The Debate between the Human Microbiota and Immune System in Treating Aerodigestive and Digestive Tract Cancers: A Review
Abstract
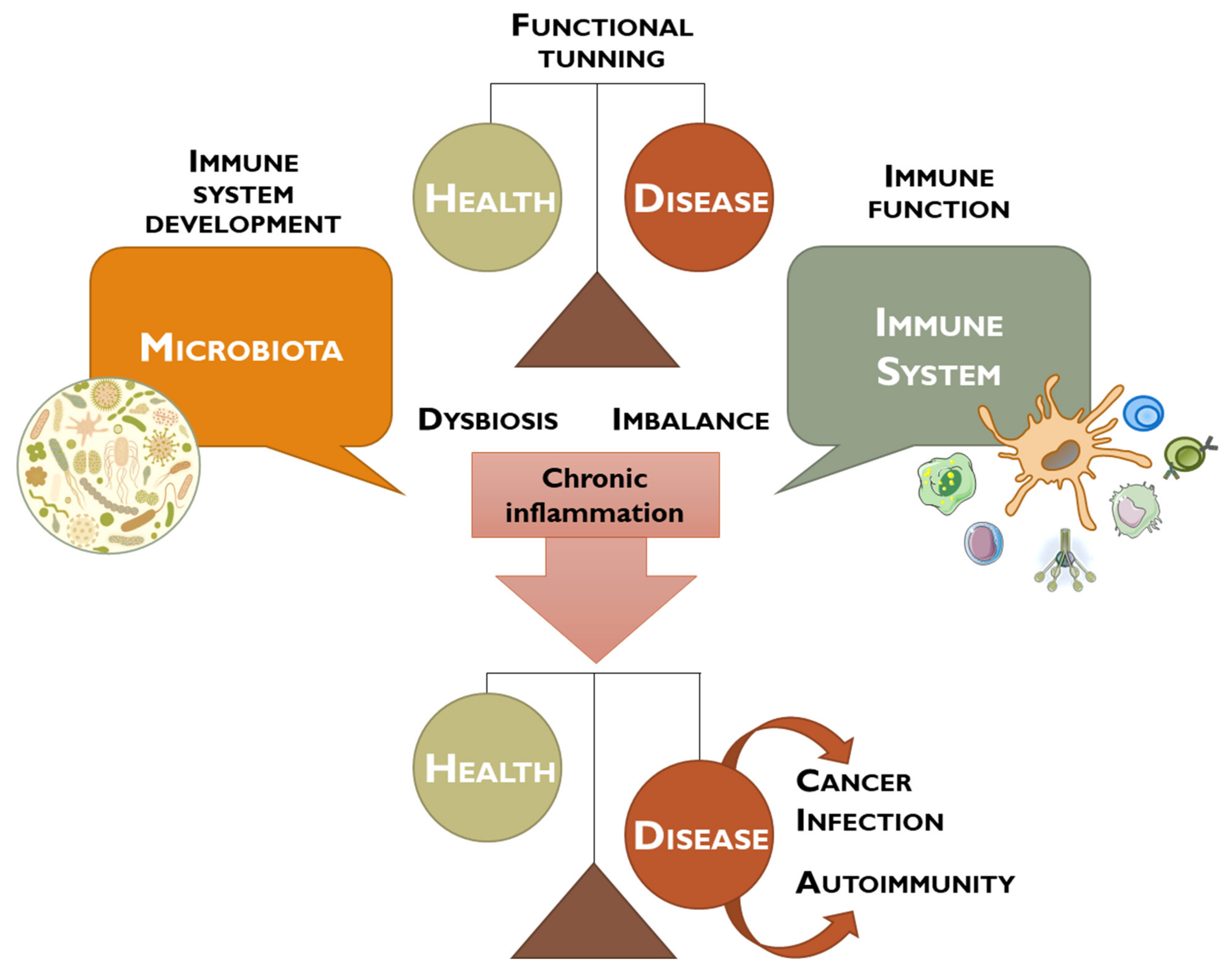
:1. Introduction
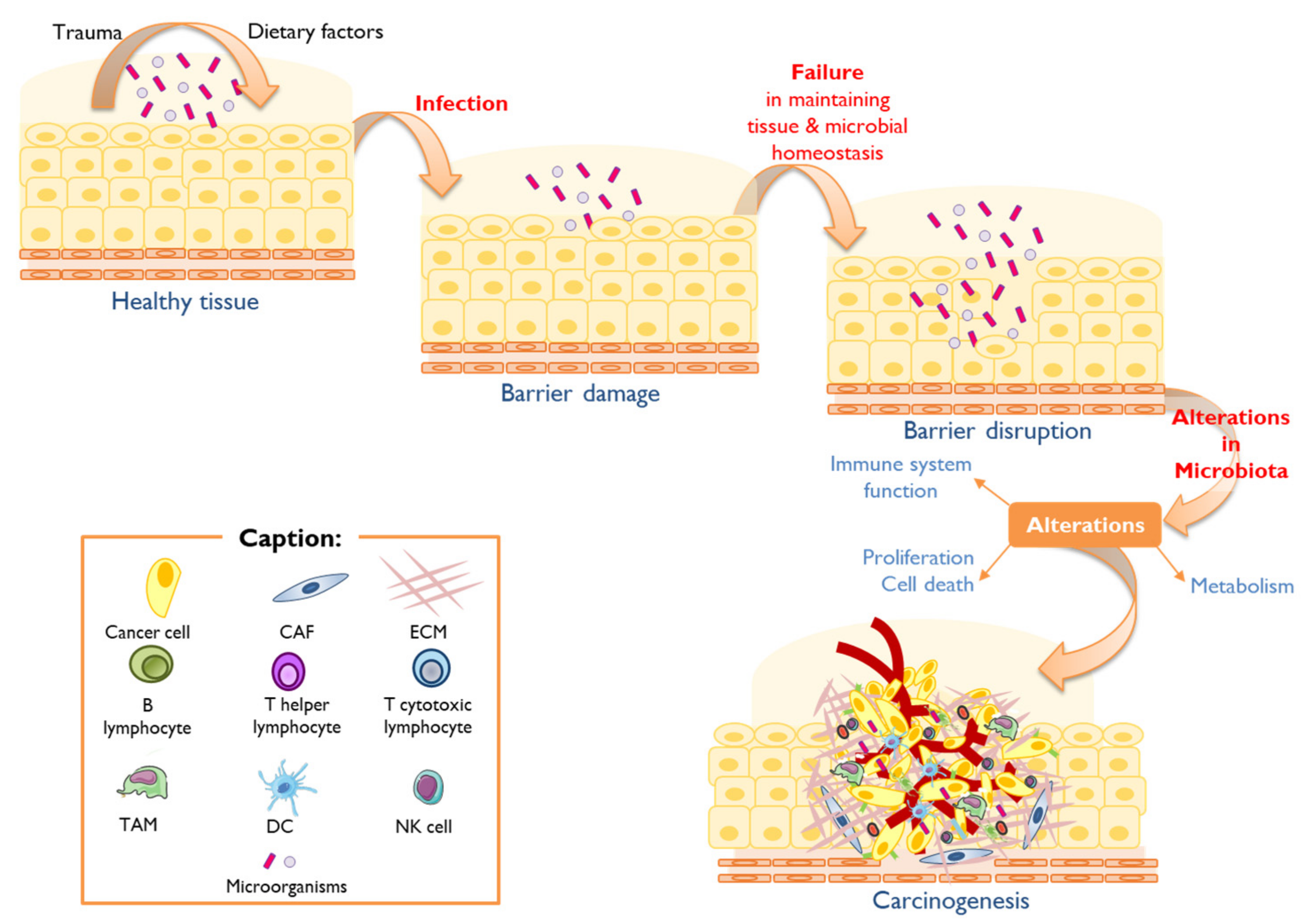
2. Human Microbiota, Dysbiosis, and Cancer
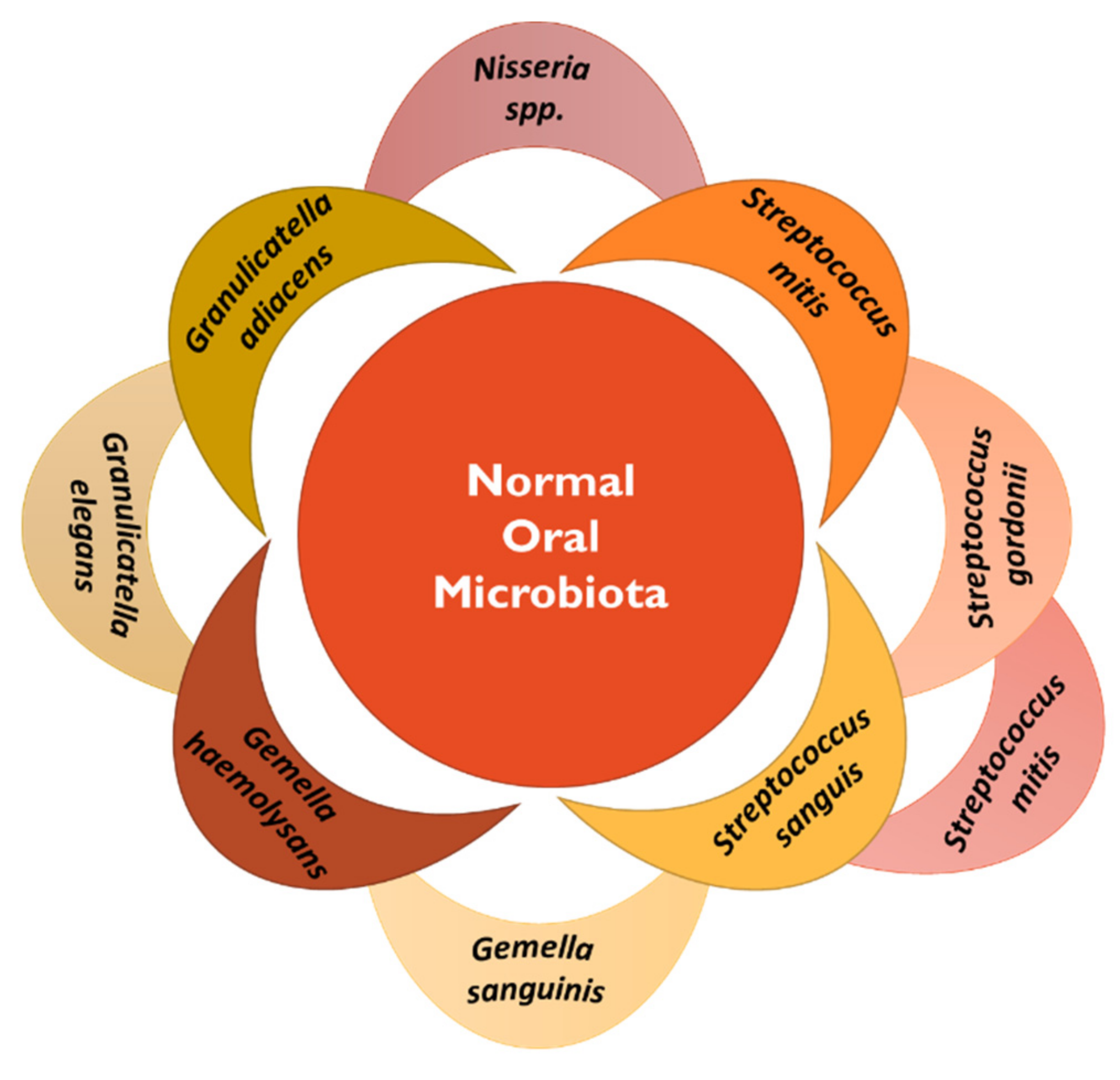
2.1. Oral Cancer
2.2. Esophageal Cancer
2.3. Stomach Cancer
2.4. Colorectal Cancer
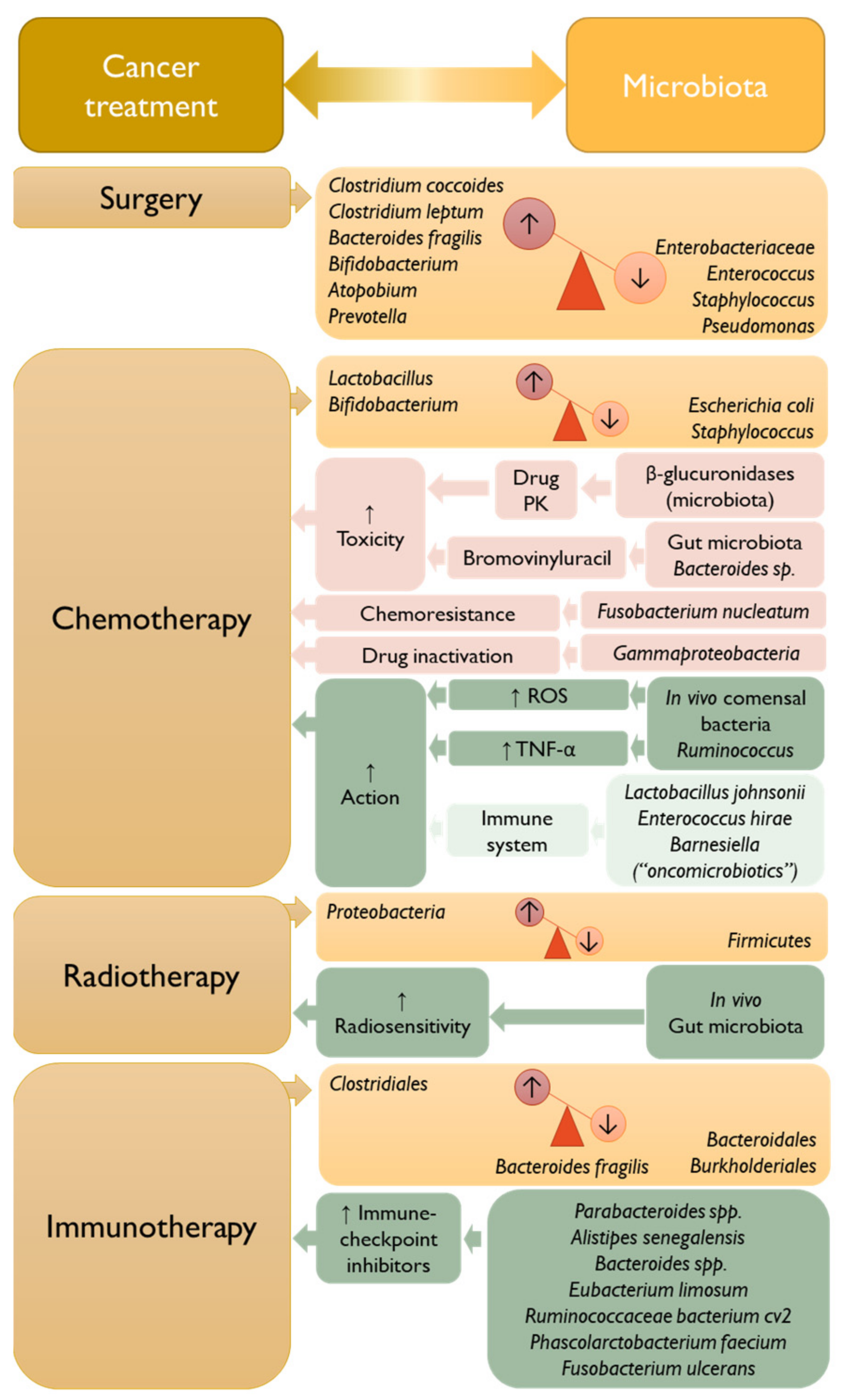
3. The Interplay between Microbiota and Cancer Treatment
3.1. Surgery
3.2. Chemotherapy
3.2.1. Platinum-Based Anticancer Therapy—Oxaliplatin and Cisplatin
3.2.2. Alkylating Agents—Cyclophosphamide
3.2.3. Irinotecan
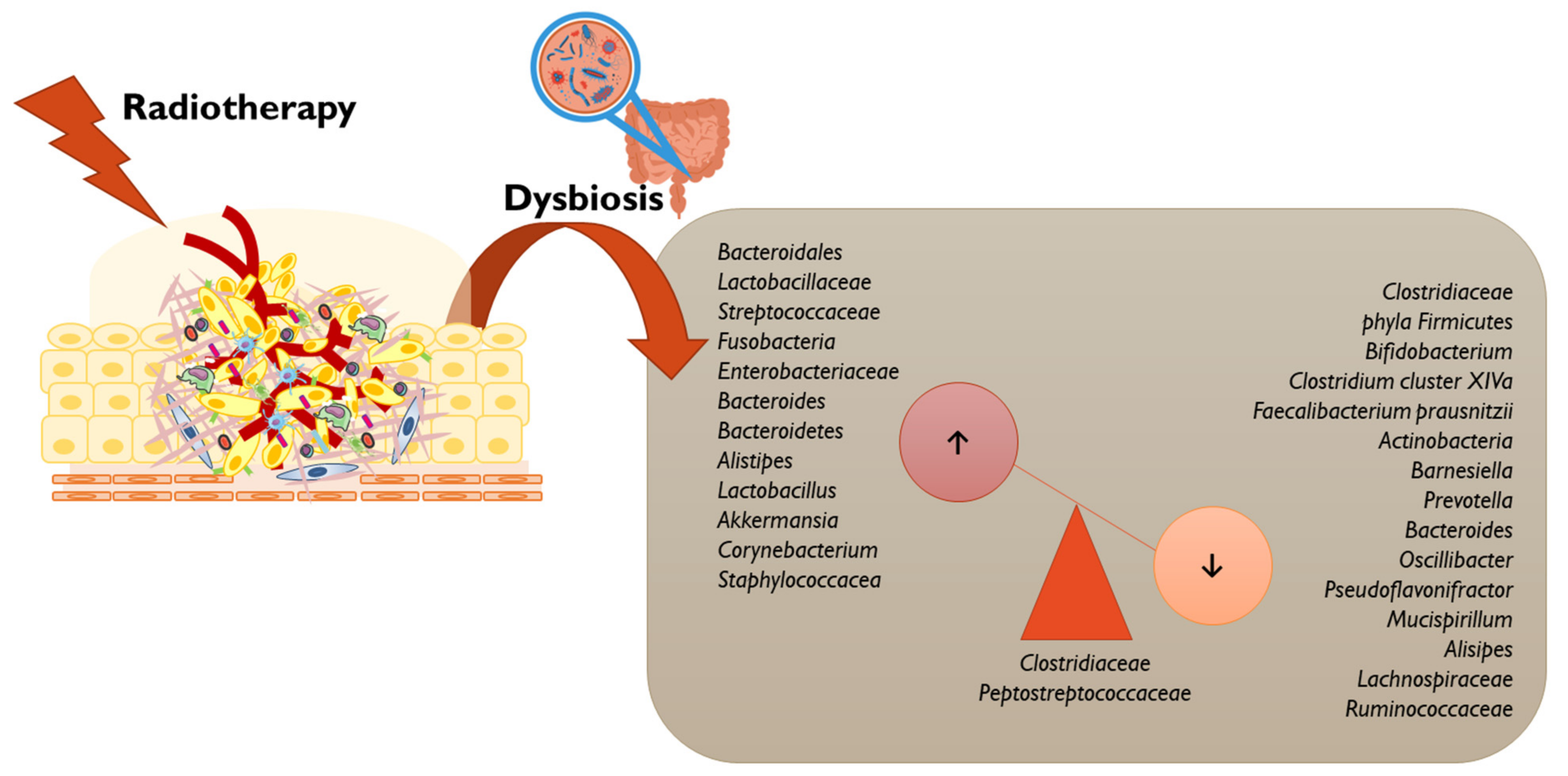
3.3. Radiotherapy
3.4. Immunotherapy
3.4.1. CpG Oligodeoxynucleotide
3.4.2. Anti-CTLA4
3.4.3. Anti-PDL1
4. The Microbiota as Target Therapy
4.1. Prebiotics
4.2. Probiotics
4.3. Fecal Microbiota Transplantation (FMT)
4.4. Nanoparticles and Microbiota
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McDonald, D.; Birmingham, A.; Knight, R. Context and the human microbiome. Microbiome 2015, 3, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roy, S.; Trinchieri, G. Microbiota: A key orchestrator of cancer therapy. Nat. Rev. Cancer 2017, 17, 271–285. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.R.P. A Microbiota Intestinal Nas Doenças Inflamatórias do Intestino e o Potencial Recurso a Probióticos e Prebióticos. Ph.D. Thesis, Universidade de Lisboa, Lisboa, Portugal, 2016. [Google Scholar]
