Invasive Meningococcal Disease and Meningococcal Serogroup B Vaccination in Adults and Their Offspring: Knowledge, Attitudes, and Practices in Italy (2019)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Sample Size
2.2. Instruments
- Demographic characteristics of the participants. Age, sex, professional qualifications, education level, household characteristics, whether they had any background (occupational or educational) in healthcare settings.
- Knowledge Test. Participants received a knowledge test, containing a total of 18 true–false statements, such as “Measles vaccine may elicit autism” (false), covering some typical misconceptions on vaccines and immunizations (items Q6 to Q18) and, more specifically, targeting IMD and meningococcal vaccines (items Q1 to Q5) [4,7,9,45,46,47,48,49]. A summary score (General Knowledge Score or GKS) was calculated as follows: when the participant provided a correct answer, +1 was added to a sum score, whereas a wrong indication or a missing answer added 0 to the sum score.
- Risk Perception. Risk perception is a significant component of attitude and has been defined as a function of the perceived probability of an event and its expected consequences, being assessed as the mathematical product of subjective probability and disease severity [43,50,51,52]. Consequently, we asked the participants about their perceived probability of (a) meningococcal infections (Einf), (b) vaccine-related adverse effects (Evac), and whether they perceived the severity of the (c) natural infection (Cinf) and (d) vaccine-related adverse effects (Cvac). In order to summarize the results, we used a fully labelled 5-point scale (“almost zero”, “low or rather low”, “moderate”, “high or rather high”, “very high”). Two risk perception scores (RPS) were eventually obtained through the formulas:
- 4.
- Attitudes toward vaccinations. Participants’ attitude towards vaccines and immunizations was asked both in general and more specifically towards MenB/MenC, through a 5-point Likert scale (“strongly against”, “against”, “neutral/no opinion”, “favorable”, “strongly favorable”). In the analyses, the results were dichotomized as somehow favorable (“strongly favorable” and “favorable”) vs. somehow against/neutral (“neutral”, “against”, “strongly against”): in both cases, a selected set of declarative sentences was presented to the participants regarding their reason to respectively accept immunizations and more specifically meningococcal vaccines (i.e., “to avoid getting vaccine preventable diseases (VPDs)”, “to avoid transmitting VPDs”, “to avoid complications of VPDs”, “to avoid VPDs in subjects who cannot be vaccinated”) or instead refuse them (e.g., “to avoid shots/medications”, “uselessness”, “fear of side effects”, “religious/ethical reasons”, etc.). Eventually, participants were asked to summarize their perceived trust towards vaccines and healthcare providers through a 5-point Likert scale (“scarce” to “optimal”).
- 5.
- Practices. All participants were asked whether they had been previously vaccinated for serogroup B meningitis. Participants having children 14 years old or younger were then asked whether their offspring had previously received MenB vaccine, or whether immunization was planned to be performed in the next six months.
2.3. Ethical Considerations
2.4. Data Analysis
3. Results
3.1. Demographics of Participants
3.2. Knowledge Test
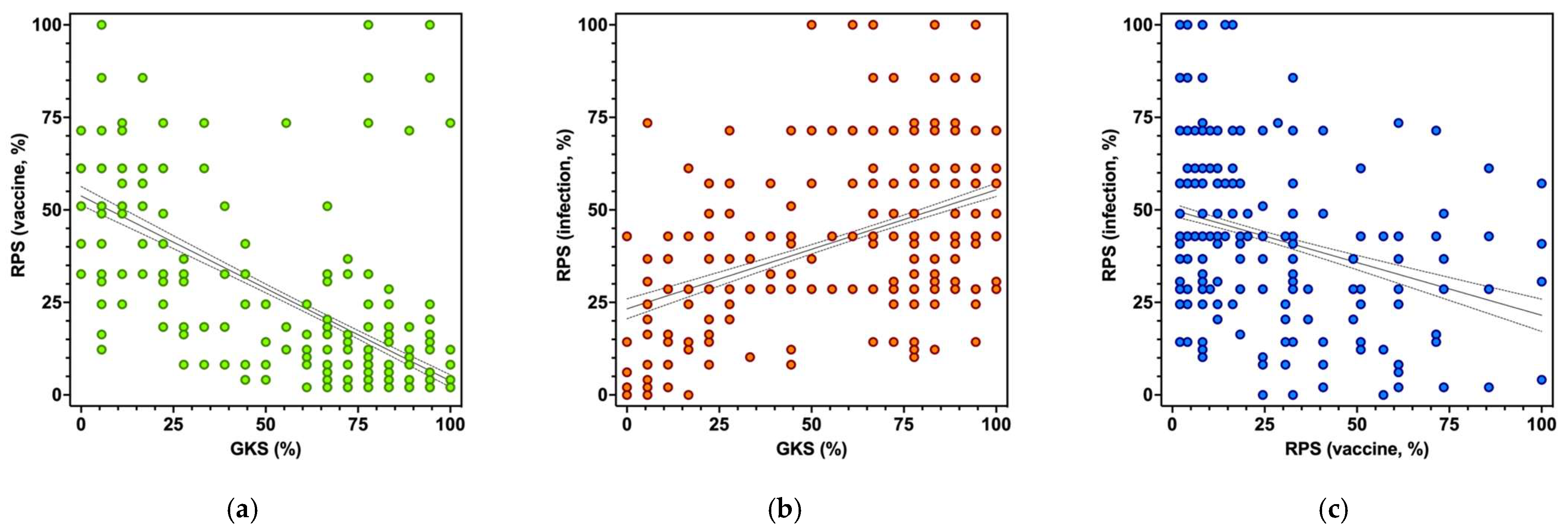
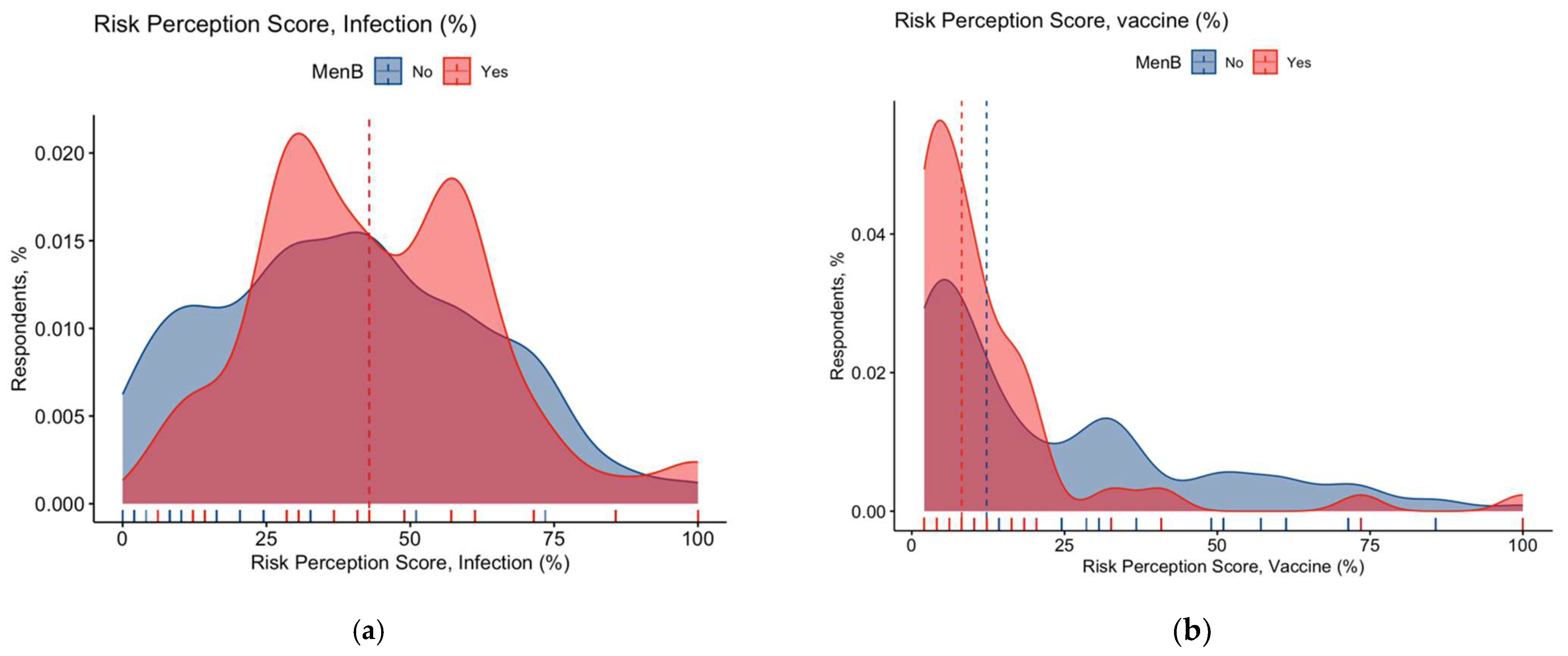
3.3. Risk Perception
3.4. Attitudes and Practices towards Meningococcal Vaccines
3.5. Univariate Analysis
3.6. Multivariable Analysis
4. Discussion
Limits
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Variable | All participants No./1010, % |
|---|---|
| Gender | |
| Male | 268, 26.5% |
| Female | 742, 73.5% |
| Age | |
| <40 years | 553, 54.8% |
| ≥40 years | 457, 45.2% |
| Literacy | |
| Primary School | 35, 3.5% |
| Secondary School | 351, 34.8% |
| University or higher | 524, 61.8% |
| Residency | |
| Municipality <5000 inhabitants | 150, 14.9% |
| Municipality 5000–15,000 inhabitants | 235, 23.3% |
| Municipality >15,000 inhabitants | 324, 32.1% |
| Main Municipality | 294, 29.1% |
| n.a. | 7, 0.7% |
| Size of Household | |
| Single | 40, 4.0% |
| 2 persons | 174, 17.2% |
| 3 persons | 330, 32.7% |
| 4 persons | 358, 35.4% |
| 5 persons or more | 97, 9.6% |
| Household including subjects <14 y.o. | 558, 55.2% |
| Household including subjects ≥65 y.o. | 168, 16.6% |
| Background in Healthcare Settings | 310, 30.7% |
| General Knowledge Score > median | 400, 39.6% |
| Confidence in Vaccination Services (high/very high) | 724, 71.7% |
| Confidence in Vaccines (high/very high) | 719, 71.2% |
| Confidence in MenB (high/very high) | 751, 74.4% |
| Risk Perception Score (Infection) > median | 441, 43.7% |
| Risk Perception Score (Vaccine) > median | 464, 45.9% |
| Perception on Neisseria meningitidis | |
| Severe/Very Severe | 927, 91.8% |
| Frequent/Highly Frequent | 196, 19.4% |
| Perception on Neisseria Vaccines’ side effects | |
| Severe/Very Severe | 214, 21.2% |
| Frequent/Highly Frequent | 184, 18.2% |
| Attitude Towards Vaccines: somehow favorable | |
| Vaccines, in general | 780, 77.2% |
| Meningitis vaccines | 753, 74.6% |
| Previously vaccinated against MenC | 285, 28.2% |
| Previously vaccinated against MenB | 150, 14.9% |
| GKS (K2, p Value) | RPS (Vaccines) (K2, p Value) | RPS (Infection) (K2, p Value) | |
|---|---|---|---|
| All participants | 149.5, p < 0.001 | 219.7, p < 0.001 | 6.830, p = 0.033 |
| Only participants with children | 8486.0, p < 0.001 | 72.75, p < 0.001 | 11.36, p = 0.003 |
| Statement | Correct Answer | All Participants No./1010, % | Only Participants with Children <14 Years No./558, % | Participants without Children <14 Years No./452, % |
|---|---|---|---|---|
| Q01. Meningococcus causes around 40–50% of all bacterial meningitis in children aged 2 years or more, and over 70% in children aged 5 years or more. Overall, it causes half of all bacterial meningitis in all infants. | TRUE ** | 471, 46.6% | 237, 42.5% | 234, 51.8% |
| Q02. The mortality of meningococcal meningitis is around 30%, irrespective of therapy. | TRUE | 591, 58.5% | 315, 56.5% | 276, 61.1% |
| Q03. Early symptoms of meningococcal meningitis are specific, allowing a prompt and appropriate treatment by healthcare providers. | FALSE ** | 632, 62.6% | 373, 66.8% | 259, 57.3% |
