BNT162b2 COVID-19 Vaccine Safety among Healthcare Workers of a Tertiary Hospital in Italy
Abstract
1. Introduction
2. Materials and Methods
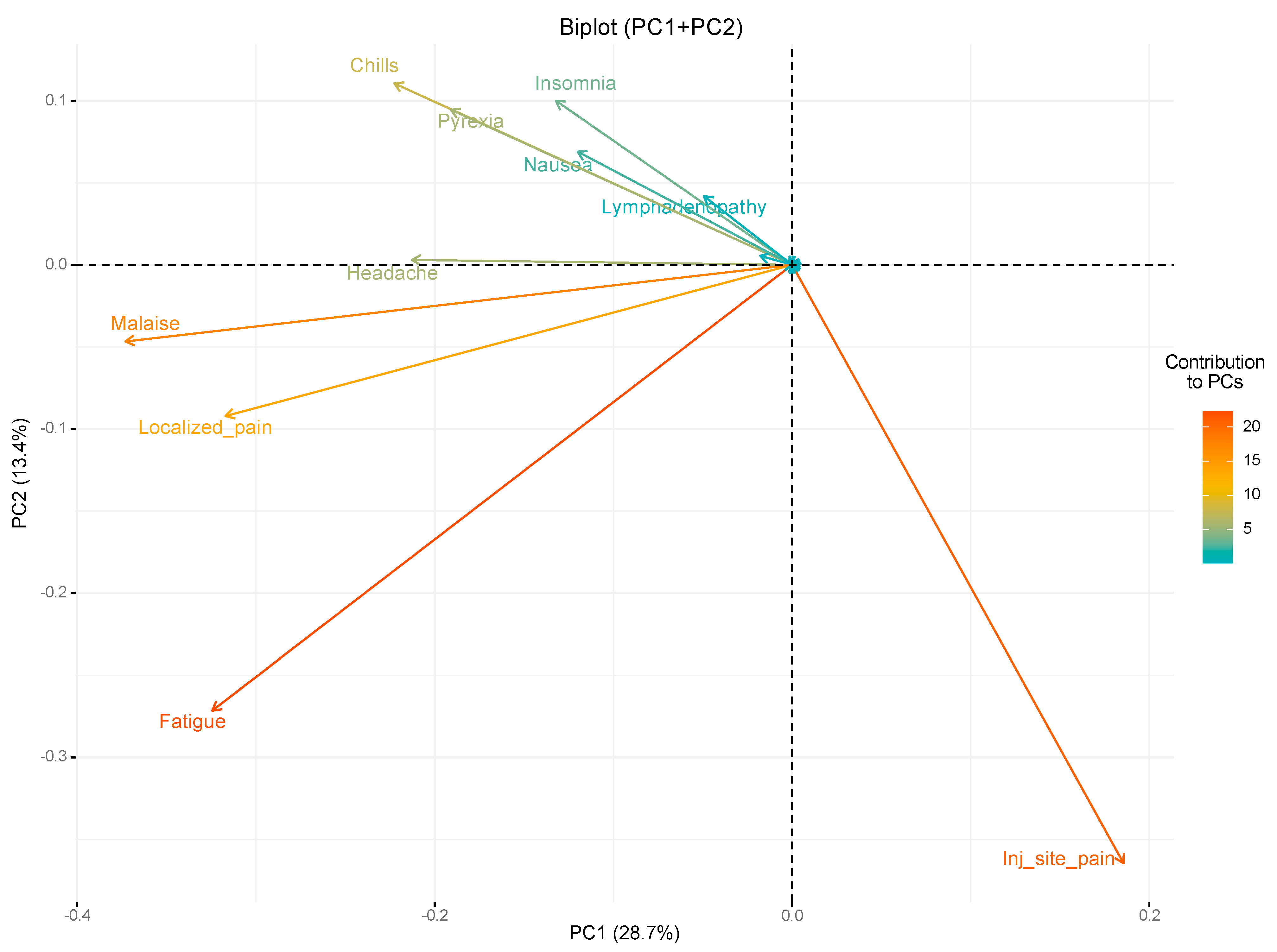
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Walker, P.G.T.; Whittaker, C.; Watson, O.J.; Baguelin, M.; Winskill, P.; Hamlet, A.; Djafaara, B.A.; Cucunubá, Z.; Mesa, D.O.; Green, W.; et al. The Impact of COVID-19 and Strategies for Mitigation and Suppression in Low- And Middle-Income Countries. Science 2020, 369, 413–422. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, G.; Pan, H.; Li, C.; Hu, Y.; Chu, K.; Han, W.; Chen, Z.; Tang, R.; Yin, W.; et al. Safety, Tolerability, and Immunogenicity of an Inactivated SARS-CoV-2 Vaccine in Healthy Adults Aged 18–59 Years: A Randomised, Double-Blind, Placebo-Controlled, Phase 1/2 Clinical Trial. Lancet Infect. Dis. 2021, 21, 181–192. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Weekly Epidemiological Update on COVID-19 - 25 January 2023. Available online: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---25-january-2023 (accessed on 26 January 2023).
- Italy: WHO Coronavirus Disease (COVID-19) Dashboard With Vaccination Data|WHO Coronavirus (COVID-19) Dashboard With Vaccination Data. Available online: https://covid19.who.int/region/euro/country/it (accessed on 28 January 2023).
- Istituto Superiore di Sanità (ISS) e Istituto Nazionale di Statistica (ISTAT). «IMPATTO DELL’EPIDEMIA COVID-19 SULLA MORTALITÀ TOTALE DELLA POPOLAZIONE RESIDENTE. ANNI 2020-2021 E GENNAIO 2022», Technical Report. 2022. Available online: https://www.istat.it/it/files//2022/03/Report_ISS_ISTAT_2022_tab3.pdf (accessed on 28 January 2023).
- Sacco, C.; Mateo-Urdiales, A.; Rota, M.C.; Fabiani, M.; Boros, S.; Bressi, M.; Fenicia Vescio, M.; Siddu, A.; Battilomo, S.; Del Manso, M..; et al. Infezioni Da SARS-CoV-2, Ricoveri e Decessi Associati a COVID-19 Direttamente Evitati Dalla Vaccinazione Italia, 27 Dicembre 2020-31 Gennaio 2022. Technical report. 2022. Available online: https://www.iss.it/documents/20126/6703853/NT_Eventi+evitati+COVID19_LAST.pdf/a140e155-bd62-adcd-1b29-d1be3464ed48?t=1649832260103 (accessed on 28 January 2023).
