The Polymorphic Membrane Protein G Has a Neutral Effect and the Plasmid Glycoprotein 3 an Antagonistic Effect on the Ability of the Major Outer Membrane Protein to Elicit Protective Immune Responses against a Chlamydia muridarum Respiratory Challenge
Abstract
:1. Introduction
2. Methods and Methods
2.1. C. muridarum Stocks
2.2. Cloning, Expression and Purification of C. muridarum Proteins
2.3. Vaccination Protocols
2.4. Determination of the Humoral Immune Responses Induced by Vaccination
2.5. Evaluation of C. muridarum—Specific Cellular Immune Responses Induced by Vaccination
2.6. Intranasal Challenge and Evaluation of the Infection and Disease
2.7. Statistical Analyses
3. Results
3.1. Analyses of the Three C. muridarum Recombinant Antigens Used for Immunization
3.2. Characterization of the Humoral Immune Responses Induced by Vaccination
3.3. Cell-Mediated Immune Responses following Vaccination
3.4. Changes in Body Weight following the Intranasal Challenge
3.5. Lung’s Weights
3.6. Burden of C. muridarum in the Lungs
3.7. Local Immune Responses in the Lungs
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- CDC. Sexually transmitted disease surveillance 2019. In Prevention DoS; U.S. Department of Health and Human Services: Atlanta, GA, USA, 2021; pp. 1–168. [Google Scholar]
- Darville, T. Recognition and treatment of chlamydial infections from birth to adolescence. Adv. Exp. Med. Biol. 2013, 764, 109–122. [Google Scholar] [PubMed]
- Adachi, K.N.; Nielsen-Saines, K.; Klausner, J.D. Chlamydia trachomatis Screening and Treatment in Pregnancy to Reduce Adverse Pregnancy and Neonatal Outcomes: A Review. Front. Public Health 2021, 9, 531073. [Google Scholar] [CrossRef] [PubMed]
- Gotz, H.; Lindback, J.; Ripa, T.; Arneborn, M.; Ramsted, K.; Ekdahl, K. Is the increase in notifications of Chlamydia trachomatis infections in Sweden the result of changes in prevalence, sampling frequency or diagnostic methods? Scand. J. Infect. Dis. 2002, 34, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Rekart, M.L.; Gilbert, M.; Meza, R.; Kim, P.H.; Chang, M.; Money, D.M.; Brunham, R.C. Chlamydia public health programs and the epidemiology of pelvic inflammatory disease and ectopic pregnancy. J. Infect. Dis. 2013, 207, 30–38. [Google Scholar] [CrossRef] [Green Version]
- Chesson, H.W.; Spicknall, I.H.; Bingham, A.; Brisson, M.; Eppink, S.T.; Farnham, P.G.; Kreisel, K.M.; Kumar, S.; Laprise, J.F.; Peterman, T.A.; et al. The Estimated Direct Lifetime Medical Costs of Sexually Transmitted Infections Acquired in the United States in 2018. Sex. Transm. Dis. 2021, 48, 215–221. [Google Scholar] [CrossRef]
- Schachter, J.; Dawson, C.R. Human Chlamydial Infections; PSG Pub. Co.: Littleton, MA, USA, 1978; pp. 121–139. [Google Scholar]
- Nichols, R.L.; Bell, S.D., Jr.; Murray, E.S.; Haddad, N.A.; Bobb, A.A. Studies on trachoma. V. Clinical observations in a field trial of bivalent trachoma vaccine at three dosage levels in Saudi Arabia. Am. J. Trop. Med. Hyg. 1966, 15, 639–647. [Google Scholar] [CrossRef]
- Taylor, H.R. Trachoma: A Blinding Scourge from the Bronze Age to the Twenty-First Century, 1st ed.; Haddington Press Pry Ltd: Victoria, Australia, 2008. [Google Scholar]
- Wang, S.P.; Grayston, J.T.; Alexander, E.R. Trachoma vaccine studies in monkeys. Am. J. Ophthalmol. 1967, 63, 1615–1630. [Google Scholar] [CrossRef]