| Q04. Vaccination against meningococcus reduces spread of bacteria, contributing to herd immunity and ultimately protecting those who cannot be vaccinated. | TRUE *** | 756, 74.9% | 368, 65.9% | 388, 85.8% |
| Q05. MenB vaccine may be associated with other vaccinations and vaccine formulates, not increasing the number of accesses to vaccination services. | TRUE *** | 480, 47.5% | 227, 40.7% | 253, 56.0% |
| Q06. Vaccine additives are not harmful to human beings. | TRUE ** | 535, 53.0% | 271, 48.6% | 264, 58.4% |
| Q07. Vaccines against measles may cause severe damages to the central nervous system. | FALSE *** | 669, 66.2% | 313, 56.1% | 356, 78.8% |
| Q08. Influenza vaccine may cause severe side effects, potentially lethal. | FALSE *** | 433, 42.9% | 210, 37.6% | 223, 49.3% |
| Q09. Measles vaccine may elicit autism. | FALSE *** | 770, 76.2% | 381, 68.3% | 389, 86.1% |
| Q10. Some vaccinations may cause diabetes. | FALSE * | 595, 58.9% | 311, 55.7% | 284, 62.8% |
| Q11. Some autoimmune diseases may be elicited by vaccines. | FALSE *** | 550, 54.5% | 252, 45.2% | 298, 65.9% |
| Q12. Vaccines are useless, as infectious diseases may be always treated with specific therapies. | FALSE *** | 895, 88.6% | 463, 83.0% | 432, 95.6% |
| Q13. Vaccination shots increase probabilities of allergic reactions. | FALSE * | 580, 57.4% | 304, 54.5% | 276, 61.1% |
| Q14. Without vaccinations, smallpox would still exist. | TRUE *** | 817, 80.9% | 398, 71.3% | 419, 92.7% |
| Q15. The efficacy of vaccines and vaccination programs has been repetitively proved. | TRUE *** | 800, 79.2% | 379, 67.9% | 421, 93.1% |
| Q16. Children would be more resistant to infectious disease without vaccinations. | FALSE *** | 736, 72.9% | 349, 62.5% | 387, 85.6% |
| Q17. Many immunizations are performed too early: as a consequence, the immune system of children is not allowed to fully develop by itself. | FALSE ** | 612, 60.6% | 315, 56.5% | 297, 65.7% |
| Q18. The immune system may be compromised by receiving too many vaccines in pediatric age. | FALSE *** | 684, 67.7% | 323, 57.9% | 361, 79.9% |
| Drivers (Vaccinated) | All Participants (No./150, %) | Only Participants with Children <14 Years (No./53, %) | Participants without Children <14 Years (No./97, %) |
|---|---|---|---|
| It is included in the National Vaccination Plan ** | 91, 60.7% | 24, 45.3% | 67, 69.1% |
| Suggested by general practitioner | 32, 21.3% | 12, 22.6% | 20, 20.6% |
| Meningitidis is a severe disease | 119, 79.3% | 38, 71.7% | 81, 83.5% |
| In order to be protected against as many infectious diseases as possible *** | 115, 76.7% | 31, 58.5% | 84, 86.6% |
| In order to be protected against meningitis | 90, 60.0% | 33, 62.3% | 57, 58.8% |
| A friend has been affected by meningitis | 22, 14.7% | 8, 15.1% | 14, 14.4% |
| In order to avoid bacterial meningitis | 110, 73.3% | 42, 79.2% | 68, 70.1% |
| In order to avoid complications of meningitis | 117, 78.0% | 39, 73.6% | 78, 80.4% |
| In order to avoid transmission of meningitis | 94, 62.7% | 30, 56.6% | 64, 66.0% |
| In order to protect subjects who cannot be vaccinated | 110, 73.3% | 41, 77.4% | 69, 71.1% |
| Barriers (Not vaccinated) | All Participants (No./860, %) | Only Participants with Children < 14 Years (No./505, %) | Participants without Children < 14 Years (No./355, %) |
| I am against vaccines (in general) * | 21, 2.4% | 17, 3.4% | 4, 1.1% |
| Preference in other preventive measures | 63, 7.3% | 33, 6.5% | 30, 8.5% |
| Greater trust in antimicrobial treatment *** | 21, 2.4% | 21, 4.2% | 0, - |
| Greater trust in alternative therapies (e.g., homeopathy) *** | 35, 4.1% | 35, 6.9% | 0, - |
| Cannot be vaccinated | 30, 3.5% * | 11, 2.2% | 19, 5.4% |
| Side effects after previous vaccination *** | 47, 5.5% | 44, 8.7% | 3, 0.8% |
| Fear of side effects *** | 152, 17.7% | 140, 27.7% | 12, 3.4% |
| Lack of trust in “experimental” vaccines *** | 173, 20.1% | 146, 28.9% | 27, 7.6% |
| Fear of neurological disorders induced by vaccines (e.g., Guillaume Barré syndrome, multiple sclerosis) *** | 155, 18.0% | 143, 28.3% | 12, 3.4% |
| Fear of developing autism after vaccine *** | 85, 9.9% | 70, 13.9% | 15, 4.2% |
| Fear of side effects elicited by vaccine adjuvants *** | 140, 16.3% | 122, 24.2% | 18, 5.1% |
| Fear of side effects elicited by heavy metals included in the vaccine *** | 146, 17.0% | 125, 24.8% | 21, 5.9% |
| Not recommended by general practitioner *** | 69, 8.0% | 8, 1.6% | 61, 17.2% |
| Aiming to reduce the number of injections | 41, 4.8% | 25, 5.0% | 16, 4.5% |
| Vaccine not affordable | 60, 7.0% *** | 13, 2.6% | 47, 13.2% |
| GKS | RPS (Vaccines) | RPS (Infection) | |
|---|---|---|---|
| rho, p Value | rho, p Value | rho, p Value | |
| All participants (No. = 1010, 100%) | |||
| GKS | - | −0.666, p < 0.001 | 0.400, p < 0.001 |
| RPS (Vaccines) | −0.666, p < 0.001 | - | −0.235, p < 0.001 |
| RPS (Infection) | 0.400, p < 0.001 | −0.235, p < 0.001 | - |
| Only participants with children (No. = 558, 55.2%) | |||
| GKS | - | −0.725, p < 0.001 | 0.432, p < 0.001 |
| RPS (Vaccines) | −0.725, p < 0.001 | - | −0.238, p < 0.001 |
| RPS (Infection) | 0.432, p < 0.001 | −0.238, p < 0.001 | - |
| GKS | RPS (Vaccines) | RPS (Infection) | ||||
|---|---|---|---|---|---|---|
| Average ± SD | M–W U, p Value | Average ± SD | M–W U, p Value | Average ± SD | M–W U, p Value | |
| All participants (No. = 1010, 100%) | ||||||
| Vaccinated against MenB | 77.6 ± 21.5 | 84,557.5 | 12.8 ± 17.6 | 47,625.0 | 50.9 ± 19.2 | 79,134.0 |
| Not vaccinated | 61.4 ± 30.8 | p < 0.001 | 23.4 ± 23.6 | p < 0.001 | 42.6 ± 21.1 | p < 0.001 |
| Only participants with children (No. = 558, 55.2%) | ||||||
| Children vaccinated for MenB | 74.7 ± 24.6 | 48,958.0 | 16.0 ± 21.3 | 19,615.0 | 44.8 ± 20.5 | 39,307.0 |
| Not vaccinated | 47.5 ± 34.5 | p < 0.001 | 32.9 ± 26.2 | p < 0.001 | 39.8 ± 23.7 | p = 0.017 |
| All participants (No. = 1010, 100%) | ||||||
| Participants with children < 14 years old | 57.6 ± 33.6 | 104,821.5 | 26.2 ± 25.8 | 148,971.0 | 46.5 ± 19.0 | 108,074.5 |
| Participants without children < 14 years old | 71.5 ± 23.1 | p < 0.001 | 16.5 ± 17.9 | p < 0.001 | 41.6 ± 22.3 | p < 0.001 |
| Vaccinated against MenB | Chi Squared Test p Value | ||
|---|---|---|---|
| Yes No./150, % | No No./860, % | ||
| Male gender | 58, 38.7% | 210, 24.4% | <0.001 |