- Wilder-Smith, A.; Chiew, C.J.; Lee, V.J. Can We Contain the COVID-19 Outbreak with the Same Measures as for SARS? Lancet Infect. Dis. 2020, 20, e102–e107. [Google Scholar] [CrossRef] [PubMed]
- Ripabelli, G.; Tamburro, M.; Buccieri, N.; Adesso, C.; Caggiano, V.; Cannizzaro, F.; di Palma, M.A.; Mantuano, G.; Montemitro, V.G.; Natale, A.; et al. Active Surveillance of Adverse Events in Healthcare Workers Recipients After Vaccination with COVID-19 BNT162b2 Vaccine (Pfizer-BioNTech, Comirnaty): A Cross-Sectional Study. J. Community Health 2022, 47, 211–225. [Google Scholar] [CrossRef]
- Haque, A.; Pant, A.B. Efforts at COVID-19 Vaccine Development: Challenges and Successes. Vaccines 2020, 8, 739. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-D.; Hwang, I.; Ku, K.B.; Lee, S.; Kim, S.-J.; Kim, C. Progress and Challenges in the Development of COVID-19 Vaccines and Current Understanding of SARS-CoV-2- Specific Immune Responses. J. Microbiol. Biotechnol. 2020, 30, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Guo, P.; Zhang, X.; Yu, Z.; Zhang, W.; Sun, H. SARS-CoV-2 Vaccine Candidates in Rapid Development. Hum. Vaccines Immunother. 2020, 17, 644–653. [Google Scholar] [CrossRef]
- Ferrara, P.; Albano, L. COVID-19 and Healthcare Systems: What Should We Do Next? Public Health 2020, 185, 1. [Google Scholar] [CrossRef]
- Della Valle, P.; Fabbri, M.; Madotto, F.; Ferrara, P.; Cozzolino, P.; Calabretto, E.; D’orso, M.I.; Longhi, E.; Polosa, R.; Riva, M.A.; et al. Occupational Exposure in the Lombardy Region (Italy) to SARS-CoV-2 Infection: Results from the MUSTANG–OCCUPATION–COVID-19 Study. Int. J. Environ. Res. Public Health 2021, 18, 2567. [Google Scholar] [CrossRef]
- Ponticelli, D.; Madotto, F.; Conti, S.; Antonazzo, I.C.; Vitale, A.; della Ragione, G.; Romano, M.L.; Borrelli, M.; Schiavone, B.; Polosa, R.; et al. Response to BNT162b2 MRNA COVID-19 Vaccine among Healthcare Workers in Italy: A 3-Month Follow-Up. Intern. Emerg. Med. 2022, 17, 481–486. [Google Scholar] [CrossRef]