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Bars, P.; Matamoros, S.; Montassier, E.; Le Vacon, F.; Potel, G.; Soueidan, A.; Jordana, F.; de La Cochetière, M.-F. The oral cavity microbiota: Between health, oral disease, and cancers of the aerodigestive tract. Can. J. Microbiol. 2017, 63, 475–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilbert, J.A.; Blaser, M.J.; Caporaso, J.G.; Jansson, J.K.; Lynch, S.V.; Knight, R. Current understanding of the human microbiome. Nat. Med. 2018, 24, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Serdoura, S.V. Microbiota Intestinal e Obesidade. Ph.D. Thesis, Universidade do Porto, Porto, Portugal, 2017. [Google Scholar]
- van den Elsen, L.W.; Poyntz, H.C.; Weyrich, L.S.; Young, W.; Forbes-Blom, E.E. Embracing the gut microbiota: The new frontier for inflammatory and infectious diseases. Clin. Transl. Immunol. 2017, 6, e125. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yao, M.; Lv, L.; Ling, Z.; Li, L. The Human Microbiota in Health and Disease. Engineering 2017, 3, 71–82. [Google Scholar] [CrossRef]
- Pope, J.L.; Tomkovich, S.; Yang, Y.; Jobin, C. Microbiota as a mediator of cancer progression and therapy. Transl. Res. 2017, 179, 139–154. [Google Scholar] [CrossRef] [Green Version]
- Garrett, W.S. Cancer and the microbiota. Science 2015, 348, 80–86. [Google Scholar] [CrossRef] [Green Version]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Dzutsev, A.; Goldszmid, R.S.; Viaud, S.; Zitvogel, L.; Trinchieri, G. The role of the microbiota in inflammation, carcinogenesis, and cancer therapy. Eur. J. Immunol. 2015, 45, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Markets and Markets Human Microbiome Market—Global Forecast to 2029. Available online: https://www.marketsandmarkets.com/Market-Reports/human-microbiome-market-37621904.html (accessed on 27 December 2022).