- Woolridge, R.L.; Grayston, J.T.; Chang, I.H.; Cheng, K.H.; Yang, C.Y.; Neave, C. Field trial of a monovalent and of a bivalent mineral oil adjuvant trachoma vaccine in Taiwan school children. Am. J. Ophthalmol. 1967, 63, 1645–1650. [Google Scholar] [CrossRef]
- Farris, C.M.; Morrison, R.P. Vaccination against Chlamydia Genital Infection Utilizing the Murine C. muridarum Model. Infect. Immun. 2011, 79, 986–996. [Google Scholar] [CrossRef] [Green Version]
- Brunham, R.C.; Rey-Ladino, J. Immunology of Chlamydia infection: Implications for a Chlamydia trachomatis vaccine. Nat. Rev. Immunol. 2005, 5, 149–161. [Google Scholar] [CrossRef]
- Morrison, R.P.; Lyng, K.; Caldwell, H.D. Chlamydial disease pathogenesis. Ocular hypersensitivity elicited by a genus-specific 57-kD protein. J. Exp. Med. 1989, 169, 663–675. [Google Scholar] [CrossRef] [Green Version]
- Rockey, D.D.; Wang, J.; Lei, L.; Zhong, G. Chlamydia vaccine candidates and tools for chlamydial antigen discovery. Expert Rev. Vaccines 2009, 8, 1365–1377. [Google Scholar] [CrossRef]
- Abraham, S.; Juel, H.B.; Bang, P.; Cheeseman, H.M.; Dohn, R.B.; Cole, T.; Kristiansen, M.P.; Korsholm, K.S.; Lewis, D.; Olsen, A.W.; et al. Safety and immunogenicity of the chlamydia vaccine candidate CTH522 adjuvanted with CAF01 liposomes or aluminium hydroxide: A first-in-human, randomised, double-blind, placebo-controlled, phase 1 trial. Lancet Infect. Dis. 2019, 19, 1091–1100. [Google Scholar] [CrossRef]
- de la Maza, L.M.; Darville, T.L.; Pal, S. Chlamydia trachomatis vaccines for genital infections: Where are we and how far is there to go? Expert Rev. Vaccines 2021, 20, 421–435. [Google Scholar] [CrossRef]
- Fitch, W.M.; Peterson, E.M.; de la Maza, L.M. Phylogenetic analysis of the outer-membrane-protein genes of Chlamydiae, and its implication for vaccine development. Mol. Biol. Evol. 1993, 10, 892–913. [Google Scholar]
- Stephens, R.S.; Kalman, S.; Lammel, C.; Fan, J.; Marathe, R.; Aravind, L.; Mitchell, W.; Olinger, L.; Tatusov, R.L.; Zhao, Q.; et al. Genome sequence of an obligate intracellular pathogen of humans: Chlamydia trachomatis. Science 1998, 282, 754–759. [Google Scholar] [CrossRef] [Green Version]
- Stephens, R.S.; Sanchez-Pescador, R.; Wagar, E.A.; Inouye, C.; Urdea, M.S. Diversity of Chlamydia trachomatis major outer membrane protein genes. J. Bacteriol. 1987, 169, 3879–3885. [Google Scholar] [CrossRef] [Green Version]
- Sun, G.; Pal, S.; Sarcon, A.K.; Kim, S.; Sugawara, E.; Nikaido, H.; Cocco, M.J.; Peterson, E.M.; de la Maza, L.M. Structural and functional analyses of the major outer membrane protein of Chlamydia trachomatis. J. Bacteriol. 2007, 189, 6222–6235. [Google Scholar] [CrossRef] [Green Version]
- Feher, V.A.; Randall, A.; Baldi, P.; Bush, R.M.; de la Maza, L.M.; Amaro, R.E. A 3-dimensional trimeric beta-barrel model for Chlamydia MOMP contains conserved and novel elements of Gram-negative bacterial porins. PLoS ONE 2013, 8, e68934. [Google Scholar] [CrossRef]
- Caldwell, H.D.; Perry, L.J. Neutralization of Chlamydia trachomatis infectivity with antibodies to the major outer membrane protein. Infect. Immun. 1982, 38, 745–754. [Google Scholar] [CrossRef] [Green Version]