- Shimabukuro, T.; Nair, N. Allergic Reactions Including Anaphylaxis After Receipt of the First Dose of Pfizer-BioNTech COVID-19 Vaccine. JAMA 2021, 325, 780–781. [Google Scholar] [CrossRef]
- EMA Recommends First COVID-19 Vaccine for Authorisation in the EU | European Medicines Agency. Available online: https://www.ema.europa.eu/en/news/ema-recommends-first-covid-19-vaccine-authorisation-eu (accessed on 28 January 2023).
- BioNTech/Pfizer Vaccine Authorised Answers to Frequently Asked Questions on AIFA’s Website. Available online: https://www.aifa.gov.it/en/-/autorizzato-il-vaccino-biontech-pfizer (accessed on 28 January 2023).
- Ministero della Salute Decreto 2 Gennaio 2021—Adozione Piano Strategico per La Vaccinazione Anti-SARS-CoV-2/COVID-19. Available online: https://www.trovanorme.salute.gov.it/norme/renderNormsanPdf?anno=2021&codLeg=78657&parte=1%20&serie=null (accessed on 28 January 2023).
- Dooling, K.; McClung, N.; Chamberland, M.; Marin, M.; Wallace, M.; Bell, B.P.; Lee, G.M.; Talbot, H.K.; Romero, J.R.; Oliver, S.E. The Advisory Committee on Immunization Practices’ Interim Recommendation for Allocating Initial Supplies of COVID-19 Vaccine—United States, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 1857–1859. [Google Scholar] [CrossRef]
- Amit, S.; Beni, S.A.; Biber, A.; Grinberg, A.; Leshem, E.; Regev-Yochay, G. Postvaccination COVID-19 among Healthcare Workers, Israel—Volume 27, Number 4—April 2021—Emerging Infectious Diseases Journal—CDC. Emerg. Infect. Dis. 2021, 27, 1220–1222. [Google Scholar] [CrossRef] [PubMed]
- Ministero della Salute; Presidenza del Consiglio dei Ministri; Istituto Superiore di Sanità; Agenzia Nazionale per i Servizi Sanitari Regionali Vaccinazione Anti-SARS-CoV-2/COVID-19. Piano Strategico. Elementi Di Preparazione e Di Implementazione Della Strategia Vaccinale. Aggiornamento Del 12 Dicembre 2020. Gazzetta Ufficiale Della Repubblica Italiana Serie Generale—N. 72 Del 24-3-2021. Available online: https://www.trovanorme.salute.gov.it/norme/renderPdf.spring?seriegu=SG&datagu=24/03/2021&redaz=21A01802&artp=1&art=1&subart=1&subart1=10&vers=1&prog=001 (accessed on 28 January 2023).
