 in Mice
3.5. Mucosal Immunogenicity of Plant-Produced HBcAg-mZP3 in Mice
3.6. Binding of Antibodies to Native Zona Pellucida In Vitro
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Firth, C.; Bhat, M.; Firth, M.A.; Williams, S.H.; Frye, M.J.; Simmonds, P.; Conte, J.M.; Ng, J.; Garcia, J.; Bhuva, N.P.; et al. Detection of zoonotic pathogens and characterization of novel viruses carried by commensal Rattus norvegicus in New York City. mBio 2014, 5, e01933-14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacob, J. Response of small rodents to manipulations of vegetation height in agro-ecosystems. Integr. Zool. 2008, 3, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Singleton, G.R.; Brown, P.R.; Jacob, J.; Aplin, K.P. Unwanted and unintended effects of culling: A case for ecologically-based rodent management. Integr. Zool. 2007, 2, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Wondifraw, B.T.; Tamene, M.Y.; Simegn, A.B. Assessment of crop damage by rodent pests from experimental barley crop fields in Farta District, South Gondar, Ethiopia. PLoS ONE 2021, 16, e0255372. [Google Scholar] [CrossRef]
- Swanepoel, L.H.; Swanepoel, C.M.; Brown, P.R.; Eiseb, S.J.; Goodman, S.M.; Keith, M.; Kirsten, F.; Leirs, H.; Mahlaba, T.A.M.; Makundi, R.H.; et al. A systematic review of rodent pest research in Afro-Malagasy small-holder farming systems: Are we asking the right questions? PLoS ONE 2017, 12, e0174554. [Google Scholar] [CrossRef] [Green Version]
- Brown, P.R.; Henry, S. Impacts of House Mice on Sustainable Fodder Storage in Australia. Agronomy 2022, 12, 254. [Google Scholar] [CrossRef]
- Singleton, G.R.; Lorica, R.P.; Htwe, N.M.; Stuart, A.M. Rodent management and cereal production in Asia: Balancing food security and conservation. Pest Manag. Sci. 2021, 77, 4249–4261. [Google Scholar] [CrossRef] [PubMed]
- Singleton, G.R.; Belmain, S.; Brown, P.R.; Aplin, K.; Htwe, N.M. Impacts of rodent outbreaks on food security in Asia. Wildl. Res. 2010, 37, 355. [Google Scholar] [CrossRef]
- Jacoblinnert, K.; Jacob, J.; Zhang, Z.; Hinds, L.A. The status of fertility control for rodents-recent achievements and future directions. Integr. Zool. 2021, 17, 964–980. [Google Scholar] [CrossRef]
- Tran, T.T.; Hinds, L.A. Fertility control of rodent pests: A review of the inhibitory effects of plant extracts on ovarian function. Pest Manag. Sci. 2013, 69, 342–354. [Google Scholar] [CrossRef]
- Hardy, C.M.; Hinds, L.A.; Kerr, P.J.; Lloyd, M.L.; Redwood, A.J.; Shellam, G.R.; Strive, T. Biological control of vertebrate pests using virally vectored immunocontraception. J. Reprod. Immunol. 2006, 71, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.J. Infertility in mice induced by a recombinant ectromelia virus expressing mouse zona pellucida glycoprotein 3. Biol. Reprod. 1998, 58, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Naz, R.K.; Saver, A.E. Immunocontraception for Animals: Current Status and Future Perspective. Am. J. Reprod. Immunol. 2016, 75, 426–439. [Google Scholar] [CrossRef] [PubMed]
- Kerr, L.E.; Paterson, M.; Aitken, R. Molecular basis of sperm–egg interaction and the prospects for immunocontraception. J. Reprod. Immunol. 1998, 40, 103–118. [Google Scholar] [CrossRef] [PubMed]
- Chambers, L.K.; Singleton, G.R.; Hood, G.M. Immunocontraception as a potential control method of wild rodent populations. Belg. J. Zool. 1997, 127, 145–156. [Google Scholar]
- Wassarman, P.M. Zona pellucida glycoproteins. J. Biol. Chem. 2008, 283, 24285–24289. [Google Scholar] [CrossRef] [Green Version]