- Caldwell, H.D.; Kromhout, J.; Schachter, J. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect. Immun. 1981, 31, 1161–1176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pal, S.; Peterson, E.M.; de la Maza, L.M. Vaccination with the Chlamydia trachomatis major outer membrane protein can elicit an immune response as protective as that resulting from inoculation with live bacteria. Infect. Immun. 2005, 73, 8153–8160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, G.; Pal, S.; Weiland, J.; Peterson, E.M.; de la Maza, L.M. Protection against an intranasal challenge by vaccines formulated with native and recombinant preparations of the Chlamydia trachomatis major outer membrane protein. Vaccine 2009, 27, 5020–5025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kari, L.; Whitmire, W.M.; Crane, D.D.; Reveneau, N.; Carlson, J.H.; Goheen, M.M.; Peterson, E.M.; Pal, S.; de la Maza, L.M.; Caldwell, H.D. Chlamydia trachomatis native major outer membrane protein induces partial protection in nonhuman primates: Implication for a trachoma transmission-blocking vaccine. J. Immunol. 2009, 182, 8063–8070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tifrea, D.F.; Pal, S.; Popot, J.L.; Cocco, M.J.; de la Maza, L.M. Increased immunoaccessibility of MOMP epitopes in a vaccine formulated with amphipols may account for the very robust protection elicited against a vaginal challenge with Chlamydia muridarum. J. Immunol. 2014, 192, 5201–5213. [Google Scholar] [CrossRef] [Green Version]
- Teng, A.; Cruz-Fisher, M.I.; Cheng, C.; Pal, S.; Sun, G.; Ralli-Jain, P.; Molina, D.M.; Felgner, P.L.; Liang, X.; de la Maza, L.M. Proteomic identification of immunodominant chlamydial antigens in a mouse model. J. Proteom. 2012, 77, 176–186. [Google Scholar] [CrossRef] [Green Version]
- Caldwell, H.D.; Schachter, J. Antigenic analysis of the major outer membrane protein of Chlamydia spp. Infect. Immun. 1982, 35, 1024–1031. [Google Scholar] [CrossRef] [Green Version]
- O’Meara, C.P.; Armitage, C.W.; Kollipara, A.; Andrew, D.W.; Trim, L.; Plenderleith, M.B.; Beagley, K.W. Induction of partial immunity in both males and females is sufficient to protect females against sexual transmission of Chlamydia. Mucosal Immunol. 2016, 9, 1076–1088. [Google Scholar] [CrossRef] [Green Version]
- Hickey, D.K.; Aldwell, F.E.; Beagley, K.W. Transcutaneous immunization with a novel lipid-based adjuvant protects against Chlamydia genital and respiratory infections. Vaccine 2009, 27, 6217–6225. [Google Scholar] [CrossRef]
- Wang, S.P.; Grayston, J.T. Classification of Trachoma Virus Strains by Protection of Mice from Toxic Death. J. Immunol. 1963, 90, 849–856. [Google Scholar] [CrossRef]
- Wang, S.-P.; Grayston, J. Microimmunofluorescence serology of Chlamydia trachomatis. In Medical Virology III; Elsevier Science Pub.: New York, NY, USA, 1984. [Google Scholar]
- Wang, S.P.; Kuo, C.C.; Barnes, R.C.; Stephens, R.S.; Grayston, J.T. Immunotyping of Chlamydia trachomatis with monoclonal antibodies. J. Infect. Dis. 1985, 152, 791–800. [Google Scholar] [CrossRef]
- Tifrea, D.F.; Pal, S.; de la Maza, L.M. A Recombinant Chlamydia trachomatis MOMP Vaccine Elicits Cross-serogroup Protection in Mice against Vaginal Shedding and Infertility. J. Infect. Dis. 2020, 221, 191–200. [Google Scholar] [CrossRef]
- Tifrea, D.F.; Sun, G.; Pal, S.; Zardeneta, G.; Cocco, M.J.; Popot, J.L.; de la Maza, L.M. Amphipols stabilize the Chlamydia major outer membrane protein and enhance its protective ability as a vaccine. Vaccine 2011, 29, 4623–4631. [Google Scholar] [CrossRef] [Green Version]
- Carmichael, J.R.; Pal, S.; Tifrea, D.; de la Maza, L.M. Induction of protection against vaginal shedding and infertility by a recombinant Chlamydia vaccine. Vaccine 2011, 29, 5276–5283. [Google Scholar] [CrossRef] [Green Version]