- Gazzetta Ufficiale della Repubblica Italiana DECRETO-LEGGE 24 Marzo 2022, n. 24 Disposizioni Urgenti per Il Superamento Delle Misure Di Contrasto Alla Diffusione Dell’epidemia Da COVID-19, in Conseguenza Della Cessazione Dello Stato Di Emergenza. (22G00034) (GU Serie Generale n.70 Del 24-03-2022). Available online: https://www.gazzettaufficiale.it/eli/id/2022/03/24/22G00034/sg (accessed on 28 January 2023).
- Riccardo, F.; Guzzetta, G.; Urdiales, A.M.; del Manso, M.; Andrianou, X.D.; Bella, A.; Pezzotti, P.; Carbone, S.; de Vito, T.; Maraglino, F.; et al. COVID-19 Response: Effectiveness of Weekly Rapid Risk Assessments, Italy. Bull. World Health Organ. 2022, 100, 161–167. [Google Scholar] [CrossRef]
- Nurchis, M.C.; Lontano, A.; Pascucci, D.; Sapienza, M.; Marziali, E.; Castrini, F.; Messina, R.; Regazzi, L.; Causio, F.A.; di Pilla, A.; et al. COVID-19 Vaccination Campaign among the Health Workers of Fondazione Policlinico Universitario Agostino Gemelli IRCCS: A Cost–Benefit Analysis. Int. J. Environ. Res. Public Health 2022, 19, 7848. [Google Scholar] [CrossRef] [PubMed]
- Rief, W. Fear of Adverse Effects and COVID-19 Vaccine Hesitancy: Recommendations of the Treatment Expectation Expert Group. JAMA Health Forum 2021, 2, e210804. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.K.H.; Lau, K.T.K.; Xiong, X.; Au, I.C.H.; Lai, F.T.T.; Wan, E.Y.F.; Chui, C.S.L.; Li, X.; Chan, E.W.Y.; Gao, L.; et al. Adverse Events of Special Interest and Mortality Following Vaccination with MRNA (BNT162b2) and Inactivated (CoronaVac) SARS-CoV-2 Vaccines in Hong Kong: A Retrospective Study. PLoS Med. 2022, 19, e1004018. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, S.; Nagpal, R.; Marya, C.M.; Taneja, P.; Kataria, S. Adverse Events Occurring Post-Covid-19 Vaccination among Healthcare Professionals—A Mixed Method Study. Int. Immunopharmacol. 2021, 100, 108136. [Google Scholar] [CrossRef] [PubMed]
- Welcome|Public Health Ontario. Available online: https://www.publichealthontario.ca/ (accessed on 28 January 2023).
- World Health Organization. Global Manual on Surveillance of Adverse Events Following Immunization, 2016 Update. 2014. Available online: https://apps.who.int/iris/handle/10665/206144 (accessed on 28 January 2023).
- Bhandari, B.; Rayamajhi, G.; Lamichhane, P.; Shenoy, A.K. Adverse Events Following Immunization with COVID-19 Vaccines: A Narrative Review. Biomed. Res. Int. 2022, 2022, 2911333. [Google Scholar] [CrossRef] [PubMed]
- Tozzi, A.E.; Gesualdo, F.; D’Ambrosio, A.; Pandolfi, E.; Agricola, E.; Lopalco, P. Can Digital Tools Be Used for Improving Immunization Programs? Front. Public Health 2016, 4, 36. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Covid-19 Vaccines: Safety Surveillance Manual; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Adverse Events Following Immunization (AEFI). Available online: https://www.who.int/teams/regulation-prequalification/regulation-and-safety/pharmacovigilance/health-professionals-info/aefi (accessed on 28 January 2023).