- Barber, M.R.; Fayrer-Hosken, R.A. Possible mechanisms of mammalian immunocontraception. J. Reprod. Immunol. 2000, 46, 103–124. [Google Scholar] [CrossRef]
- Clydesdale, G. Contraception in mice immunized with recombinant zona pellucida subunit 3 proteins correlates with Th2 responses and the levels of interleukin 4 expressed by CD4+ cells. Reprod. 2004, 128, 737–745. [Google Scholar] [CrossRef] [Green Version]
- Lloyd, M.L.; Shellam, G.R.; Papadimitriou, J.M.; Lawson, M.A. Immunocontraception is induced in BALB/c mice inoculated with murine cytomegalovirus expressing mouse zona pellucida 3. Biol. Reprod. 2003, 68, 2024–2032. [Google Scholar] [CrossRef]
- Arukha, A.P.; Minhas, V.; Shrestha, A.; Gupta, S.K. Contraceptive efficacy of recombinant fusion protein comprising zona pellucida glycoprotein-3 fragment and gonadotropin releasing hormone. J. Reprod. Immunol. 2016, 114, 18–26. [Google Scholar] [CrossRef]
- Avella, M.A.; Xiong, B.; Dean, J. The molecular basis of gamete recognition in mice and humans. Mol. Hum. Reprod. 2013, 19, 279–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiu, P.; Lam, K.; Wong, R.; Yeung, W. The identity of zona pellucida receptor on spermatozoa: An unresolved issue in developmental biology. Semin. Cell Dev. Biol. 2014, 30, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Rankin, T.; Familari, M.; Lee, E.; Ginsberg, A.; Dwyer, N.; Blanchette-Mackie, J.; Drago, J.; Westphal, H.; Dean, J. Mice homozygous for an insertional mutation in the Zp3 gene lack a zona pellucida and are infertile. Development 1996, 122, 2903–2910. [Google Scholar] [CrossRef] [PubMed]
- Hardy, C.M.; Have, J.F.M., ten; Pekin, J.; Beaton, S.; Jackson, R.J.; Clydesdale, G. Contraceptive responses of mice immunized with purified recombinant mouse zona pellucida subunit 3 (mZP3) proteins. Reproduction 2003, 126, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Ferro, V.A.; Mordini, E. Peptide vaccines in immunocontraception. Curr. Opin. Mol. Ther. 2004, 6, 83–89. [Google Scholar] [PubMed]
- Hardy, C.M.; Clydesdale, G.; Mobbs, K.J. Development of mouse-specific contraceptive vaccines: Infertility in mice immunized with peptide and polyepitope antigens. Reproduction 2004, 128, 395–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paterson, M.; Jennings, Z.A.; Wilson, M.R.; Aitken, R. The contraceptive potential of ZP3 and ZP3 peptides in a primate model. J. Reprod. Immunol. 2002, 53, 99–107. [Google Scholar] [CrossRef]
- Hardy, C.M.; Beaton, S.; Hinds, L.A. Immunocontraception in mice using repeated, multi-antigen peptides: Immunization with purified recombinant antigens. Mol. Reprod. Dev. 2008, 75, 126–135. [Google Scholar] [CrossRef]
- Gupta, S.; Srivastava, N.; Choudhury, S.; Rath, A.; Sivapurapu, N.; Gahlay, G.; Batra, D. Update on zona pellucida glycoproteins based contraceptive vaccine. J. Reprod. Immunol. 2004, 62, 79–89. [Google Scholar] [CrossRef]
- Sun, W. A Contraceptive Peptide Vaccine Targeting Sulfated Glycoprotein ZP2 of the Mouse Zona Pellucida. Biol. Reprod. 1999, 60, 900–907. [Google Scholar] [CrossRef] [Green Version]
- Hardy, C.M.; Have, J.F., ten; Mobbs, K.J.; Hinds, L. Assessment of the immunocontraceptive effect of a zona pellucida 3 peptide antigen in wild mice. Reprod. Fertil. Dev. 2002, 14, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.; Ang, J.; Thai, H.; McElveen, F.; Tung, K.S. A zona pellucida 3 peptide vaccine induces antibodies and reversible infertility without ovarian pathology. J. Immunol. 1995, 155, 2715–2720. [Google Scholar] [CrossRef] [PubMed]