- Bruneau, A.; Baylatry, M.-T.; Joly, A.C.; Sokol, H. Le microbiote intestinal: Quels impacts sur la carcinogenèse et le traitement du cancer colorectal? Bull. Cancer 2018, 105, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.L.; Wilson, I.D.; Teare, J.; Marchesi, J.R.; Nicholson, J.K.; Kinross, J.M. Gut microbiota modulation of chemotherapy efficacy and toxicity. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; Fang, L.; Lee, M.H. Dysbiosis of gut microbiota in promoting the development of colorectal cancer. Gastroenterol. Rep. 2018, 6, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bull, M.J.; Plummer, N.T. Part 1: The Human Gut Microbiome in Health and Disease. Integr. Med. 2014, 13, 17–22. [Google Scholar]
- Lim, Y.; Totsika, M.; Morrison, M.; Punyadeera, C. Oral microbiome: A new biomarker reservoir for oral and oropharyngeal cancers. Theranostics 2017, 7, 4313–4321. [Google Scholar] [CrossRef]
- Cani, P.D. Human gut microbiome: Hopes, threats and promises. Gut 2018, 67, 1716–1725. [Google Scholar] [CrossRef] [Green Version]
- Schwabe, R.F.; Jobin, C. The microbiome and cancer. Nat. Rev. Cancer 2013, 13, 800–812. [Google Scholar] [CrossRef] [Green Version]
- Rajagopala, S.V.; Vashee, S.; Oldfield, L.M.; Suzuki, Y.; Venter, J.C.; Telenti, A.; Nelson, K.E. The human microbiome and cancer. Cancer Prev. Res. 2017, 10, 226–234. [Google Scholar] [CrossRef] [Green Version]
- Herrero, R.; Park, J.Y.; Forman, D. The fight against gastric cancer—The IARC Working Group report. Best Pract. Res. Clin. Gastroenterol. 2014, 28, 1107–1114. [Google Scholar] [CrossRef]
- Zhou, P.; Hu, Y.; Wang, X.; Shen, L.; Liao, X.; Zhu, Y.; Yu, J.; Zhao, F.; Zhou, Y.; Shen, H.; et al. Microbiome in cancer: An exploration of carcinogenesis, immune responses and immunotherapy. Front. Immunol. 2022, 13, 4397. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Cancer Research (IACR) List of Classifications—IARC Monographs on the Identification of Carcinogenic Hazards to Humans. Available online: https://monographs.iarc.who.int/list-of-classifications (accessed on 19 December 2022).
- International Agency for Cancer Research (IACR). List of Classifications by Cancer Sites with Sufficient or Limited Evidence in Humans, IARC Monographs; IACR: Lyon, France, 2022; Volumes 1–132a. [Google Scholar]
- Biernat, M.M.; Wróbel, T. Bacterial infection and non-hodgkin b-cell lymphoma: Interactions between pathogen, host and the tumor environment. Int. J. Mol. Sci. 2021, 22, 7372. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Zhang, Y.; Zhang, X.; Fu, K. Gastric mucosa-associated lymphoid tissue lymphoma and Helicobacter pylori infection: A review of current diagnosis and management. Biomark. Res. 2016, 4, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zullo, A. Gastric low-grade mucosal-associated lymphoid tissue-lymphoma: Helicobacter pylori and beyond. World J. Gastrointest. Oncol. 2010, 2, 181. [Google Scholar] [CrossRef] [PubMed]
- Wotherspoon, A.C. Gastric lymphoma of mucosa-associated lymphoid tissue and Helicobacter pylori. Annu. Rev. Med. 1998, 49, 289–299. [Google Scholar] [CrossRef]
- Shannon-Lowe, C.; Rickinson, A.B.; Bell, A.I. Epstein-barr virus-associated lymphomas. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160271. [Google Scholar] [CrossRef] [Green Version]
- Ayee, R.; Ofori, M.E.O.; Wright, E.; Quaye, O. Epstein Barr virus associated lymphomas and epithelia cancers in humans. J. Cancer 2020, 11, 1737–1750. [Google Scholar] [CrossRef] [Green Version]
- Carpén, T.; Syrjänen, S.; Jouhi, L.; Randen-Brady, R.; Haglund, C.; Mäkitie, A.; Mattila, P.S.; Hagström, J. Epstein–Barr virus (EBV) and polyomaviruses are detectable in oropharyngeal cancer and EBV may have prognostic impact. Cancer Immunol. Immunother. 2020, 69, 1615–1626. [Google Scholar] [CrossRef] [Green Version]
- Migliaro, M.; Massuh, D.; Infante, M.F.; Brahm, A.M.; San Martín, M.T.; Ortuño, D. Role of Epstein-Barr Virus and Human Papilloma Virus in the Development of Oropharyngeal Cancer: A Literature Review. Int. J. Dent. 2022, 2022, 3191569. [Google Scholar] [CrossRef]
- Torresi, J.; Tran, B.M.; Christiansen, D.; Earnest-Silveira, L.; Schwab, R.H.M.; Vincan, E. HBV-related hepatocarcinogenesis: The role of signalling pathways and innovative ex vivo research models. BMC Cancer 2019, 19, 707. [Google Scholar] [CrossRef] [Green Version]
- Ringehan, M.; McKeating, J.A.; Protzer, U. Viral hepatitis and liver cancer. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, P.P.; Sadrani, V.J.; Gupta, P.P.; Parab, M.M.; Bastikar, V.A. Hepatitis C virus and hepatocellular carcinoma. In Oncogenic Viruses Volume 1: Fundamentals of Oncoviruses; Elsevier: Amsterdam, The Netherlands, 2022; Volume 82, pp. 243–262. ISBN 9780128241523. [Google Scholar]
- Tasleem, S.; Sood, G.K. Hepatitis c associated b-cell non-hodgkin lymphoma: Clinical features and the role of antiviral therapy. J. Clin. Transl. Hepatol. 2015, 3, 134–139. [Google Scholar] [CrossRef] [Green Version]
- Khaled, H.; Abu-Taleb, F.; Haggag, R. Hepatitis C virus and non-Hodgkin’s lymphomas: A minireview. J. Adv. Res. 2017, 8, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Isaguliants, M.; Bayurova, E.; Avdoshina, D.; Kondrashova, A.; Chiodi, F.; Palefsky, J.M. Oncogenic effects of HIV-1 proteins, mechanisms behind. Cancers 2021, 13, 305. [Google Scholar] [CrossRef] [PubMed]
- Ficarra, G.; Eversole, L.E. HIV-related tumors of the oral cavity. Crit. Rev. Oral Biol. Med. 1994, 5, 159–185. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, A.R.; Tortolero-Luna, G.; Ferrer, E.; Burchell, A.N.; de Sanjose, S.; Kjaer, S.K.; Muñoz, N.; Schiffman, M.; Bosch, F.X. Epidemiology of Human Papillomavirus Infection in Men, Cancers other than Cervical and Benign Conditions. Vaccine 2008, 26, K17. [Google Scholar] [CrossRef] [Green Version]
- Formana, D.; de Martel, C.; Lacey, C.J.; Soerjomatarama, I.; Lortet-Tieulent, J.; Bruni, L.; Vignat, J.; Ferlay, J.; Bray, F.; Plummer, M.; et al. Global burden of human papillomavirus and related diseases. Vaccine 2012, 30, F12–F23. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.; Franceschi, S.; Clifford, G.M. Human papillomavirus types from infection to cancer in the anus, according to sex and HIV status: A systematic review and meta-analysis. Lancet Infect. Dis. 2018, 18, 198–206. [Google Scholar] [CrossRef] [Green Version]
- Chen, A.A.; Gheit, T.; Franceschi, S.; Tommasino, M.; Clifford, G.M. Human Papillomavirus 18 Genetic Variation and Cervical Cancer Risk Worldwide. J. Virol. 2015, 89, 10680–10687. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Pan, Y.; Gao, W.; Ke, Y.; Lu, Z. Whole-genome analysis of human papillomavirus types 16, 18, and 58 isolated from cervical precancer and cancer samples in Chinese women. Sci. Rep. 2017, 7, 263. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Qiu, Y.; Yuan, S.; Wang, H. Prognostic implication of human papillomavirus types in cervical cancer patients: A systematic review and meta-analysis. Infect. Agent. Cancer 2020, 15, 66. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Yin, Y.; Li, P.; Zhang, X.; Chen, D.; Liu, Y.; Wang, J.; Guo, L. Prevalence of human papillomavirus type-18 in head and neck cancer among the Chinese population. Medicine 2019, 98, e14551. [Google Scholar] [CrossRef] [PubMed]
- Petrelli, F.; De Santi, G.; Rampulla, V.; Ghidini, A.; Mercurio, P.; Mariani, M.; Manara, M.; Rausa, E.; Lonati, V.; Viti, M.; et al. Human papillomavirus (HPV) types 16 and 18 infection and esophageal squamous cell carcinoma: A systematic review and meta-analysis. J. Cancer Res. Clin. Oncol. 2021, 147, 3011–3023. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, N.; Bosch, F.X.; de Sanjosé, S.; Herrero, R.; Castellsagué, X.; Shah, K.V.; Snijders, P.J.F.; Meijer, C.J.L.M. Epidemiologic Classification of Human Papillomavirus Types Associated with Cervical Cancer. N. Engl. J. Med. 2003, 348, 518–527. [Google Scholar] [CrossRef] [Green Version]
- Halec, G.; Alemany, L.; Lloveras, B.; Schmitt, M.; Alejo, M.; Bosch, F.X.; Tous, S.; Klaustermeier, J.E.; Guimerà, N.; Grabe, N.; et al. Pathogenic role of the eight probably/possibly carcinogenic HPV types 26, 53, 66, 67, 68, 70, 73 and 82 in cervical cancer. J. Pathol. 2014, 234, 441–451. [Google Scholar] [CrossRef]
- Wang, X.; Song, Y.; Wei, X.; Wang, G.; Sun, R.; Wang, M.; Zhao, L. Prevalence and distribution of human papillomavirus genotypes among women attending gynecology clinics in northern Henan Province of China. Virol. J. 2022, 19, 6. [Google Scholar] [CrossRef]
- Burd, E.M. Human papillomavirus and cervical cancer. Clin. Microbiol. Rev. 2003, 16, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Pfister, H. Chapter 8: Human papillomavirus and skin cancer. J. Natl. Cancer Inst. Monogr. 2003, 31, 52–56. [Google Scholar] [CrossRef] [Green Version]
- Martin, F.; Gilks, C.F.; Gibb, R.; Jenkins, A.; Protani, M.; Francis, F.; Redmond, A.M.; Neilsen, G.; Mudge, D.; Wolley, M.; et al. Human T-cell leukaemia virus type 1 and Adult T-cell leukaemia/lymphoma in Queensland, Australia: A retrospective cross-sectional study. Sex. Transm. Infect. 2022, 99, 50–52. [Google Scholar] [CrossRef]
- Goncalves, P.H.; Ziegelbauer, J.; Uldrick, T.S.; Yarchoan, R. Kaposi sarcoma herpesvirus-associated cancers and related diseases. Curr. Opin. HIV AIDS 2017, 12, 47–56. [Google Scholar] [CrossRef] [Green Version]
- Won, J.; Cho, Y.; Lee, D.; Jeon, B.Y.; Ju, J.-W.; Chung, S.; Pak, J.H. Clonorchis sinensis excretory-secretory products increase malignant characteristics of cholangiocarcinoma cells in three-dimensional co-culture with biliary ductal plates. PLoS Pathog. 2019, 15, e1007818. [Google Scholar] [CrossRef]
- Chang, J.-I.; Lee, K.; Kim, D.; Yang, J.-I.; Park, J.K.; Choi, K.; Kang, S.H.; Lee, K.H.; Lee, K.T.; Lee, J.K.; et al. Clinical Characteristics of Clonorchis sinensis-Associated Cholangiocarcinoma: A Large-Scale, Single-Center Study. Front. Med. 2021, 8, 741. [Google Scholar] [CrossRef]
- Sripa, B.; Brindley, P.J.; Mulvenna, J.; Laha, T.; Smout, M.J.; Mairiang, E.; Bethony, J.M.; Loukas, A. The tumorigenic liver fluke Opisthorchis viverrini—Multiple pathways to cancer. Trends Parasitol. 2012, 28, 395–407. [Google Scholar] [CrossRef] [Green Version]