- Murthy, A.K.; Li, W.; Guentzel, M.N.; Zhong, G.; Arulanandam, B.P. Vaccination with the defined chlamydial secreted protein CPAF induces robust protection against female infertility following repeated genital chlamydial challenge. Vaccine 2011, 29, 2519–2522. [Google Scholar] [CrossRef] [Green Version]
- Tan, C.; Hsia, R.C.; Shou, H.; Haggerty, C.L.; Ness, R.B.; Gaydos, C.A.; Dean, D.; Scurlock, A.M.; Wilson, D.P.; Bavoil, P.M. Chlamydia trachomatis-infected patients display variable antibody profiles against the nine-member polymorphic membrane protein family. Infect. Immun. 2009, 77, 3218–3226. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Zhang, Y.; Lu, C.; Lei, L.; Yu, P.; Zhong, G. A genome-wide profiling of the humoral immune response to Chlamydia trachomatis infection reveals vaccine candidate antigens expressed in humans. J. Immunol. 2010, 185, 1670–1680. [Google Scholar] [CrossRef] [Green Version]
- Cruz-Fisher, M.I.; Cheng, C.; Sun, G.; Pal, S.; Teng, A.; Molina, D.M.; Kayala, M.A.; Vigil, A.; Baldi, P.; Felgner, P.L.; et al. Identification of immunodominant antigens by probing a whole Chlamydia trachomatis open reading frame proteome microarray using sera from immunized mice. Infect. Immun. 2011, 79, 246–257. [Google Scholar] [CrossRef] [Green Version]
- Donati, M.; Sambri, V.; Comanducci, M.; Di Leo, K.; Storni, E.; Giacani, L.; Ratti, G.; Cevenini, R. DNA immunization with pgp3 gene of Chlamydia trachomatis inhibits the spread of chlamydial infection from the lower to the upper genital tract in C3H/HeN mice. Vaccine 2003, 21, 1089–1093. [Google Scholar] [CrossRef]
- Luan, X.; Peng, B.; Li, Z.; Tang, L.; Chen, C.; Chen, L.; Wu, H.; Sun, Z.; Lu, C. Vaccination with MIP or Pgp3 induces cross-serovar protection against chlamydial genital tract infection in mice. Immunobiology 2019, 224, 223–230. [Google Scholar] [CrossRef]
- Vasilevsky, S.; Stojanov, M.; Greub, G.; Baud, D. Chlamydial polymorphic membrane proteins: Regulation, function and potential vaccine candidates. Virulence 2016, 7, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Paes, W.; Brown, N.; Brzozowski, A.M.; Coler, R.; Reed, S.; Carter, D.; Bland, M.; Kaye, P.M.; Lacey, C.J. Recombinant polymorphic membrane protein D in combination with a novel, second-generation lipid adjuvant protects against intra-vaginal Chlamydia trachomatis infection in mice. Vaccine 2016, 34, 4123–4131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muller, T.; Becker, E.; Stallmann, S.; Waldhuber, A.; Rommler-Dreher, F.; Albrecht, S.; Mohr, F.; Hegemann, J.H.; Miethke, T. Vaccination with the polymorphic membrane protein A reduces Chlamydia muridarum induced genital tract pathology. Vaccine 2017, 35, 2801–2810. [Google Scholar] [CrossRef] [PubMed]
- Inic-Kanada, A.; Stojanovic, M.; Schlacher, S.; Stein, E.; Belij-Rammerstorfer, S.; Marinkovic, E.; Lukic, I.; Montanaro, J.; Schuerer, N.; Bintner, N.; et al. Delivery of a Chlamydial Adhesin N-PmpC Subunit Vaccine to the Ocular Mucosa Using Particulate Carriers. PLoS ONE 2015, 10, e0144380. [Google Scholar] [CrossRef] [Green Version]
- Tan, C.; Hsia, R.C.; Shou, H.; Carrasco, J.A.; Rank, R.G.; Bavoil, P.M. Variable expression of surface-exposed polymorphic membrane proteins in in vitro-grown Chlamydia trachomatis. Cell. Microbiol. 2010, 12, 174–187. [Google Scholar] [CrossRef] [Green Version]
