Impact of MERS-CoV and SARS-CoV-2 Viral Infection on Immunoglobulin-IgG Cross-Reactivity
Abstract
:1. Introduction
2. Subjects and Methods
2.1. Study Design and Settings
2.2. Study Participants, Inclusion, and Exclusion Criteria
2.3. Detection of MERS-CoV-IgG Antibodies Using Enzyme-Linked Immunosorbent Assay
2.4. Detection of SARS-CoV-2-IgG Antibodies Using Enzyme-Linked Immunosorbent Assay
2.5. Statistical Analysis
3. Results
3.1. Demographics and Distribution
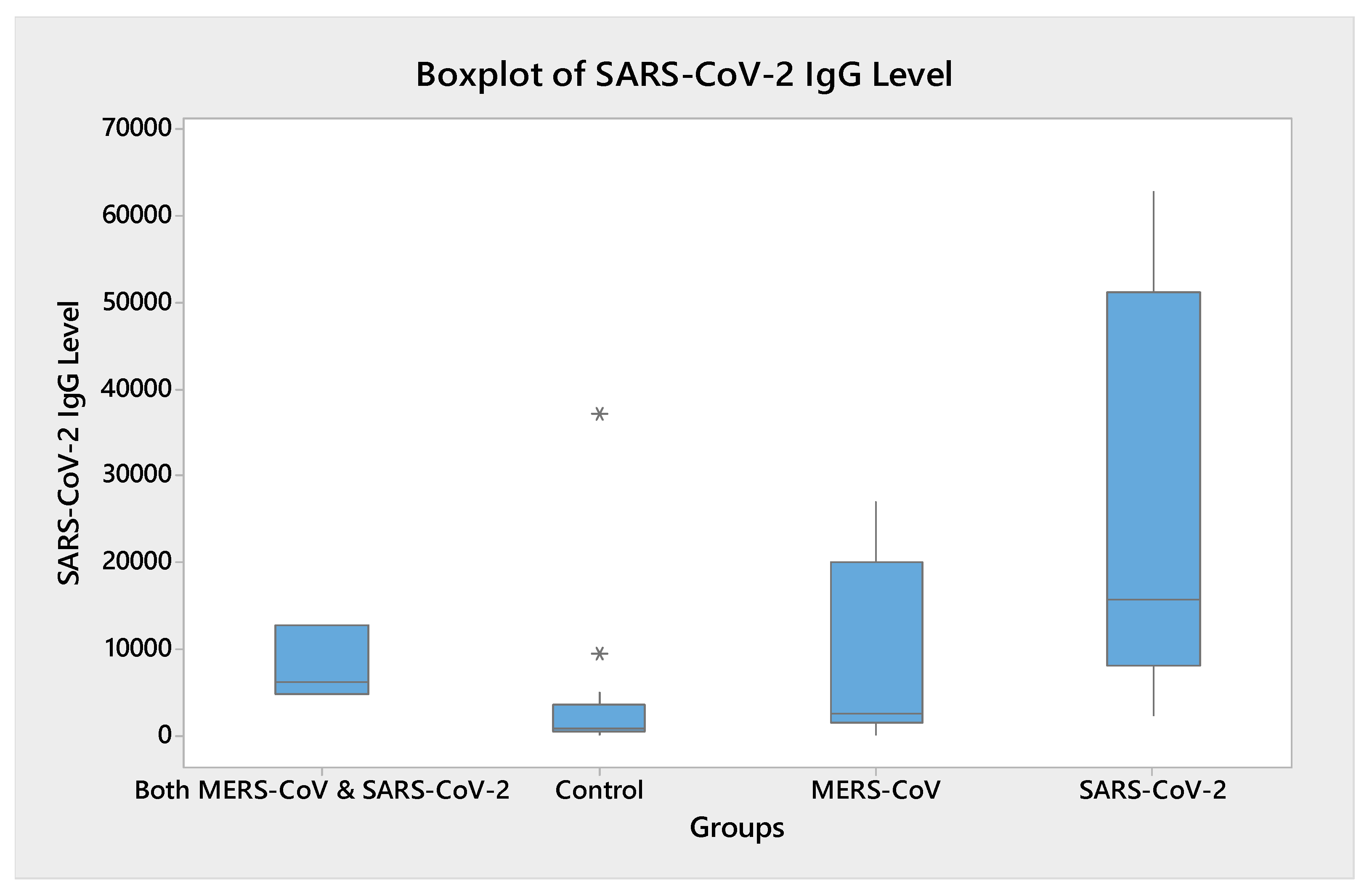
3.2. Humoral Immune Response to SARS-CoV-2
3.3. Humoral Immune Response MERS-CoV
3.4. Cross-Immunity between SARS-CoV-2 and MERS-CoV
4. Discussion
Study Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meo, S.A.; Al-Khlaiwi, T.; Usmani, A.M.; Meo, A.S.; Klonoff, D.C.; Hoang, T.D. Biological and epidemiological trends in the prevalence and mortality due to outbreaks of novel coronavirus COVID-19. J. King Saud Univ. Sci. 2020, 32, 2495–2499. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.H.; Cheng, V.C.; Woo, P.C.; Lau, S.K.; Poon, L.L.; Guan, Y.; Seto, W.H.; Yuen, K.Y.; Peiris, J.S. Serological responses in patients with severe acute respiratory syndrome coronavirus infection and cross-reactivity with human coronaviruses 229E, OC43, and NL6. Clin. Vaccine Immunol. 2005, 12, 1317–1321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gill, E.P.; Dominguez, E.A.; Greenberg, S.B.; Atmar, R.L.; Hogue, B.G.; Baxter, B.D.; Couch, R.B. Development and application of an enzyme immunoassay for coronavirus OC43 antibody in acute respiratory illness. J. Clin. Microbiol. 