- Santos, L.L.; Santos, J.; Gouveia, M.J.; Bernardo, C.; Lopes, C.; Rinaldi, G.; Brindley, P.J.; da Costa, J.M.C. Review urogenital schistosomiasis—History, pathogenesis, and bladder cancer. J. Clin. Med. 2021, 10, 205. [Google Scholar] [CrossRef]
- Hamid, H.K.S. Review article schistosoma japonicum associated colorectal cancer: A review. Am. J. Trop. Med. Hyg. 2019, 100, 501–505. [Google Scholar] [CrossRef] [Green Version]
- Verma, D.; Garg, P.K.; Dubey, A.K. Insights into the human oral microbiome. Arch. Microbiol. 2018, 200, 525–540. [Google Scholar] [CrossRef]
- Caselli, E.; Fabbri, C.; D’Accolti, M.; Soffritti, I.; Bassi, C.; Mazzacane, S.; Franchi, M. Defining the oral microbiome by whole-genome sequencing and resistome analysis: The complexity of the healthy picture. BMC Microbiol. 2020, 20, 120. [Google Scholar] [CrossRef] [PubMed]
- la Rosa, G.R.M.; Gattuso, G.; Pedullà, E.; Rapisarda, E.; Nicolosi, D.; Salmeri, M. Association of oral dysbiosis with oral cancer development (Review). Oncol. Lett. 2020, 19, 3045–3058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irfan, M.; Delgado, R.Z.R.; Frias-Lopez, J. The Oral Microbiome and Cancer. Front. Immunol. 2020, 11, 2720. [Google Scholar] [CrossRef] [PubMed]
- Domingues, C.S.d.C.; Serambeque, B.P.; Laranjo Cândido, M.S.; Marto, C.M.M.; Veiga, F.J.d.B.; Sarmento Antunes Cruz Ribeiro, A.B.; Figueiras, A.R.R.; Botelho, M.F.R.; Dourado, M.d.A.R.F. Epithelial-mesenchymal transition and microRNAs: Challenges and future perspectives in oral cancer. Head Neck 2018, 40, 2304–2313. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Chu, M.; Huang, Z.; Yang, X.; Ran, S.; Hu, B.; Zhang, C.; Liang, J. Variations in oral microbiota associated with oral cancer. Sci. Rep. 2017, 7, 11773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, W.H.; Chen, H.M.; Yang, S.F.; Liang, C.; Peng, C.Y.; Lin, F.M.; Tsai, L.L.; Wu, B.C.; Hsin, C.H.; Chuang, C.Y.; et al. Bacterial alterations in salivary microbiota and their association in oral cancer. Sci. Rep. 2017, 7, 16540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Börnigen, D.; Ren, B.; Pickard, R.; Li, J.; Ozer, E.; Hartmann, E.M.; Xiao, W.; Tickle, T.; Rider, J.; Gevers, D.; et al. Alterations in oral bacterial communities are associated with risk factors for oral and oropharyngeal cancer. Sci. Rep. 2017, 7, 17686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gholizadeh, P.; Eslami, H.; Yousefi, M.; Asgharzadeh, M.; Aghazadeh, M.; Kafil, H.S. Role of oral microbiome on oral cancers, a review. Biomed. Pharmacother. 2016, 84, 552–558. [Google Scholar] [CrossRef]
- Wong, S.H.; Kwong, T.N.Y.; Wu, C.Y.; Yu, J. Clinical applications of gut microbiota in cancer biology. Semin. Cancer Biol. 2019, 55, 28–36. [Google Scholar] [CrossRef]
- Santosh, A.B.R.; Jones, T.; Harvey, J. A review on oral cancer biomarkers: Understanding the past and learning from the present. J. Cancer Res. Ther. 2016, 12, 486–492. [Google Scholar] [CrossRef]
- Abdulkareem, A.A.; Shelton, R.M.; Landini, G.; Cooper, P.R.; Milward, M.R. Potential role of periodontal pathogens in compromising epithelial barrier function by inducing epithelial-mesenchymal transition. J. Periodontal Res. 2018, 53, 565–574. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Li, C.; Liu, J.; Geng, F.; Shi, X.; Li, Q.; Lu, Z.; Pan, Y. Fusobacterium nucleatum promotes epithelial-mesenchymal transiton through regulation of the lncRNA MIR4435-2HG/miR-296-5p/Akt2/SNAI1 signaling pathway. FEBS J. 2020, 287, 4032–4047. [Google Scholar] [CrossRef]
- Zhou, J.; Sun, S.; Luan, S.; Xiao, X.; Yang, Y.; Mao, C.; Chen, L.; Zeng, X.; Zhang, Y.; Yuan, Y. Gut Microbiota for Esophageal Cancer: Role in Carcinogenesis and Clinical Implications. Front. Oncol. 2021, 11, 717242. [Google Scholar] [CrossRef]
- Lin, Z.; Rao, W.; Xiang, Z.; Zeng, Q.; Liu, S.; Yu, K.; Zhou, J.; Wang, J.; Chen, W.; Chen, Y.; et al. Characteristics and interplay of esophageal microbiota in esophageal squamous cell carcinoma. BMC Cancer 2022, 22, 696. [Google Scholar] [CrossRef]
- Peter, S.; Pendergraft, A.; VanDerPol, W.; Wilcox, C.M.; Kyanam Kabir Baig, K.R.; Morrow, C.; Izard, J.; Mannon, P.J. Mucosa-Associated Microbiota in Barrett’s Esophagus, Dysplasia, and Esophageal Adenocarcinoma Differ Similarly Compared With Healthy Controls. Clin. Transl. Gastroenterol. 2020, 11, e00199. [Google Scholar] [CrossRef] [PubMed]
- Elliott, D.R.F.; Walker, A.W.; O’Donovan, M.; Parkhill, J.; Fitzgerald, R.C. A non-endoscopic device to sample the oesophageal microbiota: A case-control study. Lancet Gastroenterol. Hepatol. 2017, 2, 32–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.S.; Tian, D.P.; Guan, X.Y.; Yun, H.; Wang, H.T.; Xiao, Y.; Bi, C.; Ying, S.; Su, M. Esophageal intraepithelial invasion of Helicobacter pylori correlates with atypical hyperplasia. Int. J. Cancer 2014, 134, 2626–2632. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Li, S.; Ma, Z.; Liang, S.; Shan, T.; Zhang, M.; Zhu, X.; Zhang, P.; Liu, G.; Zhou, F.; et al. Presence of Porphyromonas gingivalis in esophagus and its association with the clinicopathological characteristics and survival in patients with esophageal cancer. Infect. Agent. Cancer 2016, 11, 268. [Google Scholar] [CrossRef] [Green Version]
- Shao, D.; Vogtmann, E.; Liu, A.; Qin, J.; Chen, W.; Abnet, C.C.; Wei, W. Microbial characterization of esophageal squamous cell carcinoma and gastric cardia adenocarcinoma from a high-risk region of China. Cancer 2019, 125, 3993–4002. [Google Scholar] [CrossRef]
- Yang, L.; Lu, X.; Nossa, C.W.; Francois, F.; Peek, R.M.; Pei, Z. Inflammation and Intestinal Metaplasia of the Distal Esophagus Are Associated With Alterations in the Microbiome. Gastroenterology 2009, 137, 588–597. [Google Scholar] [CrossRef] [Green Version]
- Meng, C.; Bai, C.; Brown, T.D.; Hood, L.E.; Tian, Q. Human Gut Microbiota and Gastrointestinal Cancer. Genom. Proteom. Bioinform. 2018, 16, 33–49. [Google Scholar] [CrossRef]
- Bhatt, A.P.; Redinbo, M.R.; Bultman, S.J.B. The Role of the Microbiome in Cancer Development and Therapy. Anticancer Res. 2017, 67, 326–344. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Ma, R.; Liu, F.; Lee, S.A.; Zhang, L. Modulation of gut microbiota: A novel paradigm of enhancing the efficacy of programmed death-1 and programmed death ligand-1 blockade therapy. Front. Immunol. 2018, 9, 374. [Google Scholar] [CrossRef] [Green Version]
- Wroblewski, L.E.; Peek, R.M.; Wilson, K.T. Helicobacter pylori and gastric cancer: Factors that modulate disease risk. Clin. Microbiol. Rev. 2010, 23, 713–739. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Zhou, X.; Liu, X.; Ling, Z.; Ji, F. Role of the Gastric Microbiome in Gastric Cancer: From Carcinogenesis to Treatment. Front. Microbiol. 2021, 12, 519. [Google Scholar] [CrossRef] [PubMed]
- Engstrand, L.; Graham, D.Y. Microbiome and Gastric Cancer. Dig. Dis. Sci. 2020, 65, 865–873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gunathilake, M.N.; Lee, J.; Choi, I.J.; Kim, Y.I.; Ahn, Y.; Park, C.; Kim, J. Association between the relative abundance of gastric microbiota and the risk of gastric cancer: A case-control study. Sci. Rep. 2019, 9, 13589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nie, S.; Wang, A.; Yuan, Y. Comparison of clinicopathological parameters, prognosis, micro-ecological environment and metabolic function of gastric cancer with or without fusobacterium sp. infection. J. Cancer 2021, 12, 1023–1032. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Seo, H.; Kang, C.S.; Shin, T.S.; Kim, J.W.; Park, J.M.; Kim, J.G.; Kim, Y.K. Dysbiotic change in gastric microbiome and its functional implication in gastric carcinogenesis. Sci. Rep. 2022, 12, 4285. [Google Scholar] [CrossRef] [PubMed]
- Aviles-Jimenez, F.; Vazquez-Jimenez, F.; Medrano-Guzman, R.; Mantilla, A.; Torres, J. Stomach microbiota composition varies between patients with non-atrophic gastritis and patients with intestinal type of gastric cancer. Sci. Rep. 2014, 4, 4202. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.P.; Liu, J.X.; Lu, L.L.; Wang, L.L.; Xu, L.; Guo, Z.H.; Dong, Q.J. Overgrowth of Lactobacillus in gastric cancer. World J. Gastrointest. Oncol. 2021, 13, 1099–1108. [Google Scholar] [CrossRef]
- Bakhti, S.Z.; Latifi-Navid, S. Oral microbiota and Helicobacter pylori in gastric carcinogenesis: What do we know and where next? BMC Microbiol. 2021, 21, 71. [Google Scholar] [CrossRef]
- Castaño-Rodríguez, N.; Goh, K.L.; Fock, K.M.; Mitchell, H.M.; Kaakoush, N.O. Dysbiosis of the microbiome in gastric carcinogenesis. Sci. Rep. 2017, 7, 15957. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.L.; Pang, W.; Huang, Y.; Zhang, Y.; Zhang, C.J. The Gastric Microbiome Is Perturbed in Advanced Gastric Adenocarcinoma Identified Through Shotgun Metagenomics. Front. Cell. Infect. Microbiol. 2018, 8, 433. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.; Ling, Z.; Li, L. The Intestinal Microbiota and Colorectal Cancer. Front. Immunol. 2020, 11, 3100. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.H.; Yu, J. Gut microbiota in colorectal cancer: Mechanisms of action and clinical applications. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 690–704. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Lee, D.Y.; Kang, J.H.; Kim, J.H.; Jeong, J.W.; Kim, H.W.; Oh, D.H.; Yoon, S.H.; Hur, S.J. Relationship between gut microbiota and colorectal cancer: Probiotics as a potential strategy for prevention. Food Res. Int. 2022, 156, 111327. [Google Scholar] [CrossRef] [PubMed]
- Sanapareddy, N.; Legge, R.M.; Jovov, B.; McCoy, A.; Burcal, L.; Araujo-Perez, F.; Randall, T.A.; Galanko, J.; Benson, A.; Sandler, R.S.; et al. Increased rectal microbial richness is associated with the presence of colorectal adenomas in humans. ISME J. 2012, 6, 1858–1868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Vitetta, L. Inflammation-Modulating Effect of Butyrate in the Prevention of Colon Cancer by Dietary Fiber. Clin. Color. Cancer 2018, 17, e541–e544. [Google Scholar] [CrossRef]
- Gheorghe, A.S.; Negru Șerban, M.; Preda, M.; Mihăilă, R.I.; Komporaly, I.A.; Dumitrescu, E.A.; Lungulescu, C.V.; Kajanto, L.A.; Georgescu, B.; Radu, E.A.; et al. Biochemical and Metabolical Pathways Associated with Microbiota-Derived Butyrate in Colorectal Cancer and Omega-3 Fatty Acids Implications: A Narrative Review. Nutrients 2022, 14, 1152. [Google Scholar] [CrossRef]