- Carrasco, J.A.; Tan, C.; Rank, R.G.; Hsia, R.C.; Bavoil, P.M. Altered developmental expression of polymorphic membrane proteins in penicillin-stressed Chlamydia trachomatis. Cell. Microbiol. 2011, 13, 1014–1025. [Google Scholar] [CrossRef] [Green Version]
- Nunes, A.; Gomes, J.P.; Mead, S.; Florindo, C.; Correia, H.; Borrego, M.J.; Dean, D. Comparative expression profiling of the Chlamydia trachomatis pmp gene family for clinical and reference strains. PLoS ONE 2007, 2, e878. [Google Scholar] [CrossRef]
- Van Lent, S.; Creasy, H.H.; Myers, G.S.; Vanrompay, D. The Number, Organization, and Size of Polymorphic Membrane Protein Coding Sequences as well as the Most Conserved Pmp Protein Differ within and across Chlamydia Species. J. Mol. Microbiol. Biotechnol. 2016, 26, 333–344. [Google Scholar] [CrossRef]
- Crane, D.D.; Carlson, J.H.; Fischer, E.R.; Bavoil, P.; Hsia, R.C.; Tan, C.; Kuo, C.C.; Caldwell, H.D. Chlamydia trachomatis polymorphic membrane protein D is a species-common pan-neutralizing antigen. Proc. Natl. Acad. Sci. USA 2006, 103, 1894–1899. [Google Scholar] [CrossRef] [Green Version]
- Kiselev, A.O.; Stamm, W.E.; Yates, J.R.; Lampe, M.F. Expression, processing, and localization of PmpD of Chlamydia trachomatis Serovar L2 during the chlamydial developmental cycle. PLoS ONE 2007, 2, e568. [Google Scholar] [CrossRef] [Green Version]
- Swanson, K.A.; Taylor, L.D.; Frank, S.D.; Sturdevant, G.L.; Fischer, E.R.; Carlson, J.H.; Whitmire, W.M.; Caldwell, H.D. Chlamydia trachomatis polymorphic membrane protein D is an oligomeric autotransporter with a higher-order structure. Infect. Immun. 2009, 77, 508–516. [Google Scholar] [CrossRef] [Green Version]
- Favaroni, A.; Hegemann, J.H. Chlamydia trachomatis Polymorphic Membrane Proteins (Pmps) Form Functional Homomeric and Heteromeric Oligomers. Front. Microbiol. 2021, 12, 709724. [Google Scholar] [CrossRef]
- Karunakaran, K.P.; Rey-Ladino, J.; Stoynov, N.; Berg, K.; Shen, C.; Jiang, X.; Gabel, B.R.; Yu, H.; Foster, L.J.; Brunham, R.C. Immunoproteomic discovery of novel T cell antigens from the obligate intracellular pathogen Chlamydia. J. Immunol. 2008, 180, 2459–2465. [Google Scholar] [CrossRef] [Green Version]
- Pal, S.; Favaroni, A.; Tifrea, D.F.; Hanisch, P.T.; Luczak, S.E.; Hegemann, J.H.; de la Maza, L.M. Comparison of the nine polymorphic membrane proteins of Chlamydia trachomatis for their ability to induce protective immune responses in mice against a C. muridarum challenge. Vaccine 2017, 35, 2543–2549. [Google Scholar] [CrossRef] [Green Version]
- Peterson, E.M.; Markoff, B.A.; Schachter, J.; de la Maza, L.M. The 7.5-kb plasmid present in Chlamydia trachomatis is not essential for the growth of this microorganism. Plasmid 1990, 23, 144–148. [Google Scholar] [CrossRef]
- Stothard, D.R.; Williams, J.A.; Van Der Pol, B.; Jones, R.B. Identification of a Chlamydia trachomatis serovar E urogenital isolate which lacks the cryptic plasmid. Infect. Immun. 1998, 66, 6010–6013. [Google Scholar] [CrossRef] [Green Version]
- Palmer, L.; Falkow, S. A common plasmid of Chlamydia trachomatis. Plasmid 1986, 16, 52–62. [Google Scholar] [CrossRef]
