Renal Biopsy Diagnosis of Acute Tubular Injury after Pfizer-BioNTech COVID-19 Vaccination: A Case Report
Abstract
:1. Introduction
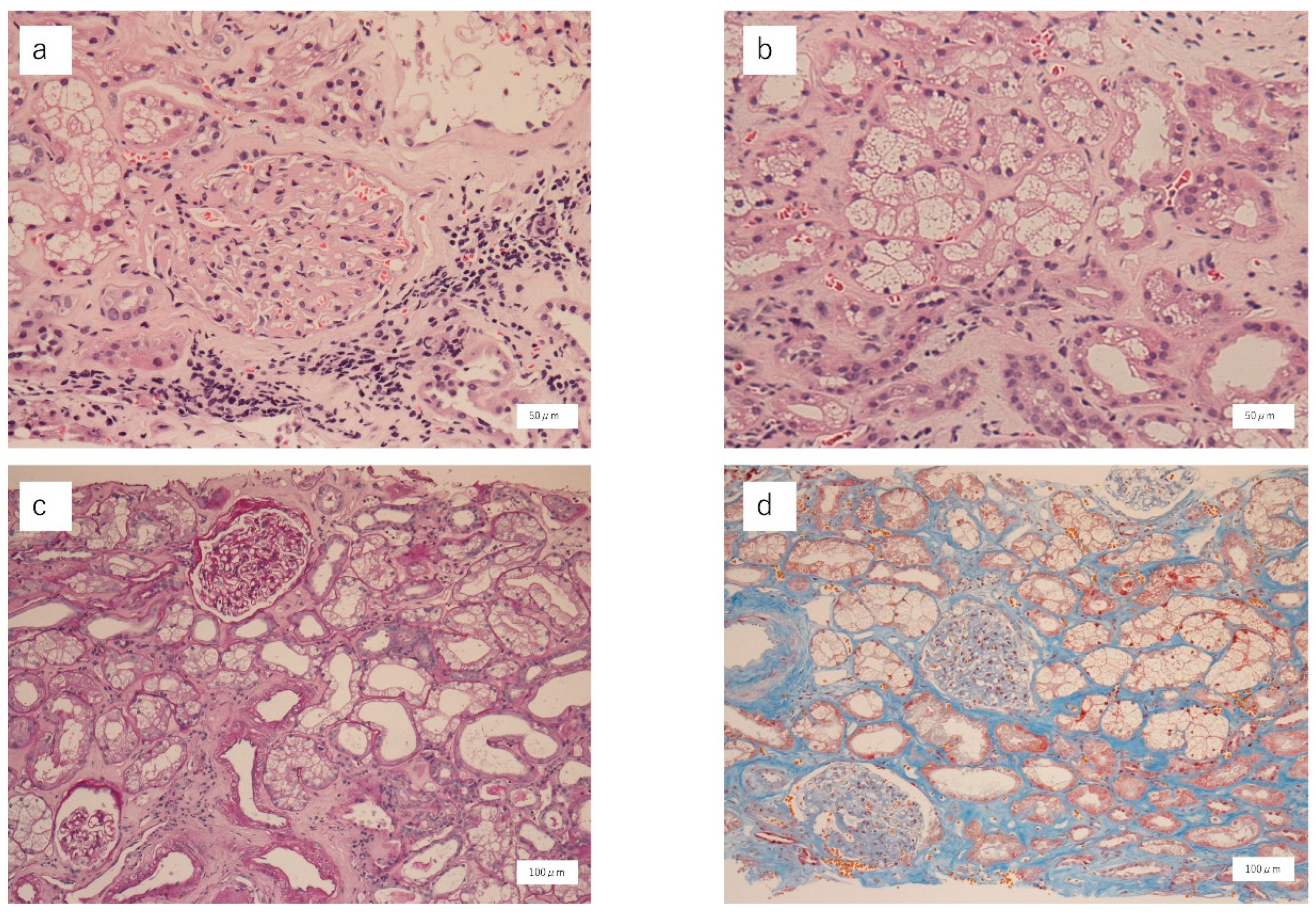
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Prescott, H.C.; Angus, D.C. Enhancing recovery from sepsis: A review. JAMA 2018, 319, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Sadoff, J.; Gray, G.; Vandebosch, A.; Cárdenas, V.; Shukarev, G.; Grinsztejn, B.; Goepfert, P.A.; Truyers, C.; Fennema, H.; Spiessens, B.; et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against COVID-19. N. Engl. J. Med. 2021, 384, 2187–2201. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. COVID Data Tracker 2021. Available online: https://covid.cdc.gov/covid-data-tracker/#datatracker-home (accessed on 10 February 2023).