- Feng, Q.; Liang, S.; Jia, H.; Stadlmayr, A.; Tang, L.; Lan, Z.; Zhang, D.; Xia, H.; Xu, X.; Jie, Z.; et al. Gut microbiome development along the colorectal adenoma-carcinoma sequence. Nat. Commun. 2015, 6, 6528. [Google Scholar] [CrossRef] [Green Version]
- Rubinstein, M.R.; Wang, X.; Liu, W.; Hao, Y.; Cai, G.; Han, Y.W. Fusobacterium nucleatum Promotes Colorectal Carcinogenesis by Modulating E-Cadherin/β-Catenin Signaling via its FadA Adhesin. Cell Host Microbe 2013, 14, 195–206. [Google Scholar] [CrossRef] [Green Version]
- Kostic, A.D.; Chun, E.; Robertson, L.; Glickman, J.N.; Gallini, C.A.; Michaud, M.; Clancy, T.E.; Chung, D.C.; Lochhead, P.; Hold, G.L.; et al. Fusobacterium nucleatum Potentiates Intestinal Tumorigenesis and Modulates the Tumor-Immune Microenvironment. Cell Host Microbe 2013, 14, 207–215. [Google Scholar] [CrossRef] [Green Version]
- Shahanavaj, K.; Gil-Bazo, I.; Castiglia, M.; Bronte, G.; Passiglia, F.; Carreca, A.P.; Del Pozo, J.L.; Russo, A.; Peeters, M.; Rolfo, C. Cancer and the microbiome: Potential applications as new tumor biomarker. Expert Rev. Anticancer Ther. 2015, 15, 317–330. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Liu, W.; Kang, W.; He, Y.; Yang, R.; Mou, X.; Zhao, W. Effects of microbiota on anticancer drugs: Current knowledge and potential applications. eBioMedicine 2022, 83, 31900056–32000096. [Google Scholar] [CrossRef] [PubMed]
- Panebianco, C.; Andriulli, A.; Pazienza, V. Pharmacomicrobiomics: Exploiting the drug-microbiota interactions in anticancer therapies. Microbiome 2018, 6, 92. [Google Scholar] [CrossRef]
- Ting, N.L.N.; Lau, H.C.H.; Yu, J. Cancer pharmacomicrobiomics: Targeting microbiota to optimise cancer therapy outcomes. Gut 2022, 71, 1412–1425. [Google Scholar] [CrossRef]
- Li, W.; Deng, X.; Chen, T. Exploring the Modulatory Effects of Gut Microbiota in Anti-Cancer Therapy. Front. Oncol. 2021, 11, 1106. [Google Scholar] [CrossRef]
- Alieva, M.; van Rheenen, J.; Broekman, M.L.D. Potential impact of invasive surgical procedures on primary tumor growth and metastasis. Clin. Exp. Metastasis 2018, 35, 319–331. [Google Scholar] [CrossRef] [Green Version]
- Sasako, M. Role of surgery in multidisciplinary treatment for solid cancers. Int. J. Clin. Oncol. 2004, 9, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Saini, K.S.; Twelves, C. Determining lines of therapy in patients with solid cancers: A proposed new systematic and comprehensive framework. Br. J. Cancer 2021, 125, 155–163. [Google Scholar] [CrossRef]
- Gaines, S.; Shao, C.; Hyman, N.; Alverdy, J.C. Gut microbiome influences on anastomotic leak and recurrence rates following colorectal cancer surgery. Br. J. Surg. 2018, 105, e131–e141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kageyama, S.; Nagao, Y.; Ma, J.; Asakawa, M.; Yoshida, R.; Takeshita, T.; Hirosue, A.; Yamashita, Y.; Nakayama, H. Compositional Shift of Oral Microbiota Following Surgical Resection of Tongue Cancer. Front. Cell. Infect. Microbiol. 2020, 10, 710. [Google Scholar] [CrossRef] [PubMed]
- Ohigashi, S.; Sudo, K.; Kobayashi, D.; Takahashi, T.; Nomoto, K.; Onodera, H. Significant Changes in the Intestinal Environment After Surgery in Patients with Colorectal Cancer. J. Gastrointest. Surg. 2013, 17, 1657–1664. [Google Scholar] [CrossRef]
- Koliarakis, I.; Athanasakis, E.; Sgantzos, M.; Mariolis-Sapsakos, T.; Xynos, E.; Chrysos, E.; Souglakos, J.; Tsiaoussis, J. Intestinal microbiota in colorectal cancer surgery. Cancers 2020, 12, 3011. [Google Scholar] [CrossRef] [PubMed]
- Bashiardes, S.; Tuganbaev, T.; Federici, S.; Elinav, E. The microbiome in anti-cancer therapy. Semin. Immunol. 2017, 32, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Iida, N.; Dzutsev, A.; Stewart, C.A.; Smith, L.; Bouladoux, N.; Weingarten, R.A.; Molina, D.A.; Salcedo, R.; Back, T.; Cramer, S.; et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science 2013, 342, 967–970. [Google Scholar] [CrossRef] [PubMed]
- Kalasabail, S.; Engelman, J.; Zhang, L.Y.; El-omar, E.; Yim, H.C.H. A perspective on the role of microbiome for colorectal cancer treatment. Cancers 2021, 13, 4623. [Google Scholar] [CrossRef] [PubMed]
- Stojanovska, V.; McQuade, R.M.; Fraser, S.; Prakash, M.; Gondalia, S.; Stavely, R.; Palombo, E.; Apostolopoulos, V.; Sakkal, S.; Nurgali, K. Oxaliplatin-induced changes in microbiota, TLR4+ cells and enhanced HMGB1 expression in the murine colon. PLoS ONE 2018, 13, e0198359. [Google Scholar] [CrossRef] [Green Version]
- Khan, O.A.; Blann, A.D.; Payne, M.J.; Middleton, M.R.; Protheroe, A.S.; Talbot, D.C.; Taylor, M.; Han, C.; Patil, M.; Harris, A.L. Continuous low-dose cyclophosphamide and methotrexate combined with celecoxib for patients with advanced cancer. Br. J. Cancer 2011, 104, 1822–1827. [Google Scholar] [CrossRef] [Green Version]
- Viaud, S.; Saccheri, F.; Mignot, G.; Yamazaki, T.; Daillère, R.; Hannani, D.; Enot, D.P.; Pfirschke, C.; Engblom, C.; Pittet, M.J.; et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science 2013, 342, 971–976. [Google Scholar] [CrossRef] [Green Version]
- Daillère, R.; Vétizou, M.; Waldschmitt, N.; Yamazaki, T.; Isnard, C.; Poirier-Colame, V.; Duong, C.P.M.; Flament, C.; Lepage, P.; Roberti, M.P.; et al. Enterococcus hirae and Barnesiella intestinihominis Facilitate Cyclophosphamide-Induced Therapeutic Immunomodulatory Effects. Immunity 2016, 45, 931–943. [Google Scholar] [CrossRef] [Green Version]
- Cheng, W.Y.; Wu, C.Y.; Yu, J. The role of gut microbiota in cancer treatment: Friend or foe? Gut 2020, 69, 1867–1876. [Google Scholar] [CrossRef]
- Yue, B.; Gao, R.; Wang, Z.; Dou, W. Microbiota-Host-Irinotecan Axis: A New Insight Toward Irinotecan Chemotherapy. Front. Cell. Infect. Microbiol. 2021, 11, 710945. [Google Scholar] [CrossRef]
- Bailly, C. Irinotecan: 25 years of cancer treatment. Pharmacol. Res. 2019, 148, 104398. [Google Scholar] [CrossRef]
- Stringer, A.M.; Gibson, R.J.; Bowen, J.M.; Logan, R.M.; Ashton, K.; Yeoh, A.S.J.; Al-Dasooqi, N.; Keefe, D.M.K. Irinotecan-induced mucositis manifesting as diarrhoea corresponds with an amended intestinal flora and mucin profile. Int. J. Exp. Pathol. 2009, 90, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Stringer, A.M.; Gibson, R.J.; Logan, R.M.; Bowen, J.M.; Yeoh, A.S.J.; Keefe, D.M.K. Faecal microflora and β-glucuronidase expression are altered in an irinotecan-induced diarrhoea model in rats. Cancer Biol. Ther. 2008, 7, 1919–1925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stringer, A. Interaction between Host Cells and Microbes in Chemotherapy-Induced Mucositis. Nutrients 2013, 5, 1488–1499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, X.B.; Dieleman, L.A.; Ketabi, A.; Bibova, I.; Sawyer, M.B.; Xue, H.; Field, C.J.; Baracos, V.E.; Gänzle, M.G. Irinotecan (CPT-11) Chemotherapy Alters Intestinal Microbiota in Tumour Bearing Rats. PLoS ONE 2012, 7, e39764. [Google Scholar] [CrossRef] [PubMed]
- Dabek, M.; McCrae, S.I.; Stevens, V.J.; Duncan, S.H.; Louis, P. Distribution of Î2-glucosidase and Î2-glucuronidase activity and of Î2-glucuronidase gene gus in human colonic bacteria. FEMS Microbiol. Ecol. 2008, 66, 487–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McIntosh, F.M.; Maison, N.; Holtrop, G.; Young, P.; Stevens, V.J.; Ince, J.; Johnstone, A.M.; Lobley, G.E.; Flint, H.J.; Louis, P. Phylogenetic distribution of genes encoding β-glucuronidase activity in human colonic bacteria and the impact of diet on faecal glycosidase activities. Environ. Microbiol. 2012, 14, 1876–1887. [Google Scholar] [CrossRef]
- Lian, Q.; Xu, J.; Yan, S.; Huang, M.; Ding, H.; Sun, X.; Bi, A.; Ding, J.; Sun, B.; Geng, M. Chemotherapy-induced intestinal inflammatory responses are mediated by exosome secretion of double-strand DNA via AIM2 inflammasome activation. Cell Res. 2017, 27, 784–800. [Google Scholar] [CrossRef]
- Wardill, H.R.; Gibson, R.J.; Van Sebille, Y.Z.A.; Secombe, K.R.; Coller, J.K.; White, I.A.; Manavis, J.; Hutchinson, M.R.; Staikopoulos, V.; Logan, R.M.; et al. Irinotecan-Induced gastrointestinal dysfunction and pain are mediated by common TLR4-dependent mechanisms. Mol. Cancer Ther. 2016, 15, 1376–1386. [Google Scholar] [CrossRef] [Green Version]
- Gibson, R.J.; Coller, J.K.; Wardill, H.R.; Hutchinson, M.R.; Smid, S.; Bowen, J.M. Chemotherapy-induced gut toxicity and pain: Involvement of TLRs. Support. Care Cancer 2016, 24, 2251–2258. [Google Scholar] [CrossRef] [Green Version]
- Wong, D.V.T.; Ribeiro-Filho, H.V.; Wanderley, C.W.S.; Leite, C.A.V.G.; Lima, J.B.; Assef, A.N.B.; Cajado, A.G.; Batista, G.L.P.; González, R.H.; Silva, K.O.; et al. SN-38, the active metabolite of irinotecan, inhibits the acute inflammatory response by targeting toll-like receptor 4. Cancer Chemother. Pharmacol. 2019, 84, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Billod, J.M.; Lacetera, A.; Guzmán-Caldentey, J.; Martín-Santamaría, S. Computational Approaches to Toll-Like Receptor 4 Modulation. Molecules 2016, 21, 994. [Google Scholar] [CrossRef] [Green Version]
- Duffy, A.G.; Greten, T.F. Immunological off-target effects of standard treatments in gastrointestinal cancers. Ann. Oncol. 2014, 25, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Mego, M.; Chovanec, J.; Vochyanova-Andrezalova, I.; Konkolovsky, P.; Mikulova, M.; Reckova, M.; Miskovska, V.; Bystricky, B.; Beniak, J.; Medvecova, L.; et al. Prevention of irinotecan induced diarrhea by probiotics: A randomized double blind, placebo controlled pilot study. Complement. Ther. Med. 2015, 23, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Quintanilha, M.F.; Miranda, V.C.; Souza, R.O.; Gallotti, B.; Cruz, C.; Santos, E.A.; Alvarez-Leite, J.I.; Jesus, L.C.L.; Azevedo, V.; Trindade, L.M.; et al. Bifidobacterium longum subsp. longum 51A attenuates intestinal injury against irinotecan-induced mucositis in mice. Life Sci. 2022, 289, 120243. [Google Scholar] [CrossRef]
- The Role of Radiation Therapy in Upper Gastrointestinal Cancers—Hematology & Oncology. Available online: https://www.hematologyandoncology.net/archives/may-2017/the-role-of-radiation-therapy-in-upper-gastrointestinal-cancers/ (accessed on 10 February 2023).