- Millar, S.E.; Chamow, S.M.; Baur, A.W.; Oliver, C.; Robey, F.; Dean, J. Vaccination with a synthetic zona pellucida peptide produces long-term contraception in female mice. Science 1989, 246, 935–938. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.; Ghosh, S.; Pagnon, J.; Colantoni, C.; Newbold, A.; Zeng, W.; Jackson, D.C. Totally synthetic peptide-based immunocontraceptive vaccines show activity in dogs of different breeds. Vaccines 2007, 25, 7111–7119. [Google Scholar] [CrossRef]
- Pinkhasov, J.; Alvarez, M.L.; Rigano, M.M.; Piensook, K.; Larios, D.; Pabst, M.; Grass, J.; Mukherjee, P.; Gendler, S.J.; Walmsley, A.M.; et al. Recombinant plant-expressed tumour-associated MUC1 peptide is immunogenic and capable of breaking tolerance in MUC1.Tg mice. Plant Biotechnol. J. 2011, 9, 991–1001. [Google Scholar] [CrossRef]
- Whitacre, D.C.; Lee, B.O.; Milich, D.R. Use of hepadnavirus core proteins as vaccine platforms. Expert Rev. Vaccines 2009, 8, 1565–1573. [Google Scholar] [CrossRef] [Green Version]
- Noad, R.; Roy, P. Virus-like particles as immunogens. Trends Microbiol. 2003, 11, 438–444. [Google Scholar] [CrossRef]
- Pumpens, P.; Grens, E. Hepatitis B core particles as a universal display model: A structure-function basis for development. FEBS Lett. 1999, 442, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Kratz, P.A.; Böttcher, B.; Nassel, M. Native display of complete foreign protein domains on the surface of hepatitis B virus capsids. Proc. Natl. Acad. Sci. USA 1999, 96, 1915–1920. [Google Scholar] [CrossRef] [Green Version]
- Francis, M.J.; Hastings, G.Z.; Brown, A.L.; Grace, K.G.; Rowlands, D.J.; Brown, F.; Clarke, B.E. Immunological properties of hepatitis B core antigen fusion proteins. Proc. Natl. Acad. Sci. USA 1990, 87, 2545–2549. [Google Scholar] [CrossRef] [Green Version]
- Ulrich, R.; Nassal, M.; Meisel, H.; Krüger, D.H. Core Particles of Hepatitis B Virus as Carrier for Foreign Epitopes. In Advances in Virus Research: Vol. 50; Maramorosch, K., Shatkin, A.J., Murphy, F.A., Eds.; Academic Press: San Diego, CA, USA; London, UK, 1998; pp. 141–182. ISBN 9780120398508. [Google Scholar]
- Grgacic, E.V.L.; Anderson, D.A. Virus-like particles: Passport to immune recognition. Methods 2006, 40, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Schödel, F.; Moriarty, A.M.; Peterson, D.L.; Zheng, J.A.; Hughes, J.L.; Will, H.; Leturcq, D.J.; McGee, J.S.; Milich, D.R. The Position of Heterologous Epitopes Inserted in Hepatitis B Virus Core Particles Determines Their Immunogenicity. Virology 1992, 66, 106–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, A.L.; Francis, M.J.; Hastings, G.Z.; Parry, N.R.; Barnett, P.V.; Rowlands, D.J.; Clarke, B.E. Foreign epitopes in immunodominant regions of hepatitis B core particles are highly immunogenic and conformationally restricted. Vaccine 1991, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Pumpens, P.; Grens, E. HBV Core Particles as a Carrier for B Cell/T Cell Epitopes. Intervirology 2001, 44, 98–114. [Google Scholar] [CrossRef]
- Daniell, H.; Singh, N.D.; Mason, H.; Streatfield, S.J. Plant-made vaccine antigens and biopharmaceuticals. Trends Plant Sci. 2009, 14, 669–679. [Google Scholar] [CrossRef] [Green Version]
- Giddings, G.; Allison, G.; Brooks, D.; Carter, A. Transgenic plants as factories for biopharmaceuticals. Nat. Biotechnol. 2000, 18, 1151–1155. [Google Scholar] [CrossRef]