1994, 32, 2372–2376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, C.M.; Tse, H.; Wong, S.S.; Woo, P.C.; Lau, S.K.; Chen, L.; Zheng, B.J.; Huang, J.D.; Yuen, K.Y. Examination of seroprevalence of coronavirus HKU1 infection with S protein-based ELISA and neutralization assay against viral spike pseudotyped virus. J. Clin. Virol. 2009, 45, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Dijkman, R.; Jebbink, M.F.; El Idrissi, N.B.; Pyrc, K.; Müller, M.A.; Kuijpers, T.W.; Zaaijer, H.L.; Van Der Hoek, L. Human coronavirus NL63 and 229E seroconversion in children. J. Clin. Microbiol. 2008, 46, 2368–2373. [Google Scholar] [CrossRef] [Green Version]
- Shirato, K.; Kawase, M.; Watanabe, O.; Hirokawa, C.; Matsuyama, S.; Nishimura, H.; Taguchi, F. Differences in neutralizing antigenicity between laboratory and clinical isolates of HCoV-229E isolated in Japan in 2004–2008 depending on the S1 region sequence of the spike protein. J. Gen. Virol. 2012, 93, 1908–1917. [Google Scholar] [CrossRef]
- Gorse, G.J.; Donovan, M.M.; Patel, G.B.; Balasubramanian, S.; Lusk, R.H. Coronavirus and other respiratory illnesses comparing older with young adults. Am. J. Med. 2015, 128, 1251.e11–1251.e20. [Google Scholar] [CrossRef] [Green Version]
- Gorse, G.J.; O’Connor, T.Z.; Hall, S.L.; Vitale, J.N.; Nichol, K.L. Human coronavirus and acute respiratory illness in older adults with chronic obstructive pulmonary disease. J. Infect. Dis. 2009, 199, 847–857. [Google Scholar] [CrossRef]
- Walsh, E.E.; Shin, J.H.; Falsey, A.R. Clinical Impact of Human Coronaviruses 229E and OC43 Infection in Diverse Adult Populations. J. Infect. Dis. 2013, 208, 1634–1642. [Google Scholar] [CrossRef] [Green Version]
- WHO Coronavirus (COVID-19) Dashboard [Internet]. World Health Organization. Available online: https://covid19.who.int/ (accessed on 27 January 2023).