- Adverse Events Following Immunisation: What Are They, and When Are They Cause for Concern?|Gavi, the Vaccine Alliance. Available online: https://www.gavi.org/vaccineswork/adverse-events-following-immunisation-what-are-they-and-when-are-they-cause-concern (accessed on 28 January 2023).
- Turner, P.J.; Ansotegui, I.J.; Campbell, D.E.; Cardona, V.; Ebisawa, M.; El-Gamal, Y.; Fineman, S.; Geller, M.; Gonzalez-Estrada, A.; Greenberger, P.A.; et al. COVID-19 Vaccine-Associated Anaphylaxis: A Statement of the World Allergy Organization Anaphylaxis Committee. World Allergy Organ. J. 2021, 14, 100517. [Google Scholar] [CrossRef] [PubMed]
- Crawford, N.W.; Clothier, H.; Hodgson, K.; Selvaraj, G.; Easton, M.L.; Buttery, J.P. Active Surveillance for Adverse Events Following Immunization. Expert Rev. Vaccines 2014, 13, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Cashman, P.; Macartney, K.; Khandaker, G.; King, C.; Gold, M.; Durrheima, D.N. Participant-Centred Active Surveillance of Adverse Events Following Immunisation: A Narrative Review. Int. Health 2017, 9, 164–176. [Google Scholar] [CrossRef] [PubMed]
- Heininger, U.; Holm, K.; Caplanusi, I.; Bailey, S.R.; Asfijah Abdoellah, S.; Arellano, F.; Arlett, P.; Ayoub, A.; Sjafri Bachtiar, N.; Bahri, P.; et al. Guide to Active Vaccine Safety Surveillance: Report of CIOMS Working Group on Vaccine Safety—Executive Summary. Vaccine 2017, 35, 3917–3921. [Google Scholar] [CrossRef] [PubMed]
- Waldman, E.A.; Luhm, K.R.; Monteiro, S.A.M.G.; Freitas, F.R.M. de Surveillance of Adverse Effects Following Vaccination and Safety of Immunization Programs. Rev. Saude Publica 2011, 45, 173–184. [Google Scholar] [CrossRef]
- Pharmacovigilance on COVID-19 Vaccines|Italian Medicines Agency. Available online: https://www.aifa.gov.it/en/farmacovigilanza-vaccini-covid-19 (accessed on 28 January 2023).
- Rosenblum, H.G.; Gee, J.; Liu, R.; Marquez, P.L.; Zhang, B.; Strid, P.; Abara, W.E.; McNeil, M.M.; Myers, T.R.; Hause, A.M.; et al. Safety of MRNA Vaccines Administered during the Initial 6 Months of the US COVID-19 Vaccination Programme: An Observational Study of Reports to the Vaccine Adverse Event Reporting System and v-Safe. Lancet Infect. Dis. 2022, 22, 802–812. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 MRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Anderson, E.J.; Rouphael, N.G.; Widge, A.T.; Jackson, L.A.; Roberts, P.C.; Makhene, M.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; Pruijssers, A.J.; et al. Safety and Immunogenicity of SARS-CoV-2 MRNA-1273 Vaccine in Older Adults. N. Engl. J. Med. 2020, 383, 2427–2438. [Google Scholar] [CrossRef]