| Age ≥40 years | 35, 23.3% | 422, 49.1% | <0.001 |
| Literacy: University or higher | 71, 47.3% | 553, 64.3% | <0.001 |
| Municipality >15,000 inh. | 104, 69.3% | 514, 59.8% | 0.027 |
| Migration Background | 0, - | 21, 2.4% | 0.053 |
| Household Characteristics | |||
| … including 3 or more people | 121, 80.7% | 664, 77.2% | 0.348 |
| … including subjects <14 y.o. | 53, 35.3% | 505, 58.7% | <0.001 |
| … including subjects ≥65 y.o. | 40, 26.7% | 128, 14.9% | <0.001 |
| Background in Healthcare Settings | 47, 31.3% | 263, 30.6% | 0.854 |
| General Knowledge Score > median | 76, 50.7% | 324, 37.7% | 0.003 |
| Risk Perception Score > median | |||
| Natural infection | 79, 52.7% | 362, 42.1% | 0.016 |
| Vaccines | 40, 26.7% | 424, 49.3% | <0.001 |
| Attitude Towards Vaccines: somehow favorable | |||
| Vaccines, in general | 142, 94.7% | 638, 74.2% | <0.001 |
| Meningitis vaccines | 143, 95.3% | 610, 70.9% | <0.001 |
| Confidence in Vaccination Services (high/very high) | 143, 95.3% | 581, 67.6% | <0.001 |
| Confidence in Vaccines (high/very high) | 135, 90.0% | 584, 67.9% | <0.001 |
| Confidence in MenB Vaccines (high/very high) | 143, 95.3% | 608, 70.7% | <0.001 |
| Previously vaccinated against MenC | 124, 82.7% | 161, 18.7% | <0.001 |
| Variable | B | S.E. | Wald Coefficient | p Value |
|---|---|---|---|---|
| Male gender | 1.158 | 0.299 | 15.002 | <0.001 |
| Municipality >15,000 inhabitants | 0.516 | 0.238 | 4.708 | 0.030 |
| General Knowledge Score > median | −0.440 | 0.290 | 2.314 | 0.128 |
| Risk Perception Score for Vaccines > median | −0.846 | 0.295 | 8.233 | 0.004 |
| Attitude Towards Vaccines: somehow favorable | ||||
| Vaccines, in general | −0.536 | 0.729 | 0.541 | 0.462 |
| Meningitis vaccines | 2.523 | 0.722 | 12.219 | <0.001 |
| Confidence in Vaccination Services (high/very high) | −0.070 | 0.450 | 0.024 | 0.877 |
| Confidence in Vaccines (high/very high) | 0.488 | 0.488 | 0.999 | 0.318 |
| Confidence in MenB Vaccines (high/very high) | −1.591 | 0.826 | 3.712 | 0.054 |
| Previously vaccinated against MenB | 1.727 | 0.544 | 10.077 | 0.002 |
| Previously vaccinated against MenC | 0.975 | 0.311 | 9.854 | 0.002 |
| Previously vaccinated children against MenC | 1.885 | 0.301 | 39.113 | <0.001 |
| Constant | −2.466 | 0.434 | 32.273 | <0.001 |
| Variable | aOR | 95% Confidence Interval | |
|---|---|---|---|
| Male gender | 1.447 | 0.908 | 2.308 |
| Age ≥40 years | 0.514 | 0.312 | 0.847 |
| Literacy: University or higher | 0.657 | 0.413 | 1.045 |
| Municipality >15,000 inh. | 1.350 | 0.816 | 2.235 |
| Household Characteristics | |||
| … including subjects <14 y.o. | 0.672 | 0.425 | 1.063 |
| … including subjects ≥65 y.o. | 1.107 | 0.645 | 1.899 |
| General Knowledge Score > median | 0.759 | 0.472 | 1.222 |
| Risk Perception Score > median | |||
| Natural infection | 1.043 | 0.660 | 1.649 |
| Vaccines | 0.977 | 0.548 | 1.742 |
| Attitude Towards Vaccines: somehow favorable | |||
| Vaccines, in general | 0.325 | 0.073 | 1.460 |
| Meningitis vaccines | 5.355 | 1.565 | 18.328 |
| Confidence in Vaccination Services (high/very high) | 10.325 | 2.783 | 38.308 |
| Confidence in Vaccines, in general (high/very high) | 0.365 | 0.131 | 1.015 |
| Confidence in MenB Vaccine (high/very high) | 0.643 | 0.168 | 2.458 |
| Previously vaccinated against MenC | 14.978 | 8.983 | 24.974 |


References
- Alderson, M.R.; Arkwright, P.D.; Bai, X.; Black, S.; Borrow, R.; Caugant, D.A.; Dinleyici, E.C.; Harrison, L.H.; Lucidarme, J.; McNamara, L.A.; et al. Surveillance and Control of Meningococcal Disease in the COVID-19 Era: A Global Meningococcal Initiative Review. J. Infect. 2022, 84, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Halperin, S.A.; Bettinger, J.A.; Greenwood, B.; Harrison, L.H.; Jelfs, J.; Ladhani, S.N.; McIntyre, P.; Ramsay, M.E.; Sáfadi, M.A.P. The Changing and Dynamic Epidemiology of Meningococcal Disease. Vaccine 2012, 30, B26–B36. [Google Scholar] [CrossRef] [PubMed]
- Pizza, M.; Bekkat-Berkani, R.; Rappuoli, R. Vaccines against Meningococcal Diseases. Microorganisms 2020, 8, 1521. [Google Scholar] [CrossRef]
- Whittaker, R.; Dias, J.G.; Ramliden, M.; Ködmön, C.; Economopoulou, A.; Beer, N.; Pastore Celentano, L. The Epidemiology of Invasive Meningococcal Disease in EU/EEA Countries, 2004–2014. Vaccine 2017, 35, 2034–2041. [Google Scholar] [CrossRef]
- Hasbun, R. Progress and Challenges in Bacterial Meningitis: A Review. JAMA 2022, 328, 2147–2154. [Google Scholar] [CrossRef]
- Stephens, D.S.; Greenwood, B.; Brandtzaeg, P. Epidemic Meningitis, Meningococcemia, and Neisseria Meningitidis. Lancet 2007, 369, 2196–2210. [Google Scholar] [CrossRef]
- McGill, F.; Heyderman, R.S.; Panagiotou, S.; Tunkel, A.R.; Solomon, T. Acute Bacterial Meningitis in Adults. Lancet 2016, 388, 3036–3047. [Google Scholar] [CrossRef]
- Sridhar, S.; Greenwood, B.; Head, C.; Plotkin, S.A.; Sáfadi, M.A.; Saha, S.; Taha, M.K.; Tomori, O.; Gessner, B.D. Global Incidence of Serogroup B Invasive Meningococcal Disease: A Systematic Review. Lancet Infect. Dis. 2015, 15, 1334–1346. [Google Scholar] [CrossRef]
- Christensen, H.; May, M.; Bowen, L.; Hickman, M.; Trotter, C.L. Meningococcal Carriage by Age: A Systematic Review and Meta-Analysis. Lancet Infect. Dis. 2010, 10, 853–861. [Google Scholar] [CrossRef]
- Stahl, J.P.; Cohen, R.; Denis, F.; Gaudelus, J.; Lery, T.; Lepetit, H.; Martinot, A. Vaccination against Meningococcus C. Vaccinal Coverage in the French Target Population. Med. Mal. Infect. 2013, 43, 75–80. [Google Scholar] [CrossRef]
- Finn, A.; Morales-Aza, B.; Sikora, P.; Giles, J.; Lethem, R.; Marlais, M.; Thors, V.; Pollard, A.J.; Faust, S.; Heath, P.; et al. Density Distribution of Pharyngeal Carriage of Meningococcus in Healthy Young Adults. Pediatr. Infect. Dis. J. 2016, 35, 1080–1085. [Google Scholar] [CrossRef]
- Grogan, J.; Roos, K. Serogroup B Meningococcus Outbreaks, Prevalence, and the Case for Standard Vaccination. Curr. Infect. Dis. Rep. 2017, 19, 30. [Google Scholar] [CrossRef] [PubMed]
- Jafri, R.Z.; Ali, A.; Messonnier, N.E.; Tevi-Benissan, C.; Durrheim, D.; Eskola, J.; Fermon, F.; Klugman, K.P.; Ramsay, M.; Sow, S.; et al. Global Epidemiology of Invasive Meningococcal Disease. Popul. Health Metr. 2013, 11, 17. [Google Scholar] [CrossRef] [PubMed]