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef]
- Wu, F.; Zhao, S.; Yu, B.; Chen, Y.M.; Wang, W.; Song, Z.G.; Hu, Y.; Tao, Z.W.; Tian, J.H.; Pei, Y.Y.; et al. A new coronavirus associated with human respiratory disease in China. Nature 2020, 579, 265–269. [Google Scholar] [CrossRef] [Green Version]
- Zaki, A.M.; Van Boheemen, S.; Bestebroer, T.M.; Osterhaus, A.D.; Fouchier, R.A. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 2012, 367, 1814–1820. [Google Scholar] [CrossRef]
- Al-Tawfiq, J.A.; Auwaerter, P.G. Healthcare-associated infections: The hallmark of Middle East respiratory syndrome coronavirus with review of the literature. J. Hosp. Infect. 2019, 101, 20–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Centre for Disease Prevention and Control. MERS-CoV Worldwide Overview. Available online: https://www.ecdc.europa.eu/en/middle-east-respiratory-syndrome-coronavirus-mers-cov-situation-update (accessed on 23 January 2023).
- Assiri, A. Hospital Outbreak of Middle East Respiratory Syndrome Coronavirus. N. Engl. J. Med. 2013, 369, 407–416. [Google Scholar] [CrossRef]
- Thompson, R.N.; Southall, E.; Daon, Y.; Lovell-Read, F.A.; Iwami, S.; Thompson, C.P.; Obolski, U. The impact of cross-reactive immunity on the emergence of SARS-CoV-2 variants. Front. Immunol. 2023, 13, 1049458. [Google Scholar] [CrossRef] [PubMed]
- Yaqinuddin, A. Cross-immunity between respiratory coronaviruses may limit COVID-19 fatalities. Med. Hypotheses 2020, 144, 110049. [Google Scholar] [CrossRef] [PubMed]
- Al Maani, A.; Al-Jardani, A.; Karrar, H.; Petersen, E.; Al Abri, S. COVID-19 in a case previously infected with MERS-CoV: No cross-immunity. J. Infect. 2021, 82, e28–e29. [Google Scholar] [CrossRef]
- English, E.; Cook, L.E.; Piec, I.; Dervisevic, S.; Fraser, W.D.; John, W.G. Performance of the Abbott SARS-CoV-2 IgG II Quantitative Antibody Assay Including the New Variants of Concern, VOC 202012/V1 (United Kingdom) and VOC 202012/V2 (South Africa), and First Steps towards Global Harmonization of COVID-19 Antibody Methods. J. Clin. Microbiol. 2021, 59, e0028821. [Google Scholar] [CrossRef]
- Chowdhury, M.A.; Hossain, N.; Kashem, M.A.; Shahid, M.A.; Alam, A. Immune Response in COVID-19: A Review. J. Infect. Public Health 2020, 13, 1619–1629. [Google Scholar] [CrossRef]
- Azhar, E.I.; Lanini, S.; Ippolito, G.; Zumla, A. The Middle East respiratory syndrome coronavirus–a continuing risk to global health security. In Emerging and Re-Emerging Viral Infections; Springer: Cham, Switzerland, 2016; pp. 49–60. [Google Scholar]
- Zhao, X.; Sehgal, M.; Hou, Z.; Cheng, J.; Shu, S.; Wu, S.; Guo, F.; Le Marchand, S.J.; Lin, H.; Chang, J.; et al. Identification of residues controlling restriction versus enhancing activities of IFITM proteins on entry of human coronaviruses. J. Virol. 2018, 92, e01535-17. [Google Scholar] [CrossRef] [Green Version]
- Yaqinuddin, A.; Kashir, J. Innate immunity in COVID-19 patients mediated by NKG2A receptors, and potential treatment using Monalizumab, Cholroquine, and antiviral agents. Med. Hypotheses 2020, 140, 109777. [Google Scholar] [CrossRef]