- Rosales-Mendoza, S.; Nieto-Gómez, R. Green Therapeutic Biocapsules: Using Plant Cells to Orally Deliver Biopharmaceuticals. Trends Biotechnol. 2018, 36, 1054–1067. [Google Scholar] [CrossRef]
- Streatfield, S.J.; Howard, J.A. Plant-based vaccines. Int. J. Parasitol. 2003, 33, 479–493. [Google Scholar] [CrossRef]
- Sack, M.; Rademacher, T.; Spiegel, H.; Boes, A.; Hellwig, S.; Drossard, J.; Stoger, E.; Fischer, R. From gene to harvest: Insights into upstream process development for the GMP production of a monoclonal antibody in transgenic tobacco plants. Plant Biotechnol J. 2015, 13, 1094–1105. [Google Scholar] [CrossRef]
- Chan, H.-T.; Daniell, H. Plant-made oral vaccines against human infectious diseases—Are we there yet? Plant Biotechnol. J. 2015, 13, 1056–1070. [Google Scholar] [CrossRef] [Green Version]
- Kwon, K.-C.; Daniell, H. Low-cost oral delivery of protein drugs bioencapsulated in plant cells. Plant Biotechnol. J. 2015, 13, 1017–1022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, K.-C.; Verma, D.; Singh, N.D.; Herzog, R.; Daniell, H. Oral delivery of human biopharmaceuticals, autoantigens and vaccine antigens bioencapsulated in plant cells. Adv. Drug Deliv. Rev. 2013, 65, 782–799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komarova, T.V.; Baschieri, S.; Donini, M.; Marusic, C.; Benvenuto, E.; Dorokhov, Y.L. Transient expression systems for plant-derived biopharmaceuticals. Expert Rev. Vaccines. 2010, 9, 859–876. [Google Scholar] [CrossRef] [PubMed]
- Kjemtrup, S.; Talarico, T.L.; Ursin, V. Biotechnology: Pharming. In Encyclopedia of Agriculture and Food Systems; van Alfen, N.K., Ed.; Elsevier/Academic Press: Amsterdam, The Netherlands, 2014; pp. 117–133. ISBN 9780080931395. [Google Scholar]
- Sheludko, Y.V.; Sindarovska, Y.R.; Gerasymenko, I.M.; Bannikova, M.A.; Kuchuk, N.V. Comparison of several Nicotiana species as hosts for high-scale Agrobacterium-mediated transient expression. Biotechnol. Bioeng. 2007, 96, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Gleba, Y.Y.; Klimyuk, V.; Marillonnet, S. Magnifection--a new platform for expressing recombinant vaccines in plants. Vaccine 2005, 23, 2042–2048. [Google Scholar] [CrossRef]
- Marillonnet, S. In planta engineering of viral RNA replicons: Efficient assembly by recombination of DNA modules delivered by Agrobacterium. Proc. Natl. Acad. Sci. USA 2004, 101, 6852–6857. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Timko, M.P. Improving Protein Quantity and Quality-The Next Level of Plant Molecular Farming. Int. J. Mol. Sci. 2022, 23, 1326. [Google Scholar] [CrossRef]
- Karg, S.R.; Kallio, P.T. The production of biopharmaceuticals in plant systems. Biotechnol. Adv. 2009, 27, 879–894. [Google Scholar] [CrossRef]
- Engler, C.; Kandzia, R.; Marillonnet, S.; El-Shemy, H.A. A One Pot, One Step, Precision Cloning Method with High Throughput Capability. PLoS ONE 2008, 3, e3647. [Google Scholar] [CrossRef] [Green Version]
- Nausch, H.; Broer, I. Cyanophycinase CphE from P. alcaligenes produced in different compartments of N. benthamiana degrades high amounts of cyanophycin in plant extracts. Appl. Microbiol. Biotechnol. 2017, 101, 2397–2413. [Google Scholar] [CrossRef]
- Berardi, A.; Lomonossoff, G.P.; Evans, D.J.; Barker, S.A. Plant-expressed Hepatitis B core antigen virus-like particles: Characterization and investigation of their stability in simulated and pig gastro-intestinal fluids. Int. J. Pharm. 2017, 522, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Peyret, H. A protocol for the gentle purification of virus-like particles produced in plants. J. Virol. Methods 2015, 225, 59–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghasemian, K.; Broer, I.; Schön, J.; Kolp, N.; Killisch, R.; Huckauf, J. Plant-Produced Mouse-Specific Zona Pellucida 3 Peptide Induces Immune Responses in Mice. Vaccines 2023, 11, 153. [Google Scholar] [CrossRef] [PubMed]