- Galaleldeen, A.; Taylor, A.B.; Chen, D.; Schuermann, J.P.; Holloway, S.P.; Hou, S.; Gong, S.; Zhong, G.; Hart, P.J. Structure of the Chlamydia trachomatis immunodominant antigen Pgp3. J. Biol. Chem. 2013, 288, 22068–22079. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.; Lei, L.; Lu, C.; Galaleldeen, A.; Hart, P.J.; Zhong, G. Characterization of Pgp3, a Chlamydia trachomatis plasmid-encoded immunodominant antigen. J. Bacteriol. 2010, 192, 6017–6024. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Chen, D.; Zhong, Y.; Wang, S.; Zhong, G. The chlamydial plasmid-encoded protein pgp3 is secreted into the cytosol of Chlamydia-infected cells. Infect. Immun. 2008, 76, 3415–3428. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Zhong, Y.; Lei, L.; Wu, Y.; Wang, S.; Zhong, G. Antibodies from women urogenitally infected with C. trachomatis predominantly recognized the plasmid protein pgp3 in a conformation-dependent manner. BMC Microbiol. 2008, 8, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Wang, S.; Wu, Y.; Zhong, G.; Chen, D. Immunization with chlamydial plasmid protein pORF5 DNA vaccine induces protective immunity against genital chlamydial infection in mice. Sci. China C Life Sci. 2008, 51, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Tifrea, D.F.; Pal, S.; le Bon, C.; Cocco, M.J.; Zoonens, M.; de la Maza, L.M. Improved protection against Chlamydia muridarum using the native major outer membrane protein trapped in Resiquimod-carrying amphipols and effects in protection with addition of a Th1 (CpG-1826) and a Th2 (Montanide ISA 720) adjuvant. Vaccine 2020, 38, 4412–4422. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Huang, Y.; Yang, Z.; Sun, Y.; Gong, S.; Hou, S.; Chen, C.; Li, Z.; Liu, Q.; Wu, Y.; et al. Plasmid-encoded Pgp3 is a major virulence factor for Chlamydia muridarum to induce hydrosalpinx in mice. Infect. Immun. 2014, 82, 5327–5335. [Google Scholar] [CrossRef] [Green Version]
- Pal, S.; Fielder, T.J.; Peterson, E.M.; de la Maza, L.M. Protection against infertility in a BALB/c mouse salpingitis model by intranasal immunization with the mouse pneumonitis biovar of Chlamydia trachomatis. Infect. Immun. 1994, 62, 3354–3362. [Google Scholar] [CrossRef] [Green Version]
- Peterson, E.M.; Zhong, G.M.; Carlson, E.; de la Maza, L.M. Protective role of magnesium in the neutralization by antibodies of Chlamydia trachomatis infectivity. Infect. Immun. 1988, 56, 885–891. [Google Scholar] [CrossRef] [Green Version]
- Atreya, C.E.; Ducker, G.S.; Feldman, M.E.; Bergsland, E.K.; Warren, R.S.; Shokat, K.M. Combination of ATP-competitive mammalian target of rapamycin inhibitors with standard chemotherapy for colorectal cancer. Investig. New Drugs 2012, 30, 2219–2225. [Google Scholar] [CrossRef]
- Beal, S.L. Ways to fit a PK model with some data below the quantification limit. J. Pharmacokinet. Pharmacodyn. 2001, 28, 481–504. [Google Scholar] [CrossRef]
- Igietseme, J.U.; Eko, F.O.; Black, C.M. Chlamydia vaccines: Recent developments and the role of adjuvants in future formulations. Expert Rev. Vaccines 2011, 10, 1585–1596. [Google Scholar] [CrossRef]
- Hafner, L.M.; Timms, P. Development of a Chlamydia trachomatis vaccine for urogenital infections: Novel tools and new strategies point to bright future prospects. Expert Rev. Vaccines 2018, 17, 57–69. [Google Scholar] [CrossRef] [Green Version]
- de la Maza, L.M.; Pal, S.; Olsen, A.W.; Follmann, F. Chlamydia Vaccines; Tan, M., Hegemann, J.H., Sutterlin, C., Eds.; Caister Academic Press: Norfolk, UK, 2020; pp. 339–383. [Google Scholar]
- Phillips, S.; Quigley, B.L.; Timms, P. Seventy Years of Chlamydia Vaccine Research—Limitations of the Past and Directions for the Future. Front. Microbiol. 2019, 10, 70. [Google Scholar] [CrossRef] [Green Version]