- Kriz, P.; Wieffer, H.; Holl, K.; Rosenlund, M.; Budhia, S.; Vyse, A. Changing Epidemiology of Meningococcal Disease in Europe from the Mid-20th to the Early 21st Century. Expert Rev. Vaccines 2011, 10, 1477–1486. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control (ECDC). Invasive Meningococcal Disease Annual Epidemiological Report for 2018 Key Facts; European Centre for Disease Prevention and Control (ECDC): Stockhoolm, Sweden, 2022. [Google Scholar]
- Martinón-Torres, F.; Taha, M.K.; Knuf, M.; Abbing-Karahagopian, V.; Pellegrini, M.; Bekkat-Berkani, R.; Abitbol, V. Evolving Strategies for Meningococcal Vaccination in Europe: Overview and Key Determinants for Current and Future Considerations. Pathog. Glob. Health 2022, 116, 85–98. [Google Scholar] [CrossRef]
- Ponticelli, D.; D’Ambrosio, A.; Cancellieri, M.; Agozzino, E. Do HCWs Adequately Know about Meningitis and 4CMenB Vaccine and Recommend Its Use to Parents? A Cross Sectional Analysis in Campania Region, Italy. J. Prev. Med. Hyg. 2019, 60, E147–E157. [Google Scholar] [CrossRef]
- Stefanizzi, P.; Bianchi, F.P.; Martinelli, A.; di Lorenzo, A.; de Petro, P.; Graziano, G.; Lattanzio, S.; Diella, G.; Stella, P.; Ancona, D.; et al. Safety Profile of MenB-FHBp Vaccine among Adolescents: Data from Surveillance of Adverse Events following Immunization in Puglia (Italy), 2018–2020. Hum. Vaccines Immunother. 2022, 18, 2041359. [Google Scholar] [CrossRef]
- Boccalini, S.; Zanella, B.; Landa, P.; Amicizia, D.; Bechini, A.; Innocenti, M.; Iovine, M.; Lecini, E.; Marchini, F.; Paolini, D.; et al. Why the Anti-Meningococcal b Vaccination during Adolescence Should Be Implemented in Italy: An Overview of Available Evidence. Microorganisms 2020, 8, 1681. [Google Scholar] [CrossRef]
- Rappuoli, R.; Pizza, M.; Masignani, V.; Vadivelu, K. Meningococcal B Vaccine (4CMenB): The Journey from Research to Real World Experience. Expert Rev. Vaccines 2018, 17, 1111–1121. [Google Scholar] [CrossRef]
- Pezzotti, P.; Bellino, S.; Riccardo, F.; Lucaroni, F.; Cerquetti, M.; Pantosti, A.; Rezza, G.; Stefanelli, P. Vaccine Preventable Invasive Bacterial Diseases in Italy: A Comparison between the National Surveillance System and Recorded Hospitalizations, 2007–2016. Vaccine 2019, 37, 41–48. [Google Scholar] [CrossRef]
- Jachowicz, E.; Gębicka, M.; Plakhtyr, D.; Shynkarenko, M.; Urbanowicz, J.; Mach, M.; Czepiel, J.; Marchewka, J.; Wójkowska-Mach, J. Incidence of Vaccine-Preventable Childhood Diseases in the European Union and in the European Free Trade Association Countries. Vaccines 2021, 9, 796. [Google Scholar] [CrossRef]
- di Pietro, G.M.; Biffi, G.; Castellazzi, M.L.; Tagliabue, C.; Pinzani, R.; Bosis, S.; Marchisio, P.G. Meningococcal Disease in Pediatric Age: A Focus on Epidemiology and Prevention. Int. J. Environ. Res. Public Health 2022, 19, 4035. [Google Scholar] [CrossRef]
- Crum-Cianflone, N.; Sullivan, E. Meningococcal Vaccinations. Infect. Dis. Ther. 2016, 5, 89–112. [Google Scholar] [CrossRef] [PubMed]
- Bonanni, P.; Ferrero, A.; Guerra, R.; Iannazzo, S.; Odone, A.; Pompa, M.; Rizzuto, E.; Signorelli, C. Vaccine Coverage in Italy and Assessment of the 2012-2014 National Immunization Prevention Plan. Epidemiol. Prev. 2015, 39, 146–158. [Google Scholar] [CrossRef]
- Signorelli, C.; Guerra, R.; Siliquini, R.; Ricciardi, W. Italy’s Response to Vaccine Hesitancy: An Innovative and Cost Effective National Immunization Plan Based on Scientific Evidence. Vaccine 2017, 35, 4057–4059. [Google Scholar] [CrossRef]
- D’ancona, F.; D’amario, C.; Maraglino, F.; Rezza, G.; Iannazzo, S. The Law on Compulsory Vaccination in Italy: An Update 2 Years after the Introduction. Eurosurveillance 2019, 24, 1900371. [Google Scholar] [CrossRef]
- Boccalini, S.; Bechini, A.; Sartor, G.; Paolini, D.; Innocenti, M.; Bonanni, P.; Panatto, D.; Lai, P.L.; Zangrillo, F.; Marchini, F.; et al. Health Technology Assessment (HTA) Del Vaccino Anti-Meningococco B (Trumenba®) per Gli Adolescenti in Italia. J. Prev. Med. Hyg. 2019, 60, E1–E94. [Google Scholar] [CrossRef]
- de Waure, C.; Quaranta, G.; Ianuale, C.; Panatto, D.; Amicizia, D.; Apprato, L.; Campanella, P.; Colotto, M.; de Meo, C.; di Nardo, F.; et al. Knowledge, Attitudes and Behaviors of the Italian Population towards Neisseria Meningitidis, Streptococcus Pneumoniae and HPV Diseases and Vaccinations: A Cross-Sectional Multicentre Study. Public Health 2016, 141, 136–142. [Google Scholar] [CrossRef]
- Biasio, L.R.; Corsello, G.; Costantino, C.; Fara, G.M.; Giammanco, G.; Signorelli, C.; Vecchio, D.; Vitale, F. Communication about Vaccination: A Shared Responsibility. Hum. Vaccin Immunother 2016, 12, 2984–2987. [Google Scholar] [CrossRef]
- Sabbatucci, M.; Odone, A.; Signorelli, C.; Siddu, A.; Maraglino, F.; Rezza, G. Improved Temporal Trends of Vaccination Coverage Rates in Childhood after the Mandatory Vaccination Act, Italy 2014–2019. J. Clin. Med. 2021, 10, 2540. [Google Scholar] [CrossRef]
- MacDonald, N.E.; Eskola, J.; Liang, X.; Chaudhuri, M.; Dube, E.; Gellin, B.; Goldstein, S.; Larson, H.; Manzo, M.L.; Reingold, A.; et al. Vaccine Hesitancy: Definition, Scope and Determinants. Vaccine 2015, 33, 4161–4164. [Google Scholar] [CrossRef]
- Bloom, B.R.; Marcuse, E.; Mnookin, S. Addressing Vaccine Hesitancy. Science (1979) 2014, 344, 339. [Google Scholar] [CrossRef]
- Goldstein, S.; MacDonald, N.E.; Guirguis, S.; Eskola, J.; Liang, X.; Chaudhuri, M.; Dube, E.; Gellin, B.; Larson, H.; Manzo, M.L.; et al. Health Communication and Vaccine Hesitancy. Vaccine 2015, 33, 4212–4214. [Google Scholar] [CrossRef]
- Hall, C.B.; Weinberg, G.A.; Iwane, M.K.; Blumkin, A.K.; Edwards, K.M.; Staat, M.A.; Auinger, P.; Griffin, M.R.; Poehling, K.A.; Erdman, D.; et al. The Burden of Respiratory Syncytial Virus Infection in Young Children. N. Engl. J. Med. 2009, 360, 588–598. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.B. Respiratory Syncytial Virus and Parainfluenza Virus. N. Engl. J. Med. 2001, 344, 1917–1926. [Google Scholar] [CrossRef]
- Peradotto, M.; Bondi, A.; Lombardi, D.; Bottino, P.; Zanotto, E.; Barbui, A.M.; Cavallo, R. The Impact of COVID-19 Pandemic Control on Vaccine-Preventable Invasive Bacterial Diseases in Piedmont (Italy). Infection 2022, 50, 767–770. [Google Scholar] [CrossRef] [PubMed]
- Italian Health Ministry. Italian Health Ministry—Vaccination Rates in Childhood and Adolescents, 2020; Italian Health Ministry: Rome, Italy, 2021. Available online: https://www.salute.gov.it/imgs/C_17_tavole_20_9_7_file.pdf (accessed on 25 December 2022).