- Leclerc, S.; Royal, V.; Lamarche, C.; Laurin, L.P. Minimal change disease with severe acute kidney injury following the Oxford-Astra Zeneca COVID-19 vaccine: A case report. Am. J. Kidney Dis. 2021, 78, 607–610. [Google Scholar] [CrossRef]
- Hanna, C.; Hernandez, L.P.H.; Bu, L.; Kizilbash, S.; Najera, L.; Rheault, M.N.; Czyzyk, J.; Kouri, A.M. IgA nephropathy presenting as macroscopic hematuria in 2 pediatric patients after receiving the Pfizer COVID-19 vaccine. Kidney Int. 2021, 100, 705–706. [Google Scholar] [CrossRef]
- Maye, J.A.; Chong, H.P.; Rajagopal, V.; Petchey, W. Reactivation of IgA vasculitis following COVID-19 vaccination. BMJ Case Rep. 2021, 14, e247188. [Google Scholar] [CrossRef]
- Shakoor, M.T.; Birkenbach, M.P.; Lynch, M. ANCA-associated vasculitis following Pfizer-BioNTech COVID-19 vaccine. Am. J. Kidney Dis. 2021, 78, 611–613. [Google Scholar] [CrossRef]
- Dheir, H.; Sipahi, S.; Cakar, G.C.; Yaylaci, S.; Hacibekiroglu, T.; Karabay, O. Acute tubulointerstitial nephritis after COVID-19 m-RNA BNT162b2 vaccine. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 6171–6173. [Google Scholar] [CrossRef]
- De Fabritiis, M.; Angelini, M.L.; Fabbrizio, B.; Cenacchi, G.; Americo, C.; Cristino, S.; Lifrieri, M.F.; Cappuccilli, M.; Spazzoli, A.; Zambianchi, L.; et al. Renal thrombotic microangiopathy in concurrent COVID-19 vaccination and infection. Pathogens 2021, 10, 1045. [Google Scholar] [CrossRef]
- Chen, G.; Li, X.; Sun, M.; Zhou, Y.; Yin, M.; Zhao, B.; Li, X. COVID-19 mRNA vaccines are generally safe in the short term: A vaccine vigilance real-world study says. Front. Immunol. 2021, 12, 669010. [Google Scholar] [CrossRef] [PubMed]
- Klomjit, N.; Alexander, M.P.; Fervenza, F.C.; Zoghby, Z.; Garg, A.; Hogan, M.C.; Nasr, S.H.; Abu Minshar, M.; Zand, L. COVID-19 vaccination and glomerulonephritis. Kidney Int. Rep. 2021, 6, 2969–2978. [Google Scholar] [CrossRef] [PubMed]
- Lewington, A.J.; Cerda, J.; Mehta, R.L. Raising awareness of acute kidney injury: A global perspective of a silent killer. Kidney Int. 2013, 84, 457–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kellum, J.A.; Romagnani, P.; Ashuntantang, G.; Ronco, C.; Zarbock, A.; Anders, H.J. Acute kidney injury. Nat. Rev. Dis. Prim. 2021, 7, 52. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, D.; Kumar, S.V.; Marschner, J.; Desai, J.; Holderied, A.; Rath, L.; Kraft, F.; Lei, Y.; Fukasawa, Y.; Moeckel, G.W.; et al. Histones and neutrophil extracellular traps enhance tubular necrosis and remote organ injury in ischemic AKI. J. Am. Soc. Nephrol. 2017, 28, 1753–1768. [Google Scholar] [CrossRef] [Green Version]