- Kaufhold, R.M.; Boddicker, M.A.; Field, J.A.; Lucas, B.J.; Antonello, J.M.; Espeseth, A.S.; Skinner, J.M.; Heinrichs, J.H.; Smith, J.G. Evaluating potential vaccine antigens in both the Chlamydia trachomatis and Chlamydia muridarum intravaginal mouse challenge models. World J. Vaccines 2019, 9, 49–69. [Google Scholar] [CrossRef] [Green Version]
- Saka, H.A.; Thompson, J.W.; Chen, Y.S.; Kumar, Y.; Dubois, L.G.; Moseley, M.A.; Valdivia, R.H. Quantitative proteomics reveals metabolic and pathogenic properties of Chlamydia trachomatis developmental forms. Mol. Microbiol. 2011, 82, 1185–1203. [Google Scholar] [CrossRef] [Green Version]
- Sharma, J.; Zhong, Y.; Dong, F.; Piper, J.M.; Wang, G.; Zhong, G. Profiling of human antibody responses to Chlamydia trachomatis urogenital tract infection using microplates arrayed with 156 chlamydial fusion proteins. Infect. Immun. 2006, 74, 1490–1499. [Google Scholar] [CrossRef] [Green Version]
- Comanducci, M.; Manetti, R.; Bini, L.; Santucci, A.; Pallini, V.; Cevenini, R.; Sueur, J.M.; Orfila, J.; Ratti, G. Humoral immune response to plasmid protein pgp3 in patients with Chlamydia trachomatis infection. Infect. Immun. 1994, 62, 5491–5497. [Google Scholar] [CrossRef] [Green Version]
- Qiu, S.; Zhang, J.; Tian, Y.; Yang, Y.; Huang, H.; Yang, D.; Lu, M.; Xu, Y. Reduced antigenicity of naturally occurring hepatitis B surface antigen variants with substitutions at the amino acid residue 126. Intervirology 2008, 51, 400–406. [Google Scholar] [CrossRef]
- Yu, H.; Jiang, X.; Shen, C.; Karunakaran, K.P.; Jiang, J.; Rosin, N.L.; Brunham, R.C. Chlamydia muridarum T-cell antigens formulated with the adjuvant DDA/TDB induce immunity against infection that correlates with a high frequency of gamma interferon (IFN-gamma)/tumor necrosis factor alpha and IFN-gamma/interleukin-17 double-positive CD4+ T cells. Infect. Immun. 2010, 78, 2272–2282. [Google Scholar] [CrossRef] [Green Version]
- Finco, O.; Frigimelica, E.; Buricchi, F.; Petracca, R.; Galli, G.; Faenzi, E.; Meoni, E.; Bonci, A.; Agnusdei, M.; Nardelli, F.; et al. Approach to discover T- and B-cell antigens of intracellular pathogens applied to the design of Chlamydia trachomatis vaccines. Proc. Natl. Acad. Sci. USA 2011, 108, 9969–9974. [Google Scholar] [CrossRef] [Green Version]
- Coler, R.N.; Bhatia, A.; Maisonneuve, J.F.; Probst, P.; Barth, B.; Ovendale, P.; Fang, H.; Alderson, M.; Lobet, Y.; Cohen, J.; et al. Identification and characterization of novel recombinant vaccine antigens for immunization against genital Chlamydia trachomatis. FEMS Immunol. Med. Microbiol. 2009, 55, 258–270. [Google Scholar] [CrossRef] [Green Version]
- Qiu, Q.; Wang, R.Y.; Jiao, X.; Jin, B.; Sugauchi, F.; Grandinetti, T.; Alter, H.J.; Shih, J.W. Induction of multispecific Th-1 type immune response against HCV in mice by protein immunization using CpG and Montanide ISA 720 as adjuvants. Vaccine 2008, 26, 5527–5534. [Google Scholar] [CrossRef] [Green Version]