- Italian Health Ministry. Italian Health Ministry—Vaccination Rates in Childhood and Adolescents, 2021; Italian Health Ministry: Rome, Italy, 2022. Available online: https://www.salute.gov.it/imgs/C_17_tavole_20_10_7_file.pdf (accessed on 25 December 2022).
- Riccò, M.; Cattani, S.; Casagranda, F.; Gualerzi, G.; Signorelli, C. Knowledge, Attitudes, Beliefs and Practices of Occupational Physicians towards Vaccinations of Health Care Workers: A Cross Sectional Pilot Study in North-Eastern Italy. Int. J. Occup. Med. Environ. Health 2017, 30, 775–790. [Google Scholar] [CrossRef]
- Riccò, M.; Bragazzi, N.L.; Vezzosi, L.; Balzarini, F.; Colucci, M.E.; Veronesi, L. Knowledge, Attitudes, and Practices on Tick-Borne Human Diseases and Tick-Borne Encephalitis Vaccine among Farmers from North-Eastern Italy (2017). J. Agromed. 2020, 25, 73–85. [Google Scholar] [CrossRef]
- Riccò, M.; Vezzosi, L.; Gualerzi, G.; Odone, A.; Signorelli, C. Knowledge, Attitudes, and Practices of Influenza and Pneumococcal Vaccines among Agricultural Workers: Results of an Italian a Cross-Sectional Study. Acta Bio Med. Atenei Parm. 2019, 90, 439. [Google Scholar]
- Riccò, M.; Cattani, S.; Casagranda, F.; Gualerzi, G.; Signorelli, C. Knowledge, Attitudes, Beliefs and Practices of Occupational Physicians towards Seasonal Influenza Vaccination: A Cross-Sectional Study from North-Eastern Italy. J. Prev. Med. Hyg. 2017, 58, E141–E154. [Google Scholar]
- Vezzosi, L.; Riccò, M.; Agozzino, E.; Odone, A.; Signorelli, C. Knowledge, Attitudes, and Practices of General Practitioners from the Province of Parma (Northern Italy) towards Vaccinations in Adults ≥65 Year-Old. Acta Biomedica 2019, 90, 71. [Google Scholar] [CrossRef]
- Azzari, C.; Nieddu, F.; Moriondo, M.; Indolfi, G.; Canessa, C.; Ricci, S.; Bianchi, L.; Serranti, D.; Poggi, G.M.; Resti, M. Underestimation of Invasive Meningococcal Disease in Italy. Emerg. Infect. Dis. 2016, 22, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Strifler, L.; Morris, S.K.; Dang, V.; Tu, H.A.T.; Minhas, R.S.; Jamieson, F.B.; Deeks, S.L.; Crowcroft, N.S.; Sander, B. The Health Burden of Invasive Meningococcal Disease: A Systematic Review. J. Pediatr. Infect. Dis. Soc. 2016, 5, 417–430. [Google Scholar] [CrossRef]
- Parent du Chatelet, I.; Deghmane, A.E.; Antona, D.; Hong, E.; Fonteneau, L.; Taha, M.K.; Lévy-Bruhl, D. Characteristics and Changes in Invasive Meningococcal Disease Epidemiology in France, 2006–2015. J. Infect. 2017, 74, 564–574. [Google Scholar] [CrossRef] [PubMed]
- Pelton, I.S. The Global Evolution of Meningococcal Epidemiology Following the Introduction of Meningococcal Vaccines. J Afolesc Health 2016, 59 (Suppl. 2), S3–S11. [Google Scholar] [CrossRef] [PubMed]
- Anonychuk, A.; Woo, G.; Vyse, A.; Demarteau, N.; Tricco, A.C. The Cost and Public Health Burden of Invasive Meningococcal Disease Outbreaks: A Systematic Review. Pharmacoeconomics 2013, 31, 563–576. [Google Scholar] [CrossRef]
- Betsch, C.; Wicker, S. Personal Attitudes and Misconceptions, Not Official Recommendations Guide Occupational Physicians’ Vaccination Decisions. Vaccine 2014, 32, 4478–4484. [Google Scholar] [CrossRef]
- Yates, F.J.; Stone, E.R. The Risk Construct. In Risk-Taking Behaviour; Yates, F.J., Ed.; John Wiley & Sons.: Chichester, UK, 1992; pp. 1–25. ISBN 0471922501. [Google Scholar]
- Riccò, M.; Gualerzi, G.; Ranzieri, S.; Ferraro, P.; Bragazzi, N.L. Knowledge, Attitudes, Practices (KAP) of Italian Occupational Physicians towards Tick Borne Encephalitis. Trop. Med. Infect. Dis. 2020, 5, 117. [Google Scholar] [CrossRef]
- Neri, A.; Pezzotti, P.; Fazio, C.; Vacca, P.; D’Ancona, F.P.; Caporali, M.G.; Stefanelli, P. Epidemiological and Molecular Characterization of Invasive Meningococcal Disease in Italy, 2008/09-2012/13. PLoS ONE 2015, 10, e0139376. [Google Scholar] [CrossRef]
- Fazio, C.; Camilli, R.; Giufré, M.; Urciuoli, R.; Boros, S.; Neri, A.; del Grosso, M.; Vacca, P.; Giancristofaro, S.; Siddu, A.; et al. Invasive Bacterial Diseases National Surveillance: 2019–2021. Rome, Italy, 2022. [Google Scholar]
- Carannante, A.; Fazio, C.; Neri, A.; Lista, F.; Fillo, S.; Ciammaruconi, A.; Vacca, P.; Stefanelli, P. Meningococcal B Vaccine Antigen FHbp Variants among Disease-Causing Neisseria Meningitidis B Isolates, Italy, 2014–2017. PLoS ONE 2020, 15, e0241793. [Google Scholar] [CrossRef]
- Riccò, M.; Vezzosi, L.; Odone, A.; Signorelli, C. Invasive Meningococcal Disease on the Workplaces: A Systematic Review. Reggio Emilia (RE) Acta Biomed. 2017, 88, 337–351. [Google Scholar] [CrossRef]
- Gattini, V.; Napoletano, M.; Bonotti, A.; Mignani, A.; Cosentino, F.; Guglielmi, G.; Fallahi, P.; Cristaudo, A.; Foddis, R. Antimeningococcal Vaccination Coverage Among Healthcare Workers in an Italian University Hospital. Front. Public Health 2021, 9, 651100. [Google Scholar] [CrossRef] [PubMed]
- Bénard, S.; Wright, C.; Voisine, J.; Olivier, C.W.; Gaudelus, J. Lifetime Cost of Meningococcal Disease in France: Scenarios of Severe Meningitis and Septicemia with Purpura Fulminans. J. Infect. Public Health 2016, 9, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Pelullo, C.P.; Napolitano, F.; Di Giuseppe, G. Meningococcal disease and vaccination: Knowledge and acceptability among adolescents in Italy. Hum. Vaccines Immunother. 2018, 14, 1197–1202. [Google Scholar] [CrossRef]
- Janz, N.K.; Becker, M.H. The Health Belief Model: A Decade Later. Health Educ. Q. 1984, 11, 1–47. [Google Scholar] [CrossRef]
- Rosenstock, I.M. Historical Origins of the Health Belief Model. Health Educ. Monogr. 1974, 2, 328–335. [Google Scholar]
- Carpenter, C.J. A Meta-Analysis of the Effectiveness of Health Belief Model Variables in Predicting Behavior. Health Commun. 2010, 25, 661–669. [Google Scholar] [CrossRef]
- Gaube, S.; Lermer, E.; Fischer, P. The Concept of Risk Perception in Health-Related Behavior Theory and Behavior Change. In Perceived Safety. Risk Engineering; Raue, M., Streicher, B., Lermer, E., Eds.; Springer: Cham, Switzerland, 2019; pp. 101–118. ISBN 9783030114565. [Google Scholar]
- Maltezou, H.C.; Gargalianos, P.; Nikolaidis, P.; Katerelos, P.; Tedoma, N.; Maltezos, E.; Lazanas, M. Attitudes towards Mandatory Vaccination and Vaccination Coverage against Vaccine-Preventable Diseases among Health-Care Workers in Tertiary-Care Hospitals. J. Infect. 2012, 64, 319–324. [Google Scholar] [CrossRef]
- Betsch, C.; Schmid, P.; Heinemeier, D.; Korn, L.; Holtmann, C.; Böhm, R. Beyond Confidence: Development of a Measure Assessing the 5C Psychological Antecedents of Vaccination. PLoS ONE 2018, 13, e0208601. [Google Scholar] [CrossRef]
- Schmid, P.; Rauber, D.; Betsch, C.; Lidolt, G.; Denker, M.L. Barriers of Influenza Vaccination Intention and Behavior—A Systematic Review of Influenza Vaccine Hesitancy, 2005–2016. PLoS ONE 2017, 12, e0170550. [Google Scholar]