- Khan, A.A.; Alahmari, A.A.; Almuzaini, Y.; Alamri, F.; Alsofayan, Y.M.; Aburas, A.; Al-Muhsen, S.; Van Kerkhove, M.; Yezli, S.; Ciottone, G.R.; et al. Potential Cross-Reactive Immunity to COVID-19 Infection in Individuals With Laboratory-Confirmed MERS-CoV Infection: A National Retrospective Cohort Study From Saudi Arabia. Front Immunol. 2021, 12, 727989. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Aigerim, A.; Park, U.; Kim, Y.; Park, H.; Rhee, J.-Y.; Choi, J.-P.; Park, W.B.; Park, S.W.; Kim, Y.; et al. Sustained Responses of Neutralizing Antibodies Against MERS-CoV in Recovered Patients and Their Therapeutic Applicability. Clin. Infect. Dis. 2020, 27, 1472–1476. [Google Scholar] [CrossRef]
- Gorse, G.J.; Donovan, M.M.; Patel, G.B. Antibodies to coronaviruses are higher in older compared with younger adults and binding antibodies are more sensitive than neutralizing antibodies in identifying coronavirus-associated illnesses. J. Med. Virol. 2020, 92, 512–517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.; Fontanet, A.; Zhang, P.H.; Zhan, L.; Xin, Z.T.; Baril, L.; Tang, F.; Lv, H.; Cao, W.C. Two-year prospective study of the humoral immune response of patients with severe acute respiratory syndrome. J. Infect. Dis. 2006, 193, 792–795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Yi, Y.; Luo, X.; Xiong, N.; Liu, Y.; Li, S.; Sun, R.; Wang, Y.; Hu, B.; Chen, W.; et al. Development, and clinical application of a rapid IgM-IgG combined antibody test for SARS-CoV-2 infection diagnosis. J. Med. Virol. 2020, 92, 1518–1524. [Google Scholar] [CrossRef] [PubMed]
- Mveang Nzoghe, A.; Essone, P.N.; Leboueny, M.; Maloupazoa Siawaya, A.C.; Bongho, E.C.; Mvoundza Ndjindji, O.; Avome Houechenou, R.M.; Agnandji, S.T.; Djoba Siawaya, J.F. Evidence and implications of pre-existing humoral cross-reactive immunity to SARS-CoV-2. Immun. Inflamm. Dis. 2021, 9, 128–133. [Google Scholar] [CrossRef]
- Zhao, J.; Zhao, J.; Mangalam, A.K.; Channappanavar, R.; Fett, C.; Meyerholz, D.K.; Agnihothram, S.; Baric, R.S.; David, C.S.; Perlman, S. Airway memory CD4(+) T cells mediate protective immunity against emerging respiratory coronaviruses. Immunity 2016, 44, 1379–1391. [Google Scholar] [CrossRef] [Green Version]
- Mbow, M.; Lell, B.; Jochems, S.P.; Cisse, B.; Mboup, S.; Dewals, B.G.; Jaye, A.; Dieye, A.; Yazdanbakhsh, M. COVID-19 in Africa: Dampening the storm? Science 2020, 369, 624–626. [Google Scholar] [CrossRef]
- Borrega, R.; Nelson, D.K.S.; Koval, A.P.; Bond, N.G.; Heinrich, M.L.; Rowland, M.M.; Lathigra, R.; Bush, D.J.; Aimukanova, I.; Phinney, W.N.; et al. Cross-Reactive Antibodies to SARS-CoV-2 and MERS-CoV in Pre-COVID-19 Blood Samples from Sierra Leoneans. Viruses 2021, 13, 2325. [Google Scholar] [CrossRef]
- Anderson, E.M.; Goodwin, E.C.; Verma, A.; Arevalo, C.P.; Bolton, M.J.; Weirick, M.E.; Gouma, S.; McAllister, C.M.; Christensen, S.R.; Weaver, J.; et al. Seasonal human coronavirus antibodies are boosted upon SARS-CoV-2 infection but not associated with protection. Cell 2021, 184, 1858–1864.e10. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.W.; Faulkner, N.; Cornish, G.H.; Rosa, A.; Harvey, R.; Hussain, S.; Ulferts, R.; Earl, C.; Wrobel, A.G.; Benton, D.J.; et al. Preexisting and de novo humoral immunity to SARS-CoV-2 in humans. Science 2020, 370, 1339–1343. [Google Scholar] [CrossRef] [PubMed]