- Baden, L.R.; el Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the MRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Barda, N.; Dagan, N.; Ben-Shlomo, Y.; Kepten, E.; Waxman, J.; Ohana, R.; Hernán, M.A.; Lipsitch, M.; Kohane, I.; Netzer, D.; et al. Safety of the BNT162b2 MRNA Covid-19 Vaccine in a Nationwide Setting. N. Engl. J. Med. 2021, 385, 1078–1090. [Google Scholar] [CrossRef]
- Klein, N.P.; Lewis, N.; Goddard, K.; Fireman, B.; Zerbo, O.; Hanson, K.E.; Donahue, J.G.; Kharbanda, E.O.; Naleway, A.; Nelson, J.C.; et al. Surveillance for Adverse Events after COVID-19 MRNA Vaccination. JAMA—J. Am. Med. Assoc. 2021, 326, 1390–1399. [Google Scholar] [CrossRef] [PubMed]
- García-Grimshaw, M.; Ceballos-Liceaga, S.E.; Hernández-Vanegas, L.E.; Núñez, I.; Hernández-Valdivia, N.; Carrillo-García, D.A.; Michel-Chávez, A.; Galnares-Olalde, J.A.; Carbajal-Sandoval, G.; del Mar Saniger-Alba, M.; et al. Neurologic Adverse Events among 704,003 First-Dose Recipients of the BNT162b2 MRNA COVID-19 Vaccine in Mexico: A Nationwide Descriptive Study. Clin. Immunol. 2021, 229, 108786. [Google Scholar] [CrossRef] [PubMed]
- Lai, F.T.T.; Li, X.; Peng, K.; Huang, L.; Ip, P.; Tong, X.; Chui, C.S.L.; Wan, E.Y.F.; Wong, C.K.H.; Chan, E.W.Y.; et al. Carditis After COVID-19 Vaccination With a Messenger RNA Vaccine and an Inactivated Virus Vaccine A Case-Control Study. Ann. Intern. Med. 2022, 175, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Shemer, A.; Pras, E.; Einan-Lifshitz, A.; Dubinsky-Pertzov, B.; Hecht, I. Association of COVID-19 Vaccination and Facial Nerve Palsy: A Case-Control Study. JAMA Otolaryngol. Head Neck Surg. 2021, 147, 739–743. [Google Scholar] [CrossRef]
- Allegato, I. Riassunto delle Caratteristiche del Prodotto Comirnaty Original/Omicron BA.1, Summary of Product Characteristics. 2023. Available online: https://farmaci.agenziafarmaco.gov.it/aifa/servlet/PdfDownloadServlet?pdfFileName=footer_005389_050306_RCP.pdf&retry=0&sys=m0b1l3 (accessed on 28 January 2023).
- McKenzie, E.; Zhang, L.; Zaki, P.; Chan, S.; Ganesh, V.; Razvi, Y.; Tsao, M.; Barnes, E.; Hwang, M.K.; Deangelis, C.; et al. Re-Analysis of Symptom Clusters in Advanced Cancer Patients Attending a Palliative Outpatient Radiotherapy Clinic. Ann. Palliat. Med 2019, 8, 140–149. [Google Scholar] [CrossRef]
- Ye, W.; Lu, W.; Tang, Y.; Chen, G.; Li, X.; Ji, C.; Hou, M.; Zeng, G.; Lan, X.; Wang, Y.; et al. Identification of COVID-19 Clinical Phenotypes by Principal Component Analysis-Based Cluster Analysis. Front. Med. 2020, 7, 782. [Google Scholar] [CrossRef]
- Agenzia Italiana del Farmaco (AIFA). Rapporto Sulla Sorveglianza Dei Vaccini Anti-COVID-19 13 27/12/2020—26/09/2022. Technical report. 2022. Available online: https://www.aifa.gov.it/documents/20142/1315190/Rapporto_sorveglianza_vaccini_COVID-19_13.pdf (accessed on 28 January 2023).