- van Lier, A.; Ferreira, J.A.; Mollema, L.; Sanders, E.A.M.; de Melker, H.E. Intention to Vaccinate Universally against Varicella, Rotavirus Gastroenteritis, Meningococcal B Disease and Seasonal Influenza among Parents in the Netherlands: An Internet Survey. BMC Res. Notes 2017, 10, 672. [Google Scholar] [CrossRef] [PubMed]
- le Ngoc Tho, S.; Ader, F.; Ferry, T.; Floret, D.; Arnal, M.; Fargeas, S.; Chidiac, C.; Valour, F. Vaccination against Serogroup B Neisseria Meningitidis: Perceptions and Attitudes of Parents. Vaccine 2015, 33, 3463–3470. [Google Scholar] [CrossRef] [PubMed]
- Gargano, L.M.; Herbert, N.L.; Painter, J.E.; Sales, J.M.; Morfaw, C.; Rask, K.; Murray, D.; Diclemente, R.J.; Hughes, J.M. Impact of a Physician Recommendation and Parental Immunization Attitudes on Receipt or Intention to Receive Adolescent Vaccines. Hum. Vaccines Immunother. 2013, 9, 2627–2633. [Google Scholar] [CrossRef] [PubMed]
- Gualano, M.R.; Bert, F.; Voglino, G.; Buttinelli, E.; D’Errico, M.M.; de Waure, C.; di Giovanni, P.; Fantini, M.P.; Giuliani, A.R.; Marranzano, M.; et al. Attitudes towards Compulsory Vaccination in Italy: Results from the NAVIDAD Multicentre Study. Vaccine 2018, 36, 3368–3374. [Google Scholar] [CrossRef]
- Morrone, T.; Napolitano, F.; Albano, L.; di Giuseppe, G. Meningococcal Serogroup B Vaccine: Knowledge and Acceptability among Parents in Italy. Hum. Vaccines Immunother. 2017, 13, 1921–1927. [Google Scholar] [CrossRef] [PubMed]
- Riccò, M.; Vezzosi, L.; Cella, C.; Pecoraro, M.; Novembre, G.; Moreo, A.; Ognibeni, E.M.; Schellenberg, G.; Maranelli, G. Tetanus Vaccination Status in Construction Workers: Results from an Institutional Surveillance Campaign. Acta Biomed. 2019, 90, 269. [Google Scholar] [CrossRef]
- Borrow, R.; Alarcón, P.; Carlos, J.; Caugant, D.A.; Christensen, H.; Debbag, R.; de Wals, P.; Echániz-Aviles, G.; Findlow, J.; Head, C.; et al. The Global Meningococcal Initiative: Global Epidemiology, the Impact of Vaccines on Meningococcal Disease and the Importance of Herd Protection. Expert Rev. Vaccines 2017, 16, 313–328. [Google Scholar] [CrossRef]
- Harrison, L.H.; Trotter, C.L.; Ramsay, M.E. Global Epidemiology of Meningococcal Disease. Vaccine 2009, 27, B51–B63. [Google Scholar] [CrossRef]
- Stefanelli, P.; Rezza, G. Impact of Vaccination on Meningococcal Epidemiology. Hum. Vaccines Immunother. 2016, 12, 1051–1055. [Google Scholar] [CrossRef]
- Stefanelli, P.; Fazio, C.; Sofia, T.; Neri, A.; Mastrantonio, P. Serogroup C Meningococci in Italy in the Era of Conjugate MenC Vaccination. BMC Infect. Dis. 2009, 9, 135. [Google Scholar] [CrossRef]
- le Maréchal, M.; Agrinier, N.; Fressard, L.; Verger, P.; Pulcini, C. Low Uptake of Meningococcal C Vaccination in France. Pediatr. Infect. Dis. J. 2017, 36, e181–e188. [Google Scholar] [CrossRef] [PubMed]
- Stefanelli, P.; Miglietta, A.; Pezzotti, P.; Fazio, C.; Neri, A.; Vacca, P.; Voller, F.; D’Ancona, F.P.; Guerra, R.; Iannazzo, S.; et al. Increased Incidence of Invasive Meningococcal Disease of Serogroup C / Clonal Complex 11, Tuscany, Italy, 2015 to 2016. Eurosurveillance 2016, 21, 30176. [Google Scholar] [CrossRef] [PubMed]
- Alicino, C.; Iudici, R.; Barberis, I.; Paganino, C.; Cacciani, R.; Zacconi, M.; Battistini, A.; Bellina, D.; di Bella, A.M.; Talamini, A.; et al. Influenza Vaccination among Healthcare Workers in Italy: The Experience of a Large Tertiary Acute-Care Teaching Hospital. Hum. Vaccines Immunother. 2015, 11, 95–100. [Google Scholar] [CrossRef]
- Durando, P.; Dini, G.; Massa, E.; la Torre, G. Tackling Biological Risk in the Workplace: Updates and Prospects Regarding Vaccinations for Subjects at Risk of Occupational Exposure in Italy. Vaccines 2019, 7, 141. [Google Scholar] [CrossRef] [PubMed]
- Heiervang, E.; Goodman, R. Advantages and Limitations of Web-Based Surveys: Evidence from a Child Mental Health Survey. Soc. Psychiat. Epidemiol. 2011, 46, 69–76. [Google Scholar] [CrossRef]
- Huang, Y.; Xu, S.; Lei, W.; Zhao, Y.; Liu, H.; Yao, D.; Xu, Y.; Lv, Q.; Hao, G.; Xu, Y.; et al. Knowledge, Attitudes, and Practices Regarding Zika: Paper and Internet-Based Survey in Zhejiang, China. JMIR Public Health Surveill. 2017, 3, e81. [Google Scholar] [CrossRef]
- Riccò, M.; Peruzzi, S. Tetanus Vaccination Status and Vaccine Hesitancy in Amateur Basketball Players (Italy, 2020). Vaccines 2022, 10, 131. [Google Scholar] [CrossRef]
- Riccò, M.; Peruzzi, S.; Balzarini, F. Public Perceptions on Non-Pharmaceutical Interventions for West Nile Virus Infections: A Survey from an Endemic Area in Northern Italy. Trop. Med. Infect. Dis. 2021, 6, 116. [Google Scholar] [CrossRef]
- Riccò, M.; Vezzosi, L.; Marchesi, F. Vaccinating Front-Line Healthcare Workers: Results of a Pre-Pandemic Cross-Sectional Study from North-Eastern Italy on First Responders. Vaccines 2022, 10, 1492. [Google Scholar] [CrossRef]
- Riccò, M.; Ferraro, P.; Camisa, V.; Satta, E.; Zaniboni, A.; Ranzieri, S.; Baldassarre, A.; Zaffina, S.; Marchesi, F. When a Neglected Tropical Disease Goes Global: Knowledge, Attitudes and Practices of Italian Physicians towards Monkeypox, Preliminary Results. Trop. Med. Infect. Dis. 2022, 7, 135. [Google Scholar] [CrossRef]
- Riccò, M.; Ferraro, P.; Camisa, V.; di Palma, P.; Minutolo, G.; Ranzieri, S.; Zaffina, S.; Baldassarre, A.; Restivo, V. Managing of Migraine in the Workplaces: Knowledge, Attitudes and Practices of Italian Occupational Physicians. Medicina 2022, 58, 686. [Google Scholar] [CrossRef] [PubMed]
- Riccò, M.; Vezzosi, L.; Gualerzi, G.; Balzarini, F.; Capozzi, V.A.; Volpi, L. Knowledge, Attitudes, Beliefs and Practices of Obstetrics-Gynecologists on Seasonal Influenza and Pertussis Immunizations in Pregnant Women: Preliminary Results from North-Western Italy. Minerva Ginecol. 2019, 71, 288–297. [Google Scholar] [CrossRef]
- Wang, B.; Clarke, M.; Afzali, H.H.A.; Marshall, H. Community, Parental and Adolescent Awareness and Knowledge of Meningococcal Disease. Vaccine 2014, 32, 2042–2049. [Google Scholar] [CrossRef]
- MacDougall, D.M.; Langley, J.M.; Li, L.; Ye, L.; MacKinnon-Cameron, D.; Top, K.A.; McNeil, S.A.; Halperin, B.A.; Swain, A.; Bettinger, J.A.; et al. Knowledge, Attitudes, Beliefs, and Behaviors of University Students, Faculty, and Staff during a Meningococcal Serogroup B Outbreak Vaccination Program. Vaccine 2017, 35, 2520–2530. [Google Scholar] [CrossRef] [PubMed]
- Agricola, E.; Gesualdo, F.; Alimenti, L.; Pandolfi, E.; Carloni, E.; D’Ambrosio, A.; Russo, L.; Campagna, I.; Ferretti, B.; Tozzi, A.E. Knowledge Attitude and Practice toward Pertussis Vaccination during Pregnancy among Pregnant and Postpartum Italian Women. Hum. Vaccines Immunother. 2016, 12, 1982–1988. [Google Scholar] [CrossRef]
- di Giuseppe, G.; Pelullo, C.P.; della Polla, G.; Montemurro, M.V.; Napolitano, F.; Pavia, M.; Angelillo, I.F. Surveying Willingness towards SARS-CoV-2 Vaccination of Healthcare Workers in Italy. Expert Rev. Vaccines 2021, 20, 881–889. [Google Scholar] [CrossRef]