- Naupu, P.N.; van Zyl, A.R.; Rybicki, E.P.; Hitzeroth, I.I. Immunogenicity of Plant-Produced Human Papillomavirus (HPV) Virus-Like Particles (VLPs). Vaccines 2020, 8, 740. [Google Scholar] [CrossRef]
- East, I.J.; Gulyas, B.J.; Dean, J. Monoclonal antibodies to the murine zona pellucida protein with sperm receptor activity: Effects on fertilization and early development. Dev. Biol. 1985, 109, 268–273. [Google Scholar] [CrossRef]
- Koyama, K.; Hasegawa, A.; Mochida, N.; Calongos, G. Follicular dysfunction induced by autoimmunity to zona pellucida. Reprod. Biol. 2005, 5, 269–278. [Google Scholar]
- Tsugama, D.; Liu, S.; Takano, T. A rapid chemical method for lysing Arabidopsis cells for protein analysis. Plant Methods 2011, 7, 22. [Google Scholar] [CrossRef] [Green Version]
- Méchin, V.; Consoli, L.; Le Guilloux, M.; Damerval, C. An efficient solubilization buffer for plant proteins focused in immobilized pH gradients. Proteomics 2003, 3, 1299–1302. [Google Scholar] [CrossRef]
- Peyret, H. Developing a Novel Hepatitis B Core—Based Antigen Presentation System 2014. Ph.D. Thesis, University of East Anglia, Norwich, UK, September 2014. Available online: https://ueaeprints.uea.ac.uk/id/eprint/52337 (accessed on 19 January 2023).
- Pang, E.L.; Peyret, H.; Ramirez, A.; Loh, H.-S.; Lai, K.-S.; Fang, C.-M.; Rosenberg, W.M.; Lomonossoff, G.P. Epitope Presentation of Dengue Viral Envelope Glycoprotein Domain III on Hepatitis B Core Protein Virus-Like Particles Produced in Nicotiana benthamiana. Front. Plant Sci. 2019, 10, 455. [Google Scholar] [CrossRef]
- Huang, Z.; Santi, L.; LePore, K.; Kilbourne, J.; Arntzen, C.J.; Mason, H.S. Rapid, high-level production of hepatitis B core antigen in plant leaf and its immunogenicity in mice. Vaccine 2006, 24, 2506–2513. [Google Scholar] [CrossRef]
- Zahmanova, G.; Mazalovska, M.; Takova, K.; Toneva, V.; Minkov, I.; Peyret, H.; Lomonossoff, G. Efficient Production of Chimeric Hepatitis B Virus-Like Particles Bearing an Epitope of Hepatitis E Virus Capsid by Transient Expression in Nicotiana benthamiana. Life 2021, 11, 64. [Google Scholar] [CrossRef] [PubMed]
- Peyret, H.; Ponndorf, D.; Meshcheriakova, Y.; Richardson, J.; Lomonossoff, G.P. Covalent protein display on Hepatitis B core-like particles in plants through the in vivo use of the SpyTag/SpyCatcher system. Sci. Rep. 2020, 10, 17095. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Lai, H.; Sun, H.; Chen, Q. Virus-like particles that display Zika virus envelope protein domain III induce potent neutralizing immune responses in mice. Sci. Rep. 2017, 7, 7679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, F.; Ge, S.; Li, L.; Wu, X.; Liu, Z.; Wang, Z. Virus-like particles: Potential veterinary vaccine immunogens. Res. Vet. Sci. 2012, 93, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.O.; Tucker, A.; Frelin, L.; Sallberg, M.; Jones, J.; Peters, C.; Hughes, J.; Whitacre, D.; Darsow, B.; Peterson, D.L.; et al. Interaction of the hepatitis B core antigen and the innate immune system. J. Immunol. 2009, 182, 6670–6681. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Y.; Kwon, K.-C.; Hoffman, B.E.; Kamesh, A.; Jones, N.T.; Herzog, R.W.; Daniell, H. Low cost delivery of proteins bioencapsulated in plant cells to human non-immune or immune modulatory cells. Biomaterials 2016, 80, 68–79. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Lou, Y.-H.; Koopman, M.; Doggett, T.; Tung, K.; Curtiss, R. Antibody responses and infertility in mice following oral immunization with attenuated Salmonella typhimurium expressing recombinant murine ZP3. Biol. Reprod. 1997, 56, 33–41. [Google Scholar] [CrossRef]