- Huang, S.H.; O’Sullivan, B. Oral cancer: Current role of radiotherapy and chemotherapy. Med. Oral Patol. Oral Cir. Bucal 2013, 18, e233. [Google Scholar] [CrossRef]
- NICE. Cancer of the Upper Aerodigestive Tract: Assessment and Management in People Aged 16 and Over; National Institute for Health and Care Excellence (NICE): London, UK, 2018; ISBN 9781473116610. [Google Scholar]
- Mendes, F.; Domingues, C.; Rodrigues-Santos, P.; Abrantes, A.M.; Gonçalves, A.C.; Estrela, J.; Encarnação, J.; Pires, A.S.; Laranjo, M.; Alves, V.; et al. The role of immune system exhaustion on cancer cell escape and anti-tumor immune induction after irradiation. Biochim. Biophys. Acta Rev. Cancer 2016, 1865, 168–175. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Wei, K.; He, J.; Ding, N.; Hua, J.; Zhou, T.; Niu, F.; Zhou, G.; Shi, T.; et al. Review: Effect of Gut Microbiota and Its Metabolite SCFAs on Radiation-Induced Intestinal Injury. Front. Cell. Infect. Microbiol. 2021, 11, 630. [Google Scholar] [CrossRef]
- Tonneau, M.; Elkrief, A.; Pasquier, D.; Paz Del Socorro, T.; Chamaillard, M.; Bahig, H.; Routy, B. The role of the gut microbiome on radiation therapy efficacy and gastrointestinal complications: A systematic review. Radiother. Oncol. 2021, 156, 1–9. [Google Scholar] [CrossRef]
- Lam, V.; Moulder, J.E.; Salzman, N.H.; Dubinsky, E.A.; Andersen, G.L.; Baker, J.E. Intestinal microbiota as novel biomarkers of prior radiation exposure. Radiat. Res. 2012, 177, 573–583. [Google Scholar] [CrossRef] [Green Version]
- Nam, Y.D.; Kim, H.J.; Seo, J.G.; Kang, S.W.; Bae, J.W. Impact of pelvic radiotherapy on gut microbiota of gynecological cancer patients revealed by massive pyrosequencing. PLoS ONE 2013, 8, e82659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goudarzi, M.; Mak, T.D.; Jacobs, J.P.; Moon, B.H.; Strawn, S.J.; Braun, J.; Brenner, D.J.; Fornace, A.J.; Li, H.H. An Integrated Multi-Omic Approach to Assess Radiation Injury on the Host-Microbiome Axis. Radiat. Res. 2016, 186, 219–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.S.; Kim, J.; Park, S.J. High-throughput 16S rRNA gene sequencing reveals alterations of mouse intestinal microbiota after radiotherapy. Anaerobe 2015, 33, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Touchefeu, Y.; Montassier, E.; Nieman, K.; Gastinne, T.; Potel, G.; Bruley Des Varannes, S.; Le Vacon, F.; De La Cochetière, M.F. Systematic review: The role of the gut microbiota in chemotherapy- or radiation-induced gastrointestinal mucositis—Current evidence and potential clinical applications. Aliment. Pharmacol. Ther. 2014, 40, 409–421. [Google Scholar] [CrossRef] [PubMed]
- Takemura, N.; Kawasaki, T.; Kunisawa, J.; Sato, S.; Lamichhane, A.; Kobiyama, K.; Aoshi, T.; Ito, J.; Mizuguchi, K.; Karuppuchamy, T.; et al. Blockade of TLR3 protects mice from lethal radiation-induced gastrointestinal syndrome. Nat. Commun. 2014, 5, 3492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vacchelli, E.; Eggermont, A.; Sautès-Fridman, C.; Galon, J.; Zitvogel, L.; Kroemer, G.; Galluzzi, L. Trial watch toll-like receptor agonists for cancer therapy. Oncoimmunology 2013, 2, e25238. [Google Scholar] [CrossRef] [Green Version]
- Delia, P.; Sansotta, G.; Donato, V.; Frosina, P.; Messina, G.; De Renzis, C.; Famularo, G. Use of probiotics for prevention of radiation-induced diarrhea. World J. Gastroenterol. 2007, 13, 912–915. [Google Scholar] [CrossRef]
- Manichanh, C.; Varela, E.; Martinez, C.; Antolin, M.; Llopis, M.; Dor, J.; Giralt, J.; Guarner, F.; Malagelada, J.-R. The Gut Microbiota Predispose to the Pathophysiology of Acute Postradiotherapy Diarrhea. Am. J. Gastroenterol. 2008, 103, 1754–1761. [Google Scholar] [CrossRef]
- Mohan, S.; Bhaskaran, M.; George, A.; Thirutheri, A.; Somasundaran, M.; Pavithran, A. Immunotherapy in oral cancer. J. Pharm. Bioallied Sci. 2019, 11, S107–S111. [Google Scholar] [CrossRef]
- Puhr, H.C.; Preusser, M.; Ilhan-Mutlu, A. Immunotherapy for esophageal cancers: What is practice changing in 2021? Cancers 2021, 13, 4632. [Google Scholar] [CrossRef]
- Takei, S.; Kawazoe, A.; Shitara, K. The New Era of Immunotherapy in Gastric Cancer. Cancers 2022, 14, 1054. [Google Scholar] [CrossRef] [PubMed]
- Golshani, G.; Zhang, Y. Advances in immunotherapy for colorectal cancer: A review. Ther. Adv. Gastroenterol. 2020, 13, 1756284820917527. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Ying, J. Gut Microbiota and Immunotherapy. Front. Microbiol. 2022, 13, 2383. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Yuan, X.; Wang, M.; He, Z.; Li, H.; Wang, J.; Li, Q. Gut microbiota influence immunotherapy responses: Mechanisms and therapeutic strategies. J. Hematol. Oncol. 2022, 15, 47. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Zhang, J.; Wu, Q.; Fang, H.; Shi, C.; Li, Z.; Lin, C.; Tang, D.; Wang, D. Intestinal microbiota: A new force in cancer immunotherapy. Cell Commun. Signal. 2020, 18, 90. [Google Scholar] [CrossRef] [PubMed]
- Nigar, S.; Shimosato, T. Cooperation of Oligodeoxynucleotides and Synthetic Molecules as Enhanced Immune Modulators. Front. Nutr. 2019, 6, 140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.H.; Moon, C.; Oh, S.S.; Park, S.; Jeong, J.W.; Kim, S.; Lee, H.G.; Kwon, H.J.; Kim, K.D. Liposome-encapsulated CpG enhances antitumor activity accompanying the changing of lymphocyte populations in tumor via intratumoral administration. Nucleic Acid Ther. 2015, 25, 95–102. [Google Scholar] [CrossRef] [Green Version]
- Hanagata, N. CpG oligodeoxynucleotide nanomedicines for the prophylaxis or treatment of cancers, infectious diseases, and allergies. Int. J. Nanomed. 2017, 12, 515–531. [Google Scholar] [CrossRef] [Green Version]
- Guiducci, C.; Vicari, A.P.; Sangaletti, S.; Trinchieri, G.; Colombo, M.P. Redirecting in vivo elicited tumor infiltrating macrophages and dendritic cells towards tumor rejection. Cancer Res. 2005, 65, 3437–3446. [Google Scholar] [CrossRef] [Green Version]
- Viaud, S.; Daillère, R.; Boneca, I.G.; Lepage, P.; Langella, P.; Chamaillard, M.; Pittet, M.J.; Ghiringhelli, F.; Trinchieri, G.; Goldszmid, R.; et al. Gut microbiome and anticancer immune response: Really hot Sh∗t! Cell Death Differ. 2015, 22, 199–214. [Google Scholar] [CrossRef] [Green Version]
- Vétizou, M.; Pitt, J.M.; Daillère, R.; Lepage, P.; Waldschmitt, N.; Flament, C.; Rusakiewicz, S.; Routy, B.; Roberti, M.P.; Duong, C.P.M.; et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 2015, 350, 1079–1084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coutzac, C.; Jouniaux, J.M.; Paci, A.; Schmidt, J.; Mallardo, D.; Seck, A.; Asvatourian, V.; Cassard, L.; Saulnier, P.; Lacroix, L.; et al. Systemic short chain fatty acids limit antitumor effect of CTLA-4 blockade in hosts with cancer. Nat. Commun. 2020, 11, 2168. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Chen, M.; Nie, H.; Yuan, Y. PD-1 and PD-L1 in cancer immunotherapy: Clinical implications and future considerations. Hum. Vaccines Immunother. 2019, 15, 1111–1122. [Google Scholar] [CrossRef]
- Liu, J.; Chen, Z.; Li, Y.; Zhao, W.; Wu, J.B.; Zhang, Z. PD-1/PD-L1 Checkpoint Inhibitors in Tumor Immunotherapy. Front. Pharmacol. 2021, 12, 2339. [Google Scholar] [CrossRef]
- Peng, Z.; Cheng, S.; Kou, Y.; Wang, Z.; Jin, R.; Hu, H.; Zhang, X.; Gong, J.F.; Li, J.; Lu, M.; et al. The Gut microbiome is associated with clinical response to Anti-PD-1/PD-L1 immunotherapy in gastrointestinal cancer. Cancer Immunol. Res. 2020, 8, 1251–1261. [Google Scholar] [CrossRef]
- Yan, X.; Zhang, S.; Deng, Y.; Wang, P.; Hou, Q.; Xu, H. Prognostic factors for checkpoint inhibitor based immunotherapy: An update with new evidences. Front. Pharmacol. 2018, 9, 1050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Liu, Z.; Chen, T. Gut Microbiota: A Promising Milestone in Enhancing the Efficacy of PD1/PD-L1 Blockade Therapy. Front. Oncol. 2022, 12, 476. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Amatya, S.B.; Salmi, S.; Koivukangas, V.; Karihtala, P.; Reunanen, J. Microbiota and Extracellular Vesicles in Anti-PD-1/PD-L1 Therapy. Cancers 2022, 14, 5121. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, R.; Cheng, K.; Zhang, K.; Wang, Y.; Zhang, Y.; Li, Y.; Liu, G.; Xu, J.; Xu, J.; et al. Bacterial Outer Membrane Vesicles Presenting Programmed Death 1 for Improved Cancer Immunotherapy via Immune Activation and Checkpoint Inhibition. ACS Nano 2020, 14, 16698–16711. [Google Scholar] [CrossRef]