- Napolitano, F.; Navaro, M.; Vezzosi, L.; Santagati, G.; Angelillo, I.F. Primary Care Pediatricians’ Attitudes and Practice towards Hpv Vaccination: A Nationwide Survey in Italy. PLoS ONE 2018, 13, e0194920. [Google Scholar] [CrossRef]
- di Girolamo, N.; Mans, C. Research Study Design. In Miller—Fowler’s Zoo and Wild Animal Medicine Current Therapy: Volume 9; Elsevier: Amsterdam, The Netherlands, 2018; Volume 9, pp. 59–62. ISBN 9780323552288. [Google Scholar]
- Tostrud, L.; Thelen, J.; Palatnik, A. Models of Determinants of COVID-19 Vaccine Hesitancy in Non-Pregnant and Pregnant Population: Review of Current Literature. Hum. Vaccines Immunother. 2022, 18, 2138047. [Google Scholar] [CrossRef]
- Prochaska, J.O.; Velicer, W.F. The Transtheoretical Model of Health Behavior Change. Am. J. Health Promot. 1997, 12, 38–48. [Google Scholar] [CrossRef]
- Lipschitz, J.M.; Fernandez, A.C.; Elsa Larson, H.; Blaney, C.L.; Meier, K.S.; Redding, C.A.; Prochaska, J.O.; Paiva, A.L. Validation of Decisional Balance and Self-Efficacy Measures for HPV Vaccination in College Women. Am. J. Health Promot. 2013, 27, 299–307. [Google Scholar] [CrossRef]
- Arnett, D.K.; Claas, S.A. Introduction to Epidemiology. In Clinical and Translational Science: Principles of Human Research: Second Edition; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 53–69. ISBN 9780128021019. [Google Scholar]
- Caranci, N.; di Girolamo, C.; Bartolini, L.; Fortuna, D.; Berti, E.; Sforza, S.; Rossi, P.G.; Moro, M.L. General and COVID-19-Related Mortality by Pre-Existing Chronic Conditions and Care Setting during 2020 in Emilia-Romagna Region, Italy. Int. J. Environ. Res. Public Health 2021, 18, 3224. [Google Scholar] [CrossRef]
- Alicandro, G.; Remuzzi, G.; Centanni, S.; Gerli, A.; la Vecchia, C. Excess Total Mortality during the COVID-19 Pandemic in Italy: Updated Estimates Indicate Persistent Excess in Recent Months. Med. Lav. 2022, 113, e2022021. [Google Scholar] [CrossRef] [PubMed]
- Scortichini, M.; Schneider Dos Santos, R.; De’ Donato, F.; de Sario, M.; Michelozzi, P.; Davoli, M.; Masselot, P.; Sera, F.; Gasparrini, A. Excess Mortality during the COVID-19 Outbreak in Italy: A Two-Stage Interrupted Time-Series Analysis. Int. J. Epidemiol. 2020, 49, 1909–1917. [Google Scholar] [CrossRef] [PubMed]
- Mahroum, N.; Watad, A.; Rosselli, R.; Brigo, F.; Chiesa, V.; Siri, A.; Ben-Ami Shor, D.; Martini, M.; Bragazzi, N.L.; Adawi, M. An Infodemiological Investigation of the So-Called “Fluad Effect” during the 2014/2015 Influenza Vaccination Campaign in Italy: Ethical and Historical Implications. Hum. Vaccines Immunother. 2018, 14, 712–718. [Google Scholar] [CrossRef]
- Kow, R.Y.; Mohamad Rafiai, N.; Ahmad Alwi, A.A.; Low, C.L.; Ahmad, M.W.; Zakaria, Z.; Zulkifly, A.H. COVID-19 Infodemiology: Association Between Google Search and Vaccination in Malaysian Population. Cureus 2022, 14, e29515. [Google Scholar] [CrossRef]
- Bragazzi, N.L. Infodemiology and Infoveillance of Multiple Sclerosis in Italy. Mult. Scler. Int. 2013, 2013, 924029. [Google Scholar] [CrossRef]
- Ciaffi, J.; Meliconi, R.; Landini, M.P.; Mancarella, L.; Brusi, V.; Faldini, C.; Ursini, F. Seasonality of Back Pain in Italy: An Infodemiology Study. Int. J. Environ. Res. Public Health 2021, 18, 1325. [Google Scholar] [CrossRef]
- Riccò, M.; Baldassarre, A.; Provenzano, S.; Corrado, S.; Cerviere, M.P.; Parisi, S.; Marchesi, F.; Bottazzoli, M. Infodemiology of RSV in Italy (2017–2022): An Alternative Option for the Surveillance of Incident Cases in Pediatric Age? Children 2022, 9, 1984. [Google Scholar] [CrossRef]
- Riccò, M.; Valente, M.; Marchesi, F. Are Symptoms Associated with SARS-CoV-2 Infections Evolving over Time? Infect. Dis. Now 2022, 52, 110–112. [Google Scholar] [CrossRef]
- Cervellin, G.; Comelli, I.; Lippi, G. Is Google Trends a Reliable Tool for Digital Epidemiology? Insights from Different Clinical Settings. J. Epidemiol. Glob. Health 2017, 7, 185–189. [Google Scholar] [CrossRef]
- Rovetta, A. Reliability of Google Trends: Analysis of the Limits and Potential of Web Infoveillance During COVID-19 Pandemic and for Future Research. Front. Res. Metr. Anal. 2021, 6, 670226. [Google Scholar] [CrossRef]



| Variable | Only Participants with Children <14 Years No./558, % | Participants without Children <14 Years No./452, % | Chi Squared Test p Value |
|---|---|---|---|
| Gender | <0.001 | ||
| Male | 122, 21.9% | 146, 32.3% | |
| Female | 436, 78.1% | 306, 67.7% | |
| Age | 0.181 | ||
| <40 years | 295, 52.9% | 258, 57.1% | |
| ≥40 years | 263, 47.1% | 194, 42.9% | |
| Literacy | 0.030 | ||
| Primary School | 20, 3.6% | 15, 3.3% | |
| Secondary School | 174, 31.2% | 177, 39.2% | |
| University or higher | 364, 65.2% | 260, 57.5% | |
| Residency | <0.001 | ||
| Municipality <5000 inhabitants | 64, 11.5% | 86, 19.0% | |
| Municipality 5000–15,000 inhabitants | 161, 28.9% | 74, 16.4% | |
| Municipality >15,000 inhabitants (1) | 176, 31.5% | 148, 32.7% | |
| Main Municipality (2) | 157, 28.1% | 137, 30.3% | |
| n.a. | 0, - | 7, 1.5% | |
| Size of Household | <0.001 | ||
| Single | 0, - | 40, 8.8% | |
| 2 persons | 12, 2.2% | 162, 35.8% | |
| 3 persons | 230, 41.2% | 100, 22.1% | |
| 4 persons | 252, 45.2% | 106, 23.5% | |
| 5 persons or more | 60, 10.8% | 37, 8.2% | |
| n.a. | 4, 0.7% | 7, 1.5% | |
| Household including subjects ≥ 65 y.o. | 74, 13.3% | 94, 20.8% | 0.001 |
| Background in healthcare settings | 149, 26.7% | 161, 35.6% | 0.002 |
| Statement | Correct Answer | No./558, % |
|---|---|---|
| Q01. Meningococcus causes around 40–50% of all bacterial meningitis in children aged 2 years or more, and over 70% in children aged 5 years or more. Overall, it causes half of all bacterial meningitis in all infants. | TRUE | 237, 42.5% |
| Q02. The mortality of meningococcal meningitis is around 30%, irrespective of therapy. | TRUE | 315, 56.5% |
| Q03. Early symptoms of meningococcal meningitis are specific, allowing a prompt and appropriate treatment by healthcare providers. | FALSE | 373, 66.8% |
| Q04. Vaccination against meningococcus reduces spread of bacteria, contributing to herd immunity and ultimately protecting those who cannot be vaccinated. | TRUE | 368, 65.9% |
| Q05. MenB vaccine may be associated with other vaccinations and vaccine formulates, not increasing the number of accesses to vaccination services. | TRUE | 227, 40.7% |
| Q06. Vaccine additives are not harmful to human beings. | TRUE | 271, 48.6% |
| Q07. Vaccines against measles may cause severe damages to the central nervous system. | FALSE | 313, 56.1% |
| Q08. Influenza vaccine may cause severe side effects, potentially lethal. | FALSE | 210, 37.6% |
| Q09. Measles vaccine may elicit autism. | FALSE | 381, 68.3% |
| Q10. Some vaccinations may cause diabetes. | FALSE | 311, 55.7% |
| Q11. Some autoimmune diseases may be elicited by vaccines. | FALSE | 252, 45.2% |