- Pascucci, D.; Nurchis, M.C.; Sapienza, M.; Castrini, F.; Beccia, F.; D’ambrosio, F.; Grossi, A.; Castagna, C.; Pezzullo, A.M.; Zega, M.; et al. Evaluation of the Effectiveness and Safety of the Bnt162b2 Covid-19 Vaccine in the Vaccination Campaign among the Health Workers of Fondazione Policlinico Universitario Agostino Gemelli Irccs. Int. J. Environ. Res. Public Health 2021, 18, 11098. [Google Scholar] [CrossRef]
- Gringeri, M.; Mosini, G.; Battini, V.; Cammarata, G.; Guarnieri, G.; Carnovale, C.; Clementi, E.; Radice, S. Preliminary Evidence on the Safety Profile of BNT162b2 (Comirnaty): New Insights from Data Analysis in EudraVigilance and Adverse Reaction Reports from an Italian Health Facility. Hum. Vaccin. Immunother. 2021, 17, 2969. [Google Scholar] [CrossRef]
- Pietro Vigezzi, G.; Lume, A.; Minerva, M.; Nizzero, P.; Biancardi, A.; Gianfredi, V.; Odone, A.; Signorelli, C.; Moro, M. Safety Surveillance after BNT162b2 MRNA COVID-19 Vaccination: Results from a Cross-Sectional Survey among Staff of a Large Italian Teaching Hospital. Acta Biomed. 2021, 92, e2021450. [Google Scholar] [CrossRef]
- Ughi, N.; del Gaudio, F.; Dicuonzo, A.; Orso, M.; Micheloni, G.; Puoti, M.; Pani, A.; Scaglione, F.; Zoppini, L.; Rossetti, C.; et al. Host Factors and History of SARS-CoV-2 Infection Impact the Reactogenicity of BNT162b2 MRNA Vaccine: Results from a Cross-Sectional Survey on 7,014 Workers in Healthcare. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 7985–7996. [Google Scholar] [CrossRef]
- Borroni, E.; Consonni, D.; Cugno, M.; Lombardi, A.; Mangioni, D.; Bono, P.; Oggioni, M.; Renteria, S.U.; Bordini, L.; Nava, C.D.; et al. Side Effects among Healthcare Workers from a Large Milan University Hospital after Second Dose of Bnt162b2mrna Covid-19 Vaccine. Medicina del Lavoro 2021, 112, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Alomar, M.; Palaian, S.; Al-Tabakha, M.M. Open Peer Review Pharmacovigilance in Perspective: Drug Withdrawals, Data Mining and Policy Implications [Version 1; Peer Review: 2 Approved]. F1000Research 2019, 8, 2019. [Google Scholar] [CrossRef] [PubMed]
- Carnovale, C.; Gringeri, M.; Battini, V.; Mosini, G.; Invernizzi, E.; Mazhar, F.; Bergamaschi, F.; Fumagalli, M.; Zuccotti, G.; Clementi, E.; et al. Beta-Blocker-Associated Hypoglycaemia: New Insights from a Real-World Pharmacovigilance Study. Br. J. Clin. Pharmacol. 2021, 87, 3320–3331. [Google Scholar] [CrossRef]
- Aristei, L.; D’ambrosio, F.; Villani, L.; Rossi, M.F.; Daniele, A.; Amantea, C.; Damiani, G.; Laurenti, P.; Ricciardi, W.; Gualano, M.R.; et al. Public Health Regulations and Policies Dealing with Preparedness and Emergency Management: The Experience of the COVID-19 Pandemic in Italy. Int. J. Environ. Res. Public Health 2022, 19, 1091. [Google Scholar] [CrossRef] [PubMed]
- Araja, D.; Krumina, A.; Nora-Krukle, Z.; Berkis, U.; Murovska, M. Vaccine Vigilance System: Considerations on the Effectiveness of Vigilance Data Use in COVID-19 Vaccination. Vaccines 2022, 10, 2115. [Google Scholar] [CrossRef]
- Guo, Q.; Duan, S.; Liu, Y.; Yuan, Y. Adverse Drug Events in the Prevention and Treatment of COVID-19: A Data Mining Study on the FDA Adverse Event Reporting System. Front. Pharmacol. 2022, 13, 954359. [Google Scholar] [CrossRef]