- Narayanan, V.; Peppelenbosch, M.P.; Konstantinov, S.R. Human fecal microbiome-based biomarkers for colorectal cancer. Cancer Prev. Res. 2014, 7, 1108–1111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- GibsoSn, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, types, sources, mechanisms, and clinical applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liong, M.T. Roles of probiotics and prebiotics in colon cancer prevention: Postulated mechanisms and in-vivo evidence. Int. J. Mol. Sci. 2008, 9, 854–863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanders, M.E.; Merenstein, D.J.; Reid, G.; Gibson, G.R.; Rastall, R.A. Probiotics and prebiotics in intestinal health and disease: From biology to the clinic. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Chen, Y.; Cheng, M.; Zhang, X.; Zheng, X.; Zhang, Z. The modulatory effect of polyphenols from green tea, oolong tea and black tea on human intestinal microbiota in vitro. J. Food Sci. Technol. 2018, 55, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Elmén, L.; Segota, I.; Xian, Y.; Tinoco, R.; Feng, Y.; Fujita, Y.; Segura Muñoz, R.R.; Schmaltz, R.; Bradley, L.M.; et al. Prebiotic-Induced Anti-tumor Immunity Attenuates Tumor Growth. Cell Rep. 2020, 30, 1753–1766. [Google Scholar] [CrossRef] [Green Version]
- Sharma, V.; Sharma, N.; Sheikh, I.; Kumar, V.; Sehrawat, N.; Yadav, M.; Ram, G.; Sankhyan, A.; Sharma, A.K. Probiotics and Prebiotics Having Broad Spectrum Anticancer Therapeutic Potential: Recent Trends and Future Perspectives. Curr. Pharmacol. Rep. 2021, 7, 67–79. [Google Scholar] [CrossRef]
- Zitvogel, L.; Ma, Y.; Raoult, D.; Kroemer, G.; Gajewski, T.F. The microbiome in cancer immunotherapy: Diagnostic tools and therapeutic strategies. Science 2018, 359, 1366–1370. [Google Scholar] [CrossRef] [Green Version]
- Pham, F.; Moinard-Butot, F.; Coutzac, C.; Chaput, N. Cancer and immunotherapy: A role for microbiota composition. Eur. J. Cancer 2021, 155, 145–154. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, W.; Feng, C.; Kwok, L.Y.; He, Q.; Sun, Z. Stronger gut microbiome modulatory effects by postbiotics than probiotics in a mouse colitis model. npj Sci. Food 2022, 6, 53. [Google Scholar] [CrossRef]
- Kroemer, G.; Zitvogel, L. Inosine: Novel microbiota-derived immunostimulatory metabolite. Cell Res. 2020, 30, 942–943. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, H.J.; Ji, K. The Proliferation Inhibitory Effect of Postbiotics Prepared from Probiotics with Antioxidant Activity against HT-29 Cells. Appl. Sci. 2022, 12, 12519. [Google Scholar] [CrossRef]
- Xu, H.; Cao, C.; Ren, Y.; Weng, S.; Liu, L.; Guo, C.; Wang, L.; Han, X.; Ren, J.; Liu, Z. Antitumor effects of fecal microbiota transplantation: Implications for microbiome modulation in cancer treatment. Front. Immunol. 2022, 13, 4788. [Google Scholar] [CrossRef]
- Chen, D.; Wu, J.; Jin, D.; Wang, B.; Cao, H. Fecal microbiota transplantation in cancer management: Current status and perspectives. Int. J. Cancer 2019, 145, 2021–2031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gopalakrishnan, V.; Spencer, C.N.; Nezi, L.; Reuben, A.; Andrews, M.C.; Karpinets, T.V.; Prieto, P.A.; Vicente, D.; Hoffman, K.; Wei, S.C.; et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 2018, 359, 97–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baruch, E.N.; Youngster, I.; Ben-Betzalel, G.; Ortenberg, R.; Lahat, A.; Katz, L.; Adler, K.; Dick-Necula, D.; Raskin, S.; Bloch, N.; et al. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science 2021, 371, 602–609. [Google Scholar] [CrossRef]
- Smet, A.; Kupcinskas, J.; Link, A.; Hold, G.L.; Bornschein, J. The Role of Microbiota in Gastrointestinal Cancer and Cancer Treatment: Chance or Curse? CMGH 2022, 13, 857–874. [Google Scholar] [CrossRef]
- Domingues, C.; Santos, A.; Alvarez-Lorenzo, C.; Concheiro, A.; Jarak, I.; Veiga, F.; Barbosa, I.; Dourado, M.; Figueiras, A. Where Is Nano Today and Where Is It Headed? A Review of Nanomedicine and the Dilemma of Nanotoxicology. ACS Nano 2022, 16, 9994–10041. [Google Scholar] [CrossRef]
- Lamas, B.; Martins Breyner, N.; Houdeau, E. Impacts of foodborne inorganic nanoparticles on the gut microbiota-immune axis: Potential consequences for host health. Part. Fibre Toxicol. 2020, 17, 1–22. [Google Scholar] [CrossRef]
- Utembe, W.; Tlotleng, N.; Kamng’ona, A.W. A systematic review on the effects of nanomaterials on gut microbiota. Curr. Res. Microb. Sci. 2022, 3, 100118. [Google Scholar] [CrossRef]
- Richard, M.L.; Liguori, G.; Lamas, B.; Brandi, G.; da Costa, G.; Hoffmann, T.W.; Pierluigi Di Simone, M.; Calabrese, C.; Poggioli, G.; Langella, P.; et al. Mucosa-associated microbiota dysbiosis in colitis associated cancer. Gut Microbes 2018, 9, 131–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meijnikman, A.S.; Gerdes, V.E.; Nieuwdorp, M.; Herrema, H. Evaluating causality of gut microbiota in obesity and diabetes in humans. Endocr. Rev. 2018, 39, 133–153. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Wu, G.D.; Albenberg, L.; Tomov, V.T. Gut microbiota and IBD: Causation or correlation? Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 573–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, R.; Lang, T.; Yan, W.; Zhu, X.; Huang, X.; Yin, Q.; Li, Y. Gut Microbiota: Influence on Carcinogenesis and Modulation Strategies by Drug Delivery Systems to Improve Cancer Therapy. Adv. Sci. 2021, 8, 2003542. [Google Scholar] [CrossRef]
- Chen, Z.; Zhou, D.; Han, S.; Zhou, S.; Jia, G. Hepatotoxicity and the role of the gut-liver axis in rats after oral administration of titanium dioxide nanoparticles. Part. Fibre Toxicol. 2019, 16, 48. [Google Scholar] [CrossRef]
- Zhu, X.; Zhao, L.; Liu, Z.; Zhou, Q.; Zhu, Y.; Zhao, Y.; Yang, X. Long-term exposure to titanium dioxide nanoparticles promotes diet-induced obesity through exacerbating intestinal mucus layer damage and microbiota dysbiosis. Nano Res. 2021, 14, 1512–1522. [Google Scholar] [CrossRef]
- Chen, L.; Guo, Y.; Hu, C.; Lam, P.K.S.; Lam, J.C.W.; Zhou, B. Dysbiosis of gut microbiota by chronic coexposure to titanium dioxide nanoparticles and bisphenol A: Implications for host health in zebrafish. Environ. Pollut. 2018, 234, 307–317. [Google Scholar] [CrossRef]
- Auguste, M.; Lasa, A.; Pallavicini, A.; Gualdi, S.; Vezzulli, L.; Canesi, L. Exposure to TiO2 nanoparticles induces shifts in the microbiota composition of Mytilus galloprovincialis hemolymph. Sci. Total Environ. 2019, 670, 129–137. [Google Scholar] [CrossRef]
- Mu, W.; Wang, Y.; Huang, C.; Fu, Y.; Li, J.; Wang, H.; Jia, X.; Ba, Q. Effect of Long-Term Intake of Dietary Titanium Dioxide Nanoparticles on Intestine Inflammation in Mice. J. Agric. Food Chem. 2019, 67, 9382–9389. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Li, B.; Cui, J.; Gao, N.; Sun, H.; Meng, Q.; Wu, S.; Bo, J.; Yan, L.; et al. Prebiotic protects against anatase titanium dioxide nanoparticles-induced microbiota-mediated colonic barrier defects. NanoImpact 2019, 14, 100164. [Google Scholar] [CrossRef]
- Khan, S.T.; Saleem, S.; Ahamed, M.; Ahmad, J. Survival of probiotic bacteria in the presence of food grade nanoparticles from chocolates: An in vitro and in vivo study. Appl. Microbiol. Biotechnol. 2019, 103, 6689–6700. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.; Milner, J.; Boudreau, M.D.; Gokulan, K.; Cerniglia, C.E.; Khare, S. Effects of subchronic exposure of silver nanoparticles on intestinal microbiota and gut-associated immune responses in the ileum of Sprague-Dawley rats. Nanotoxicology 2015, 9, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Arvind Bharani, R.; Karthick Raja Namasivayam, S. Biogenic silver nanoparticles mediated stress on developmental period and gut physiology of major lepidopteran pest Spodoptera litura (Fab.) (Lepidoptera: Noctuidae)—An eco-friendly approach of insect pest control. J. Environ. Chem. Eng. 2017, 5, 453–467. [Google Scholar] [CrossRef]
- Han, X.; Geller, B.; Moniz, K.; Das, P.; Chippindale, A.K.; Walker, V.K. Monitoring the developmental impact of copper and silver nanoparticle exposure in Drosophila and their microbiomes. Sci. Total Environ. 2014, 487, 822–829. [Google Scholar] [CrossRef]
- Yausheva, E.; Miroshnikov, S.; Sizova, E. Intestinal microbiome of broiler chickens after use of nanoparticles and metal salts. Environ. Sci. Pollut. Res. 2018, 25, 18109–18120. [Google Scholar] [CrossRef]
- Ju, Z.; Ren, G.; Zhou, M.; Jing, J.; Xiang, J.; Liu, X.; Huang, R.; Zhou, P.K. Exposure to a combination of silica nanoparticles and low-dose radiation aggravates lung fibrosis in mice: Via gut microbiota modulation. Environ. Sci. Nano 2020, 7, 3979–3998. [Google Scholar] [CrossRef]