| Q12. Vaccines are useless, as infectious diseases may be always treated with specific therapies. | FALSE | 463, 83.0% |
| Q13. Vaccination shots increase probabilities of allergic reactions. | FALSE | 304, 54.5% |
| Q14. Without vaccinations, smallpox would still exist. | TRUE | 398, 71.3% |
| Q15. The efficacy of vaccines and vaccination programs has been repetitively proved. | TRUE | 379, 67.9% |
| Q16. Children would be more resistant to infectious disease without vaccinations. | FALSE | 349, 62.5% |
| Q17. Many immunizations are performed too early: as a consequence, the immune system of children is not allowed to fully develop by itself. | FALSE | 315, 56.5% |
| Q18. The immune system may be compromised by receiving too many vaccines in pediatric age. | FALSE | 323, 57.9% |
| Variable | Only Participants with Children <14 Years No./558, % |
|---|---|
| RPSinf > median | 210, 37.6% |
| RPSvac > median | 288, 51.6% |
| Perception on Neisseria meningitidis | |
| Severe/Very Severe | 496, 88.9% |
| Frequent/Highly Frequent | 104, 18.6% |
| Perception on Neisseria Vaccines’ side effects | |
| Severe/Very Severe | 159, 28.5% |
| Frequent/Highly Frequent | 147, 26.3% |
| Attitude Towards Vaccines: somehow favorable | |
| Vaccines, in general | 367, 65.8% |
| Meningitis vaccines | 354, 63.4% |
| Previously vaccinated against MenC | 120, 21.5% |
| Previously vaccinated against MenB | 53, 9.5% |
| Offspring vaccinated against MenB | 216, 38.7% |
| Confidence in Vaccination Services (high/very high) | 326, 58.4% |
| Confidence in Vaccines (high/very high) | 329, 59.0% |
| Confidence in MenB (high/very high) | 358, 64.2% |
| Drivers | Child Vaccinated (No./216) |
|---|---|
| It is included in the National Vaccination Plan | 77, 35.6% |
| Suggested by general practitioner/pediatrician | 44, 20.4% |
| Meningitidis is a severe disease | 139, 64.4% |
| In order to be protected against as many infectious diseases as possible | 157, 72.7% |
| In order to be protected against meningitis | 121, 56.0% |
| An acquaintance has been affected by meningitis | 16, 7.4% |
| In order to avoid bacterial meningitis | 152, 70.4% |
| In order to avoid complications of meningitis | 169, 78.2% |
| In order to avoid transmission of meningitis | 116, 53.7% |
| In order to protect subjects who cannot be vaccinated | 134, 62.2% |
| Barriers | Child not Vaccinated (No./325, %) |
| I am against vaccines (in general) | 17, 5.2% |
| Preference in other preventive measures | 25, 7.7% |
| Greater trust in antimicrobial treatment | 18, 5.5% |
| Greater trust in alternative therapies (e.g., homeopathy) | 31, 9.5% |
| Cannot be vaccinated | 4, 1.2% |
| Side effects after previous vaccination | 29, 8.9% |
| Fear of side effects | 117, 36.0% |
| Lack of trust in “experimental” vaccines | 123, 37.8% |
| Fear of neurological disorders induced by vaccines (e.g., Guillaume Barré syndrome, multiple sclerosis) | 120, 36.9% |
| Fear of developing autism after vaccine | 55, 16.9% |
| Fear of side effects elicited by vaccine adjuvants | 99, 30.5% |
| Fear of side effects elicited by heavy metals included in the vaccine | 98, 30.2% |
| Not recommended by general practitioner/pediatrician | 4, 1.2% |
| Aiming to reduce the number of injections | 18, 5.5% |
| Vaccine not affordable | 11, 3.4% |
| Vaccinated Children | Chi Squared Test p Value | ||
|---|---|---|---|
| Yes No./216, % | No No./325, % | ||
| Male gender | 64, 29.6% | 52, 16.0% | <0.001 |
| Age ≥40 years | 94, 43.5% | 156, 48.0% | 0.306 |
| Literacy: University or higher | 149, 69.0% | 206, 63.1% | 0.157 |
| Municipality >15,000 inhabitants | 139, 64.4% | 181, 55.7% | 0.045 |
| Migration Background | 4, 1.9% | 14, 4.3% | 0.119 |
| Household Characteristics | |||
| … including ≥3 people | 208, 96.3% | 317, 97.5% | 0.404 |
| … including subjects ≥65 y.o. | 32, 14.8% | 39, 12.0% | 0.342 |
| Background in Healthcare Settings | 66, 30.6% | 83, 25.5% | 0.201 |
| General Knowledge Score > median | 116, 53.7% | 97, 29.8% | <0.001 |
| Risk Perception Score > median | |||
| … of Natural infection | 91, 42.1% | 116, 35.7% | 0.131 |
| … of Vaccines | 65, 30.1% | 216, 66.5% | <0.001 |
| Attitude Towards Vaccines: somehow favorable | |||
| Vaccines, in general | 192, 88.9% | 166, 51.1% | <0.001 |
| Meningitis vaccines | 193, 89.4% | 156, 48.0% | <0.001 |
| Confidence in Vaccination Services (high/very high) | 176, 81.5% | 144, 44.3% | <0.001 |
| Confidence in Vaccines (high/very high) | 182, 84.3% | 141, 43.4% | <0.001 |
| Confidence in MenB Vaccines (high/very high) | 193, 89.4% | 155, 47.7% | <0.001 |
| Previously vaccinated against MenB | 46, 21.3% | 7, 2.2% | <0.001 |
| Previously vaccinated against MenC | 84, 38.9% | 33, 10.2% | <0.001 |
| Vaccinated children against MenC | 174, 81.7% | 123, 37.8% | <0.001 |
| Variable | aOR | 95% Confidence Interval | |
|---|---|---|---|
| Male gender | 3.184 | 1.772 | 5.721 |
| Municipality >15,000 inhabitants | 1.675 | 1.051 | 2.668 |
| General Knowledge Score > median | 0.644 | 0.365 | 1.135 |
| Risk Perception Score for Vaccines > median | 0.429 | 0.241 | 0.765 |
| Attitude Towards Vaccines: somehow favorable | |||
| Vaccines, in general | 0.585 | 0.140 | 2.441 |
| Meningitis vaccines | 12.472 | 3.030 | 51.338 |
| Confidence in Vaccination Services (high/very high) | 0.932 | 0.386 | 2.254 |
| Confidence in Vaccines (high/very high) | 1.629 | 0.626 | 4.238 |
| Confidence in MenB Vaccines (high/very high) | 0.204 | 0.040 | 1.028 |
| Previously vaccinated against MenB | 5.624 | 1.936 | 16.337 |
| Previously vaccinated against MenC | 2.652 | 1.442 | 4.874 |
| Previously vaccinated children against MenC | 6.585 | 3.648 | 11.888 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riccò, M.; Cerviere, M.P.; Marchesi, F.; Bottazzoli, M. Invasive Meningococcal Disease and Meningococcal Serogroup B Vaccination in Adults and Their Offspring: Knowledge, Attitudes, and Practices in Italy (2019). Vaccines 2023, 11, 508. https://doi.org/10.3390/vaccines11030508
Riccò M, Cerviere MP, Marchesi F, Bottazzoli M. Invasive Meningococcal Disease and Meningococcal Serogroup B Vaccination in Adults and Their Offspring: Knowledge, Attitudes, and Practices in Italy (2019). Vaccines. 2023; 11(3):508. https://doi.org/10.3390/vaccines11030508
Chicago/Turabian StyleRiccò, Matteo, Milena Pia Cerviere, Federico Marchesi, and Marco Bottazzoli. 2023. "Invasive Meningococcal Disease and Meningococcal Serogroup B Vaccination in Adults and Their Offspring: Knowledge, Attitudes, and Practices in Italy (2019)" Vaccines 11, no. 3: 508. https://doi.org/10.3390/vaccines11030508
APA StyleRiccò, M., Cerviere, M. P., Marchesi, F., & Bottazzoli, M. (2023). Invasive Meningococcal Disease and Meningococcal Serogroup B Vaccination in Adults and Their Offspring: Knowledge, Attitudes, and Practices in Italy (2019). Vaccines, 11(3), 508. https://doi.org/10.3390/vaccines11030508