- Cheng, C.; Jain, P.; Pal, S.; Tifrea, D.; Sun, G.; Teng, A.A.; Liang, X.; Felgner, P.L.; de la Maza, L.M. Assessment of the role in protection and pathogenesis of the Chlamydia muridarum V-type ATP synthase subunit A (AtpA) (TC0582). Microbes Infect. 2014, 16, 123–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Guentzel, M.N.; Seshu, J.; Zhong, G.; Murthy, A.K.; Arulanandam, B.P. Induction of cross-serovar protection against genital chlamydial infection by a targeted multisubunit vaccination approach. Clin. Vaccine Immunol. 2007, 14, 1537–1544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tifrea, D.F.; Barta, M.L.; Pal, S.; Hefty, P.S.; de la Maza, L.M. Computational modeling of TC0583 as a putative component of the Chlamydia muridarum V-type ATP synthase complex and assessment of its protective capabilities as a vaccine antigen. Microbes Infect. 2016, 18, 245–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Zhong, G.; Ren, L.; Lu, C.; Li, Z.; Wu, Y. Identification of Plasmid-Free Chlamydia muridarum Organisms Using a Pgp3 Detection-Based Immunofluorescence Assay. J. Microbiol. Biotechnol. 2015, 25, 1621–1628. [Google Scholar] [CrossRef] [PubMed]
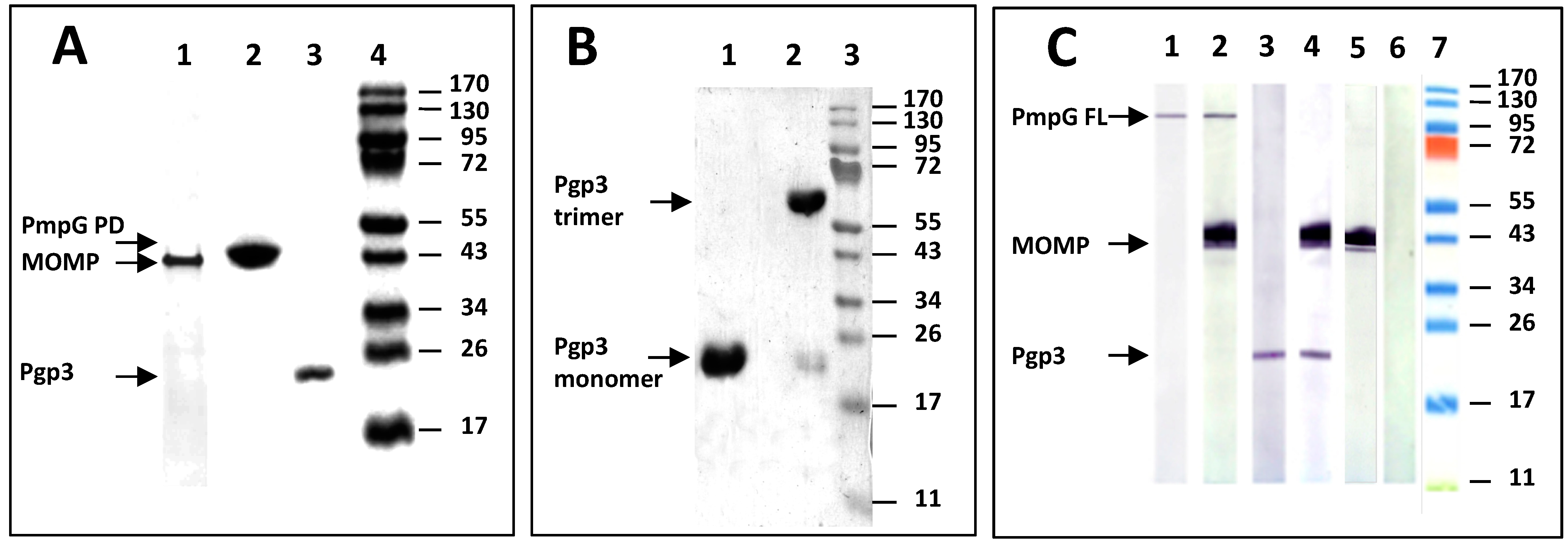
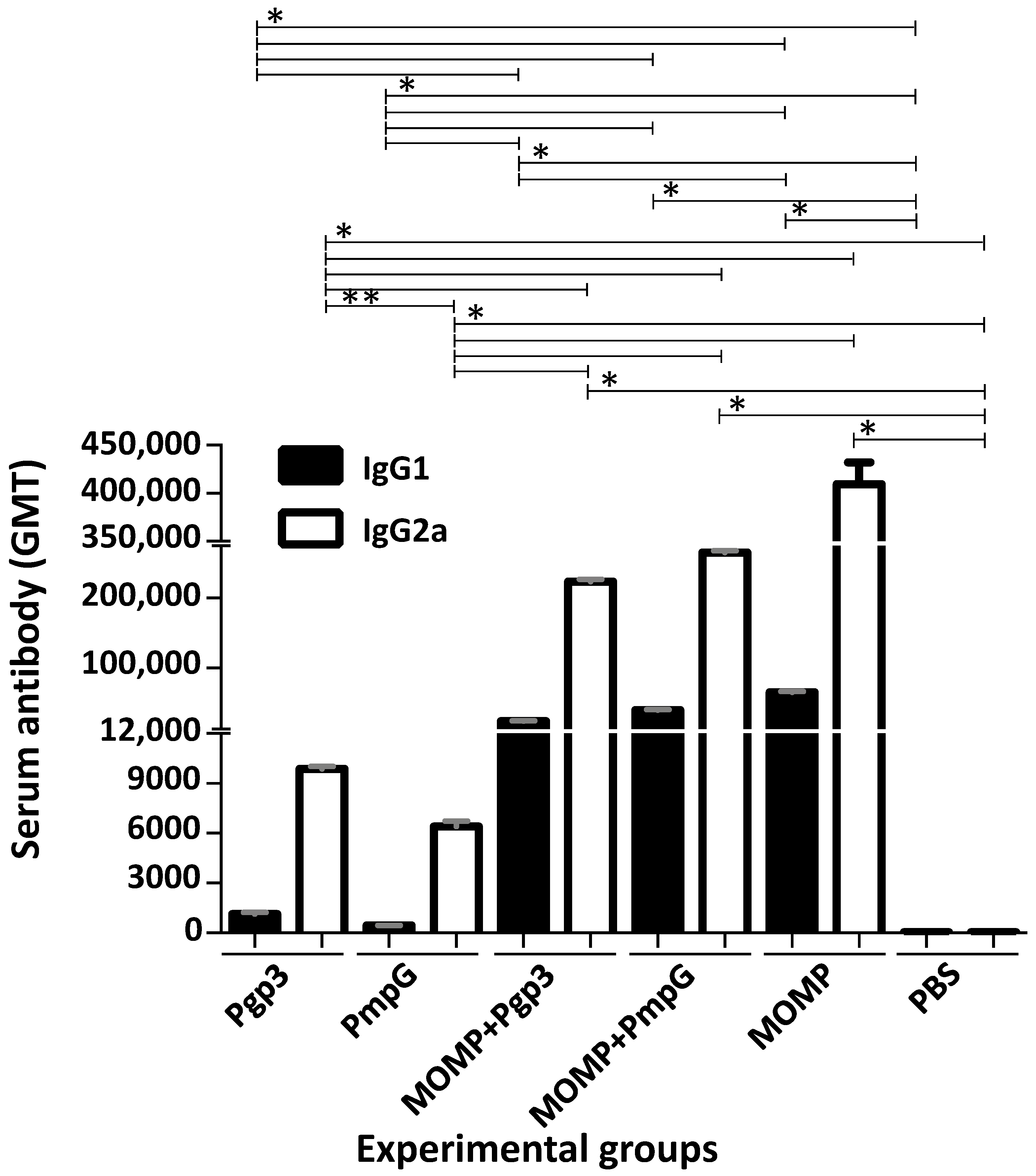
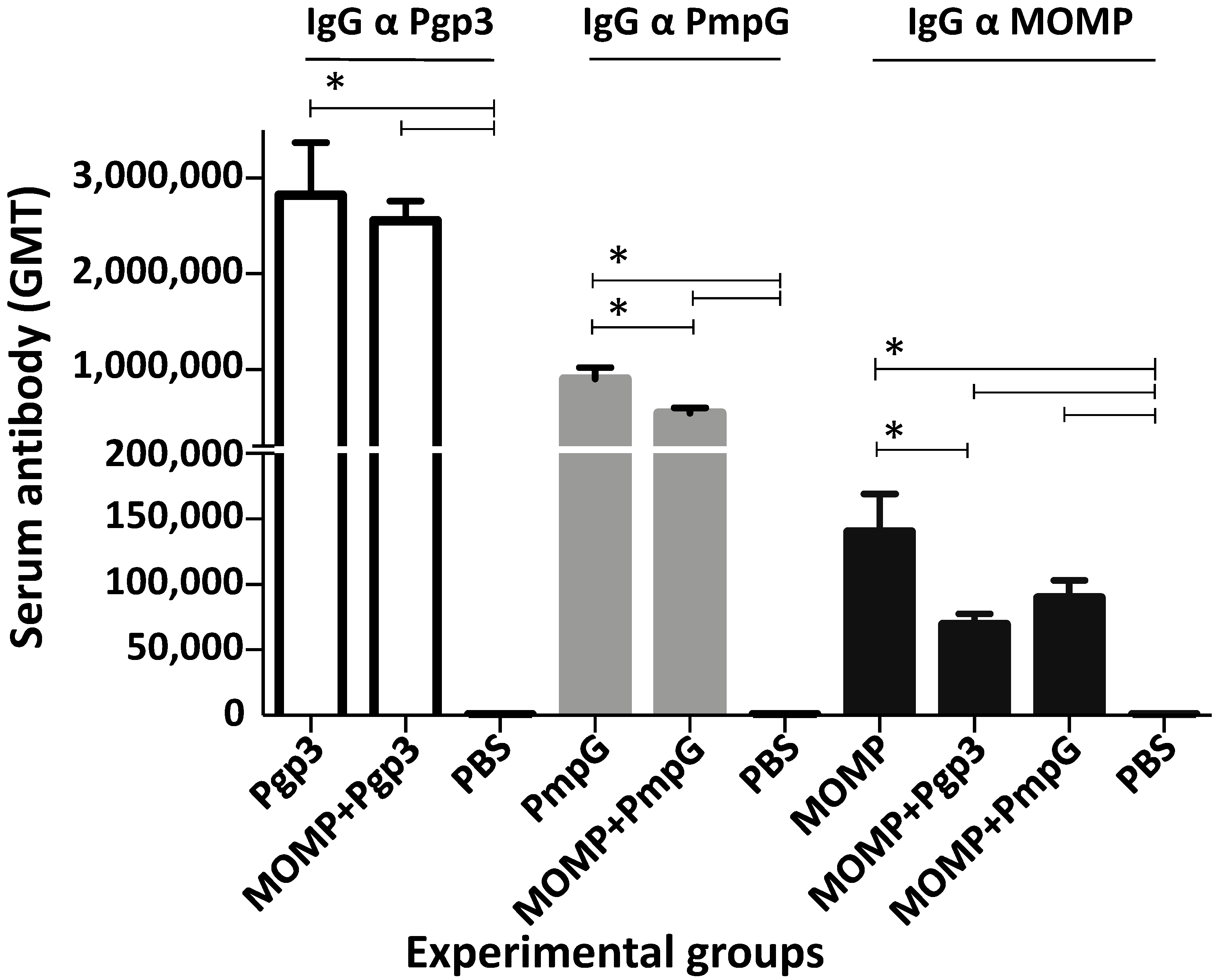



| Immunization Groups | % Change Body Weight (Mean ± 1 SE) | Lungs Weight (g) (Mean ± 1 SE) | Median Number IFU Recovered from Lungs (Min–Max) × 106 | IFN-γ (pg/mL) (Mean ± 1 SE) |
|---|---|---|---|---|
| Pgp3 | −16.4 ± 1.8 a,b,d,e | 0.33 ± 0.01 a,b,d | 114.76 (11.78–1177.80) g,h,i | 1184.8 ± 170.6 b,d |
| PmpG | −18.6 ± 2.2 b,d,e | 0.31 ± 0.01 a,b,d | 115.89 (7.40–906.00) f,g,h,i | 836.2 ± 161.0 a,b,d |
| MOMP + Pgp3 | −9.1 ± 2.5 a,c | 0.29 ± 0.02 a,b | 16.50 (0.03–154.02) f,g,h | 719.8 ± 241.0 a,b,d |
| MOMP + PmpG | −6.1 ± 0.8 a | 0.27 ± 0.00 a,b | 0.05 (BLD–5.44) f | 46.0 ± 16.9 a |
| MOMP | −4.2 ± 0.9 a | 0.23 ± 0.00 a | 0.09 (BLD–10.87) f | 112.5 ± 60.3 a |
| PBS | −21.4 ± 1.6 | 0.36 ± 0.01 | 498.30 (84.56–1087.20) | 1444.4 ± 219.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Slepenkin, A.; Pal, S.; Hoang-Phou, S.; Abisoye-Ogunniyan, A.; Rasley, A.; D’haeseleer, P.; Coleman, M.A.; de la Maza, L.M. The Polymorphic Membrane Protein G Has a Neutral Effect and the Plasmid Glycoprotein 3 an Antagonistic Effect on the Ability of the Major Outer Membrane Protein to Elicit Protective Immune Responses against a Chlamydia muridarum Respiratory Challenge. Vaccines 2023, 11, 504. https://doi.org/10.3390/vaccines11030504
Slepenkin A, Pal S, Hoang-Phou S, Abisoye-Ogunniyan A, Rasley A, D’haeseleer P, Coleman MA, de la Maza LM. The Polymorphic Membrane Protein G Has a Neutral Effect and the Plasmid Glycoprotein 3 an Antagonistic Effect on the Ability of the Major Outer Membrane Protein to Elicit Protective Immune Responses against a Chlamydia muridarum Respiratory Challenge. Vaccines. 2023; 11(3):504. https://doi.org/10.3390/vaccines11030504
Chicago/Turabian StyleSlepenkin, Anatoli, Sukumar Pal, Steven Hoang-Phou, Abisola Abisoye-Ogunniyan, Amy Rasley, Patrik D’haeseleer, Matthew A. Coleman, and Luis M. de la Maza. 2023. "The Polymorphic Membrane Protein G Has a Neutral Effect and the Plasmid Glycoprotein 3 an Antagonistic Effect on the Ability of the Major Outer Membrane Protein to Elicit Protective Immune Responses against a Chlamydia muridarum Respiratory Challenge" Vaccines 11, no. 3: 504. https://doi.org/10.3390/vaccines11030504
APA StyleSlepenkin, A., Pal, S., Hoang-Phou, S., Abisoye-Ogunniyan, A., Rasley, A., D’haeseleer, P., Coleman, M. A., & de la Maza, L. M. (2023). The Polymorphic Membrane Protein G Has a Neutral Effect and the Plasmid Glycoprotein 3 an Antagonistic Effect on the Ability of the Major Outer Membrane Protein to Elicit Protective Immune Responses against a Chlamydia muridarum Respiratory Challenge. Vaccines, 11(3), 504. https://doi.org/10.3390/vaccines11030504