- Pereira, D.I.A.; Bruggraber, S.F.A.; Faria, N.; Poots, L.K.; Tagmount, M.A.; Aslam, M.F.; Frazer, D.M.; Vulpe, C.D.; Anderson, G.J.; Powell, J.J. Nanoparticulate iron(III) oxo-hydroxide delivers safe iron that is well absorbed and utilised in humans. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 1877–1886. [Google Scholar] [CrossRef] [Green Version]
- Merrifield, D.L.; Shaw, B.J.; Harper, G.M.; Saoud, I.P.; Davies, S.J.; Handy, R.D.; Henry, T.B. Ingestion of metal-nanoparticle contaminated food disrupts endogenous microbiota in zebrafish (Danio rerio). Environ. Pollut. 2013, 174, 157–163. [Google Scholar] [CrossRef]
- Chaplin, A.; Gao, H.; Asase, C.; Rengasamy, P.; Park, B.; Skander, D.; Bebek, G.; Rajagopalan, S.; Maiseyeu, A. Systemically-delivered biodegradable PLGA alters gut microbiota and induces transcriptomic reprogramming in the liver in an obesity mouse model. Sci. Rep. 2020, 10, 13786. [Google Scholar] [CrossRef]
- Lee, H.W.; Park, Y.S.; Jung, J.S.; Shin, W.S. Chitosan oligosaccharides, dp 2-8, have prebiotic effect on the Bifidobacterium bifidium and Lactobacillus sp. Anaerobe 2002, 8, 319–324. [Google Scholar] [CrossRef]
- Zhang, C.; Jiao, S.; Wang, Z.A.; Du, Y. Exploring Effects of Chitosan Oligosaccharides on Mice Gut Microbiota in in vitro Fermentation and Animal Model. Front. Microbiol. 2018, 9, 2388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Zhang, C.; Guo, C.; Li, X. Chitosan ameliorates DSS-induced ulcerative colitis mice by enhancing intestinal barrier function and improving microflora. Int. J. Mol. Sci. 2019, 20, 5751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saleh, N.; Mahmoud, H.E.; Eltaher, H.; Helmy, M.; El-Khordagui, L.; Hussein, A.A. Prodigiosin-Functionalized Probiotic Ghosts as a Bioinspired Combination Against Colorectal Cancer Cells. Probiotics Antimicrob. Proteins 2022, 1, 3. [Google Scholar] [CrossRef] [PubMed]
- Perumal, K.; Ahmad, S.; Mohd-Zahid, M.H.; Wan Hanaffi, W.N.; Z.A., I.; Six, J.L.; Ferji, K.; Jaafar, J.; Boer, J.C.; Plebanski, M.; et al. Nanoparticles and Gut Microbiota in Colorectal Cancer. Front. Nanotechnol. 2021, 3, 46. [Google Scholar]
- Elham, N.; Naheed, M.; Elahe, M.; Hossein, M.M.; Majid, T. Selective Cytotoxic effect of Probiotic, Paraprobiotic and Postbiotics of L.casei strains against Colorectal Cancer Cells: Invitro studies. Braz. J. Pharm. Sci. 2022, 58, e19400. [Google Scholar] [CrossRef]
- An, B.C.; Yoon, Y.-S.; Park, H.J.; Park, S.; Kim, T.Y.; Ahn, J.Y.; Kwon, D.; Choi, O.; Heo, J.Y.; Ryu, Y.; et al. Toxicological Evaluation of a Probiotic-Based Delivery System for P8 Protein as an Anti-Colorectal Cancer Drug. Drug Des. Dev. Ther. 2021, 15, 4761–4793. [Google Scholar] [CrossRef]
- Turner, P.V. The role of the gut microbiota on animal model reproducibility. Anim. Model. Exp. Med. 2018, 1, 109–115. [Google Scholar] [CrossRef]


| Microbe | Group | Cancer Type | References |
|---|---|---|---|
| Bacteria | |||
| Helicobacter pylori | 1 | Non-Hodgkin lymphoma: all combined a Non-Hodgkin lymphoma: low-grade B-cell mucosa-associated lymphoid tissue (MALT) gastric lymphoma Stomach | [27,28,29,30] |
| Viruses | |||
| Epstein–Barr virus | 1 | Burkitt lymphoma Hodgkin lymphoma Lymphoepithelioma-like carcinoma (LELC) a Non-Hodgkin lymphoma Non-Hodgkin lymphoma: extranodal NK/T cell lymphoma (nasal type) Non-Hodgkin lymphoma: immunosuppression-related lymphoma Pharynx: nasopharynx | [31,32,33,34] |
| Hepatitis B virus | 1 | Liver | [35,36] |
| Hepatitis C virus | 1 | Liver Non-Hodgkin lymphoma: all combined | [37] [38,39] |
| Human immunodeficiency virus type 1 | 1 | Anus a Endothelium (Kaposi sarcoma) Eye Hodgkin lymphoma Liver a Non-Hodgkin lymphoma: all combined Skin a Uterine cervix | [40,41] |
| Human papillomavirus type 16 | 1 | Penis Pharynx: oropharynx, tonsil Reto Uterine cervix Vagina Vulva | [42,43] |
| Human papillomavirus type 33 | 1 | Anus a | [44] |
| Human papillomavirus type 18 | 1 | Anus a Oral cavity a Penis Uterine cervix | [45,46,47,48,49] |
| Human papillomavirus types 26, 53, 66, 67, 68, 70, 73, and 82 | 2B | Uterine cervix a | [50,51,52] |
| Human papillomavirus types 31, 35, 39, 45, 51, 52, 56, 58, and 59 | 1 | Uterine cervix | [53] |
| Human papillomavirus types 5 and 8 | 2B | Skin a | [54] |
| Human T cell lymphotropic virus type 1 | 1 | Adult T cell leukemia/lymphoma | [55] |
| Kaposi sarcoma herpesvirus | 1 | Endothelium (Kaposi sarcoma) Multicentric Castleman disease a Primary effusion lymphoma | [56] |
| Parasites | |||
| Clonorchis sinensis | 1 | Bile duct | [57,58] |
| Opisthorchis viverrini | 1 | Bile duct | [59] |
| Schistosoma haematobium | 1 | Urinary bladder | [60] |
| Schistosoma japonicum | 2B | Bile duct a Colon a Liver a Rectum a | [61] |
| Samples | Method | Microbiota | References |
|---|---|---|---|
| Esophageal tumor and tumor-adjacent (A-ESCC) samples obtained from patients with esophageal squamous cell carcinoma (ESCC) | 16S ribosomal RNA sequencing |
| [76] |
| Control vs. pathological esophagus | 16S ribosomal ribonucleic acid V4 gene DNA sequencing |
| [77] |
| Normal squamous controls, non-dysplastic and dysplastic Barrett’s esophagus, and esophageal adenocarcinoma | 16S rRNA gene amplicon sequencing |
| [78] |
| ESCC and A-ESCC | 16S rRNA |
| [79] |
| Esophageal tissues from ESCC patients and normal controls | Immunohistochemistry 16S rDNA |
| [80] |
| Tumor and non-tumor samples with ESCC or GCA | 16S ribosomal RNA gene |
| [81] |
| Normal, esophagitis, or Barrett’s esophagus (intestinal metaplasia) | Bacterial 16S ribosomal RNA gene survey |
| [82] |
| Nanoparticles | Animal Model | Main Result | References |
|---|---|---|---|
| TiO2 | Rats | ↑ Lactobacillus_reuteri ↓ Romboutsia Hepatotoxicity | [203] |
| Mice | ↑ Firmicutes ↓ Bacteroidetes Intestinal mucus layer damage and dysbiosis | [204] | |
| Zebrafish | TiO2 and bisphenol induced ↑ Lawsonia ↓ Hyphomicrobium | [205] | |
| Mytilus galloprovincialis | ↑ Stenotrophomonas ↓ Shewanella, Kistimonas, Vibrio TiO2 nanoparticles impact hemolymph microbiome composition that may result from the interplay between the microbiota and the immune system | [206] | |
| Mice | ↓ Bifidobacterium and Lactobacillus Exacerbated immune responses in vivo | [207] | |
| Mice | ↓ Bifidobacterium Prebiotic inulin supplementation prevented TiO2 nanoparticles-induced colonic barrier dysfunction | [208] | |
| White albino mice | TiO2 from chocolates inhibited the growth and activity of Bacillus coagulans, Enterococcus faecalis, and Enterococcus faecium | [209] | |
| AgNPs | Sprague Dawley rats | Ileal mucosal microbial populations, alterations apparent ↓ in Firmicutes phyla ↓ expression of important immunomodulatory genes, including MUC3, TLR2, TLR4, GPR43, and FOXP3 | [210] |
| Spodoptera litura | ↓ Klebsiella pneumoniae, Bacillus licheniformis, and Bacillus cereus and Citrobacter freundi, Enterobacter cloacae | [211] | |
| Drosophila melanogaster | ↓ in the diversity ↑ Lactobacillus brevis ↓ Acetobacter | [212] | |
| ZnNPs | Chicken | ↑ Bacteroides and Faecalibacterium ↓ Lactobacillus | [213] |
| SiO2 | Mice | ↑ Bacteroidetes ↓ Firmicutes | [214] |
| Iron(III) oxo-hydroxidenano | Rats | Fe(III) supplemented group ↑ Lactobacillus spp. ↓ Bacteroides spp. Compared with animals supplemented with Fe(II) sulfate | [215] |
| CuNPs | Danio rerio | Suppression in beneficial bacteria Cetobacterium somerae | [216] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domingues, C.; Cabral, C.; Jarak, I.; Veiga, F.; Dourado, M.; Figueiras, A. The Debate between the Human Microbiota and Immune System in Treating Aerodigestive and Digestive Tract Cancers: A Review. Vaccines 2023, 11, 492. https://doi.org/10.3390/vaccines11030492
Domingues C, Cabral C, Jarak I, Veiga F, Dourado M, Figueiras A. The Debate between the Human Microbiota and Immune System in Treating Aerodigestive and Digestive Tract Cancers: A Review. Vaccines. 2023; 11(3):492. https://doi.org/10.3390/vaccines11030492
Chicago/Turabian StyleDomingues, Cátia, Cristiana Cabral, Ivana Jarak, Francisco Veiga, Marília Dourado, and Ana Figueiras. 2023. "The Debate between the Human Microbiota and Immune System in Treating Aerodigestive and Digestive Tract Cancers: A Review" Vaccines 11, no. 3: 492. https://doi.org/10.3390/vaccines11030492
APA StyleDomingues, C., Cabral, C., Jarak, I., Veiga, F., Dourado, M., & Figueiras, A. (2023). The Debate between the Human Microbiota and Immune System in Treating Aerodigestive and Digestive Tract Cancers: A Review. Vaccines, 11(3), 492. https://doi.org/10.3390/vaccines11030492