- Pyrski, M.; Mieloch, A.A.; Plewiński, A.; Basińska-Barczak, A.; Gryciuk, A.; Bociąg, P.; Murias, M.; Rybka, J.D.; Pniewski, T. Parenteral-Oral Immunization with Plant-Derived HBcAg as a Potential Therapeutic Vaccine against Chronic Hepatitis, B. Vaccines 2019, 7, 211. [Google Scholar] [CrossRef] [Green Version]
- Srinivasan, J.; Tinge, S.; Wright, R.; Herr, J.; Curtiss, R. Oral immunization with attenuated Salmonella expressing human sperm antigen induces antibodies in serum and reproductive tract. Biol. Reprod. 1995, 53, 462–471. [Google Scholar] [CrossRef] [Green Version]
- Clarke, J.L.; Paruch, L.; Dobrica, M.-O.; Caras, I.; Tucureanu, C.; Onu, A.; Ciulean, S.; Stavaru, C.; Eerde, A.; Wang, Y.; et al. Lettuce-produced hepatitis C virus E1E2 heterodimer triggers immune responses in mice and antibody production after oral vaccination. Plant Biotechnol. J. 2017, 15, 1611–1621. [Google Scholar] [CrossRef] [Green Version]
- Hayden, C.A.; Fischer, M.E.; Andrews, B.L.; Chilton, H.C.; Turner, D.D.; Walker, J.H.; Tizard, I.R.; Howard, J.A. Oral delivery of wafers made from HBsAg-expressing maize germ induces long-term immunological systemic and mucosal responses. Vaccine 2015, 33, 2881–2886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pniewski, T.; Kapusta, J.; Bociąg, P.; Wojciechowicz, J.; Kostrzak, A.; Gdula, M.; Fedorowicz-Strońska, O.; Wójcik, P.; Otta, H.; Samardakiewicz, S.; et al. Low-dose oral immunization with lyophilized tissue of herbicide-resistant lettuce expressing hepatitis B surface antigen for prototype plant-derived vaccine tablet formulation. J. Appl. Genet. 2011, 52, 125–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buchanan, R.M.; Tetland, S.; Wilson, H.L. Low dose antigen exposure for a finite period in newborn rats prevents induction of mucosal tolerance. PLoS ONE 2012, 7, e51437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, R.P.; Santiago, A.F.; Ficker, S.M.; Gomes-Santos, A.C.; Faria, A.M.C. Antigen administration by continuous feeding enhances oral tolerance and leads to long-lasting effects. J. Immunol. Methods 2015, 421, 36–43. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghasemian, K.; Broer, I.; Schön, J.; Killisch, R.; Kolp, N.; Springer, A.; Huckauf, J. Oral and Subcutaneous Immunization with a Plant-Produced Mouse-Specific Zona Pellucida 3 Peptide Presented on Hepatitis B Core Antigen Virus-like Particles. Vaccines 2023, 11, 462. https://doi.org/10.3390/vaccines11020462
Ghasemian K, Broer I, Schön J, Killisch R, Kolp N, Springer A, Huckauf J. Oral and Subcutaneous Immunization with a Plant-Produced Mouse-Specific Zona Pellucida 3 Peptide Presented on Hepatitis B Core Antigen Virus-like Particles. Vaccines. 2023; 11(2):462. https://doi.org/10.3390/vaccines11020462
Chicago/Turabian StyleGhasemian, Khadijeh, Inge Broer, Jennifer Schön, Richard Killisch, Nadine Kolp, Armin Springer, and Jana Huckauf. 2023. "Oral and Subcutaneous Immunization with a Plant-Produced Mouse-Specific Zona Pellucida 3 Peptide Presented on Hepatitis B Core Antigen Virus-like Particles" Vaccines 11, no. 2: 462. https://doi.org/10.3390/vaccines11020462