- Tso, F.Y.; Lidenge, S.J.; Peña, P.B.; Clegg, A.A.; Ngowi, J.R.; Mwaiselage, J.; Ngalamika, O.; Julius, P.; West, J.T.; Wood, C. High prevalence of pre-existing serological cross-reactivity against severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) in sub-Saharan Africa. Int. J. Infect. Dis. 2021, 102, 577–583. [Google Scholar] [CrossRef]
- Murray, S.M.; Ansari, A.M.; Frater, J.; Klenerman, P.; Dunachie, S.; Barnes, E.; Ogbe, A. The impact of pre-existing cross-reactive immunity on SARS-CoV-2 infection and vaccine responses. Nat. Rev. Immunol. 2022, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Al-Salihi, K.A.; Khalaf, J.M. The emerging SARS-CoV, MERS-CoV, and SARS-CoV-2: An insight into the virus’ zoonotic aspects. Vet. World 2021, 14, 190–199. [Google Scholar] [CrossRef]
- Hajjar, S.A.; Memish, Z.A.; McIntosh, K. Middle East Respiratory Syndrome Coronavirus (MERS-CoV): A perpetual challenge. Ann. Saudi. Med. 2013, 33, 427–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Demographic Characteristics | Number (Percentage %) |
|---|---|
| Sex | |
| Male | 22 (64.70%) |
| Female | 12 (35.29%) |
| Age | |
| 18–24 | 4 (11.76%) |
| 25–34 | 11 (32.35%) |
| 35–44 | 10 (29.41%) |
| 45–54 | 4 (11.76%) |
| 55–64 | 3 (8.82%) |
| 65–74 | 2 (5.88%) |
| Educational Level | |
| College degree or higher level | 22 (64.70%) |
| High school | 8 (23.52%) |
| Middle school | 2 (5.88%) |
| Primary school | 2 (5.88%) |
| Residence Area | |
| Riyadh | 29 (85.29%) |
| Al Qassim | 5 (14.70%) |
| Occupation | |
| Healthcare worker | 13 (41.17%) |
| Non-healthcare worker | 21 (61.76%) |
| Comorbidities (diabetes mellitus, hypertension, and/or dyslipidemia) | |
| Yes | 12 (35.29%) |
| No | 22 (64.70%) |
| Vaccine Doses | |
| Vaccine Dose-1 | |
| Pfizer | 26 (76.47%) |
| AstraZeneca | 8 (23.52%) |
| Vaccine dose 2 | |
| Pfizer | 26 (76.47%) |
| AstraZeneca | 7 (20.58%) |
| Vaccine Dose 3 | |
| Pfizer | 7 (20.58%) |
| AstraZeneca | 1 (2.94%) |
| Infection history | |
| Control with no previous infection with SARS-CoV-2 or MERS-CoV (C-Gp) | 13 (38.23%) |
| Infection with MERS-CoV only (MV-Gp) | 8 (23.52%) |
| Infection with SARS-CoV-2 only (SV-Gp) | 8 (23.52%) |
| Infection with both SARS-CoV-2 and MERS-CoV (SV-MV-Gp) | 5 (14.70%) |
| Groups | Number of Days (ds) Since Last Vaccination and Collection of Samples | Number of SARS-CoV-2 Vaccines | SARS-CoV2 IgG Levels (AU/mL) | Mean (AU/mL) | Date of MERS-CoV/SARS-CoV2 Infection Where Applicable | MERS-CoV IgG Levels. (Ratio) | |
|---|---|---|---|---|---|---|---|
| Control (No previous infection with SARS-CoV-2 or MERS-CoV (C-Gp)) | 4679 | ||||||
| No. 1 | (Third dose) 184 ds | 3 | 930.1 | N/A | 0.0224 | ||
| No. 2 | (Second dose) 658 ds | 2 | 9460.6 | N/A | 0.0176 | ||
| No. 3 | (Second dose) 143 ds | 2 | 1107.2 | N/A | 0.04 | ||
| No. 4 | (Second dose) 84 ds | 2 | 364.8 | N/A | 0.3424 | ||
| No. 5 | (Second dose) 157 ds | 2 | 860.9 | N/A | 0.0352 | ||
| No. 6 | (Second dose) 156 ds | 2 | 5078.1 | N/A | 0.0224 | ||