- Luo, H.; Li, X.; Ren, Q.; Zhou, Y.; Chen, G.; Zhao, B.; Li, X. Acute kidney injury after COVID-19 vaccines: A real-world study. Ren. Fail. 2022, 44, 958–965. [Google Scholar] [CrossRef]
- Bagshaw, S.M.; George, C.; Bellomo, R.; ANZICS Database Management Committee. Early acute kidney injury and sepsis: A multicentre evaluation. Crit. Care 2008, 12, R47. [Google Scholar] [CrossRef] [Green Version]
- Mehta, R.L.; A Burdmann, E.; Cerdá, J.; Feehally, J.; Finkelstein, F.; García-García, G.; Godin, M.; Jha, V.; Lameire, N.H.; Levin, N.W.; et al. Recognition and management of acute kidney injury in the International Society of Nephrology oby25 Global Snapshot: A multinational cross-sectional study. Lancet 2016, 387, 2017–2025. [Google Scholar] [CrossRef]
- Cerda, J.; Bagga, A.; Kher, V.; Chakravarthi, R.M. The contrasting characteristics of acute kidney injury in developed and developing countries. Nat. Clin. Pract. Nephrol. 2008, 4, 138–153. [Google Scholar] [CrossRef]
- Jennette, J.C.; Nachman, P.H. ANCA glomerulonephritis and vasculitis. Clin. J. Am. Soc. Nephrol. 2017, 12, 1680–1691. [Google Scholar] [CrossRef] [Green Version]
- Pacheco, I.C.R.; Costa, D.M.D.N.; Sousa, D.S.; Salgado Filho, N.; Silva, G.E.B.; Neves, P.D.M.M. Kidney injury associated with COVID-19 infection and vaccine: A narrative review. Front. Med. 2022, 9, 956158. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Zhao, J.; Jia, X.-Y.; Zhu, S.-N.; Jin, Q.-Z.; Cheng, X.-Y.; Zhao, M.-H. Anti-glomerular basement membrane disease: Outcomes of different therapeutic regimens in a large single-center Chinese cohort study. Medicine 2011, 90, 303–311. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soma, Y.; Kitaji, D.; Hoshino, K.; Sunohara, S.; Iwano, T.; Kawano, N. Renal Biopsy Diagnosis of Acute Tubular Injury after Pfizer-BioNTech COVID-19 Vaccination: A Case Report. Vaccines 2023, 11, 464. https://doi.org/10.3390/vaccines11020464
Soma Y, Kitaji D, Hoshino K, Sunohara S, Iwano T, Kawano N. Renal Biopsy Diagnosis of Acute Tubular Injury after Pfizer-BioNTech COVID-19 Vaccination: A Case Report. Vaccines. 2023; 11(2):464. https://doi.org/10.3390/vaccines11020464
Chicago/Turabian StyleSoma, Yu, Daiyu Kitaji, Kaoru Hoshino, Sumire Sunohara, Takehisa Iwano, and Naomi Kawano. 2023. "Renal Biopsy Diagnosis of Acute Tubular Injury after Pfizer-BioNTech COVID-19 Vaccination: A Case Report" Vaccines 11, no. 2: 464. https://doi.org/10.3390/vaccines11020464
APA StyleSoma, Y., Kitaji, D., Hoshino, K., Sunohara, S., Iwano, T., & Kawano, N. (2023). Renal Biopsy Diagnosis of Acute Tubular Injury after Pfizer-BioNTech COVID-19 Vaccination: A Case Report. Vaccines, 11(2), 464. https://doi.org/10.3390/vaccines11020464