- Beccia, F.; di Pilla, A.; Causio, F.A.; Federico, B.; Specchia, M.L.; Favaretti, C.; Boccia, S.; Damiani, G. Narrative Review of the COVID-19 Pandemic’s First Two Years in Italy. Int. J. Environ. Res. Public Health 2022, 19, 15443. [Google Scholar] [CrossRef]

| System Organ Class | Very Common (≥1/10) | Common (≥1/100 to <1/10) | Uncommon (≥1/1000 to <1/100) | Rare (≥1/10,000 to <1/1000) | Very Rare (<1/10,000) | Not Known § |
|---|---|---|---|---|---|---|
| Blood and lymphatic system disorders | – | – | Lymphadenopathy 9.10% | – | – | – |
| Immune system disorders | – | – | Hypersensitivity reactions (e.g., rash, pruritus,) 6.71% | Hypersensitivity reactions (urticaria, angioedema) NR | – | Anaphylaxis NR |
| Metabolism and nutrition disorders | – | – | Decreased appetite NR | – | – | – |
| Psychiatric disorders | – | – | Insomnia 20.06% | – | – | – |
| Nervous system disorders | Headache 27.68% (ref. >50%) | – | Lethargy NR | Acute peripheral facial paralysis NR Photophobia 0.08% Presyncope 0.04% | – | Paresthesia 0.25% Hypoaesthesia 0.04% |
| Cardiac disorders | Tachycardia 0.12% (ref. >60%) | – | – | Myocarditis; Pericarditis NR | ||
| Respiratory disorders * | – | – | – | Sinus congestion 0.08% Cough 0.08% | – | – |
| Gastrointestinal disorders | Diarrhea 0.21% | Nausea 13.96% Vomiting 0.04% | Gastrointestinal disorders 0.12% | – | – | – |
| Skin and subcutaneous tissue disorder | Hyperhidrosis 0.08% Night sweats 0.12% | Aphtha 0.04% | – | Eritema multiforme NR | ||
| Musculoskeletal and connective tissue disorders | Arthralgia 0.12% (ref. >20%) Myalgia 0.04% (ref. >40%) Localized pain 36.90% | – | Pain in extremity NR | – | – | – |
| General disorders and administration site conditions | Injection site pain 63.92% (ref. >80%) Fatigue 48.23% (ref. >60%) Chills 21.29% (ref. >30%) Pyrexia 16.89 (ref. >10%) Injection site swelling 0.04% (ref.>10%) | Injection site redness 0.04% | Malaise 37.77% Injection site pruritus NR Asthenia 0.12% | Pustule 0.04% | – | Extensive swelling of vaccinated limb NR Facial swelling NR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beccia, F.; Regazzi, L.; Marziali, E.; Beccia, V.; Pascucci, D.; Mores, N.; Vetrugno, G.; Laurenti, P. BNT162b2 COVID-19 Vaccine Safety among Healthcare Workers of a Tertiary Hospital in Italy. Vaccines 2023, 11, 477. https://doi.org/10.3390/vaccines11020477
Beccia F, Regazzi L, Marziali E, Beccia V, Pascucci D, Mores N, Vetrugno G, Laurenti P. BNT162b2 COVID-19 Vaccine Safety among Healthcare Workers of a Tertiary Hospital in Italy. Vaccines. 2023; 11(2):477. https://doi.org/10.3390/vaccines11020477
Chicago/Turabian StyleBeccia, Flavia, Luca Regazzi, Eleonora Marziali, Viria Beccia, Domenico Pascucci, Nadia Mores, Giuseppe Vetrugno, and Patrizia Laurenti. 2023. "BNT162b2 COVID-19 Vaccine Safety among Healthcare Workers of a Tertiary Hospital in Italy" Vaccines 11, no. 2: 477. https://doi.org/10.3390/vaccines11020477
APA StyleBeccia, F., Regazzi, L., Marziali, E., Beccia, V., Pascucci, D., Mores, N., Vetrugno, G., & Laurenti, P. (2023). BNT162b2 COVID-19 Vaccine Safety among Healthcare Workers of a Tertiary Hospital in Italy. Vaccines, 11(2), 477. https://doi.org/10.3390/vaccines11020477