| No. 7 | (Second dose) 93 ds | 2 | 635.6 | N/A | 0.024 | ||
| No. 8 | (Second dose) 141 ds | 2 | 2064.6 | N/A | 0.0176 | ||
| No. 9 | (Second dose) 63 ds | 2 | 37,272.1 | N/A | 0.0224 | ||
| No. 10 | (Second dose) 157 ds | 2 | 269.1 | N/A | 0.0256 | ||
| No. 11 | (Second dose) 157 ds | 2 | 1626.4 | N/A | 0.0432 | ||
| No. 12 | (Second dose) 188 ds | 2 | 639.1 | N/A | 0.0192 | ||
| No. 13 | (Second dose) 288 ds | 2 | 518.3 | N/A | 0.0176 | ||
| Infection with SARS-CoV-2 only (SV-Gdsp) | 27,854 | ||||||
| No. 1 | (Third dose) 150 ds | 3 | 10,621.3 | 6/10/2020 | 0.0368 | ||
| No. 2 | (Second dose) 100 ds | 2 | 7369.8 | 9/2/2020 | 0.032 | ||
| No. 3 | (Second dose) 112 ds | 2 | 19,165.2 | 6/30/2021 | 0.0464 | ||
| No. 4 | (Second dose) 123 ds | 2 | 2380.2 | 5/14/2020 | 0.032 | ||
| No. 5 | (Third dose) 41 ds | 3 | 62,790.1 | 9/23/2021 | 0.0448 | ||
| No. 6 | (Second dose) 65 ds | 2 | 12,164.3 | 7/15/2020 | 0.0336 | ||
| No. 7 | (Third dose) 53 ds | 3 | 56,728.8 | 7/14/2020 | 0.0448 | ||
| No. 8 | (First dose) 132 ds | 1 | 34,380.4 | 9/2/2020 | 0.024 | ||
| Infection with MERS-CoV only (MV-Gp) | 10,292 | ||||||
| No. 1 | (Second dose) 90 ds | 2 | 2807.1 | 1/17/2016 | 0.0336 | ||
| No. 2 | (Second dose) 256 ds | 2 | 1503.1 | 11/20/2018 | 0.9504 (Borderline) | ||
| No. 3 | (Second dose) 23 ds | 2 | 26,955.9 | 9/20/2014 | 0.0288 | ||
| No. 4 | (Third dose) 55 ds | 3 | 19,770.4 | 4/29/2014 | 0.2368 | ||
| No. 5 | (Second dose) 159 ds | 2 | 1589 | 8/15/2015 | 0.3264 | ||
| No. 6 | (Second dose) 523 ds | 3 | 2335.7 | 12/2/2015 | 0.5872 | ||
| No. 7 | (Third dose) 52 ds | 2 | 21,158.1 | 9/25/2015 | 0.84 (Borderline) | ||
| No. 8 | (Second dose) 99 ds | 2 | 6219.3 | 3/4/2016 | 1.4768 (Pos) | ||
| Both SARS-CoV-2 and MERS-CoV (SV-MV-Gp) | 7986 | SARS-CoV2 | MERS-CoV | ||||
| No. 1 | (Second dose) 44 ds | 2 | 6272.4 | 7/13/2021 | 6/20/2014 | 0.3088 | |
| No. 2 | (Third dose) 1 ds | 3 | 12,817.2 | 4/202021 | 5/20/2014 | 0.7136 (Borderline) | |
| No. 3 | (Second dose) 313 ds | 2 | 4869.6 | 6/1/2021 | 6/7/2017 | 0.8688 (Borderline) | |
| No. 4 | (Second dose) 153 ds | 2 | 1812.7 | 12/30/2021 | 2/20/2015 | 0.1616 | |
| No. 5 | (Second dose) 153 ds | 2 | 139.3 | 12/30/2021 | 2/20/2015 | 0.4400 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
AlKhalifah, J.M.; Seddiq, W.; Alshehri, M.A.; Alhetheel, A.; Albarrag, A.; Meo, S.A.; Al-Tawfiq, J.A.; Barry, M. Impact of MERS-CoV and SARS-CoV-2 Viral Infection on Immunoglobulin-IgG Cross-Reactivity. Vaccines 2023, 11, 552. https://doi.org/10.3390/vaccines11030552
AlKhalifah JM, Seddiq W, Alshehri MA, Alhetheel A, Albarrag A, Meo SA, Al-Tawfiq JA, Barry M. Impact of MERS-CoV and SARS-CoV-2 Viral Infection on Immunoglobulin-IgG Cross-Reactivity. Vaccines. 2023; 11(3):552. https://doi.org/10.3390/vaccines11030552
Chicago/Turabian StyleAlKhalifah, Joud Mohammed, Waleed Seddiq, Mohammed Abdullah Alshehri, Abdulkarim Alhetheel, Ahmed Albarrag, Sultan Ayoub Meo, Jaffar A. Al-Tawfiq, and Mazin Barry. 2023. "Impact of MERS-CoV and SARS-CoV-2 Viral Infection on Immunoglobulin-IgG Cross-Reactivity" Vaccines 11, no. 3: 552. https://doi.org/10.3390/vaccines11030552







