Heart Rate Variability in Subjects with Severe Allergic Background Undergoing COVID-19 Vaccination
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
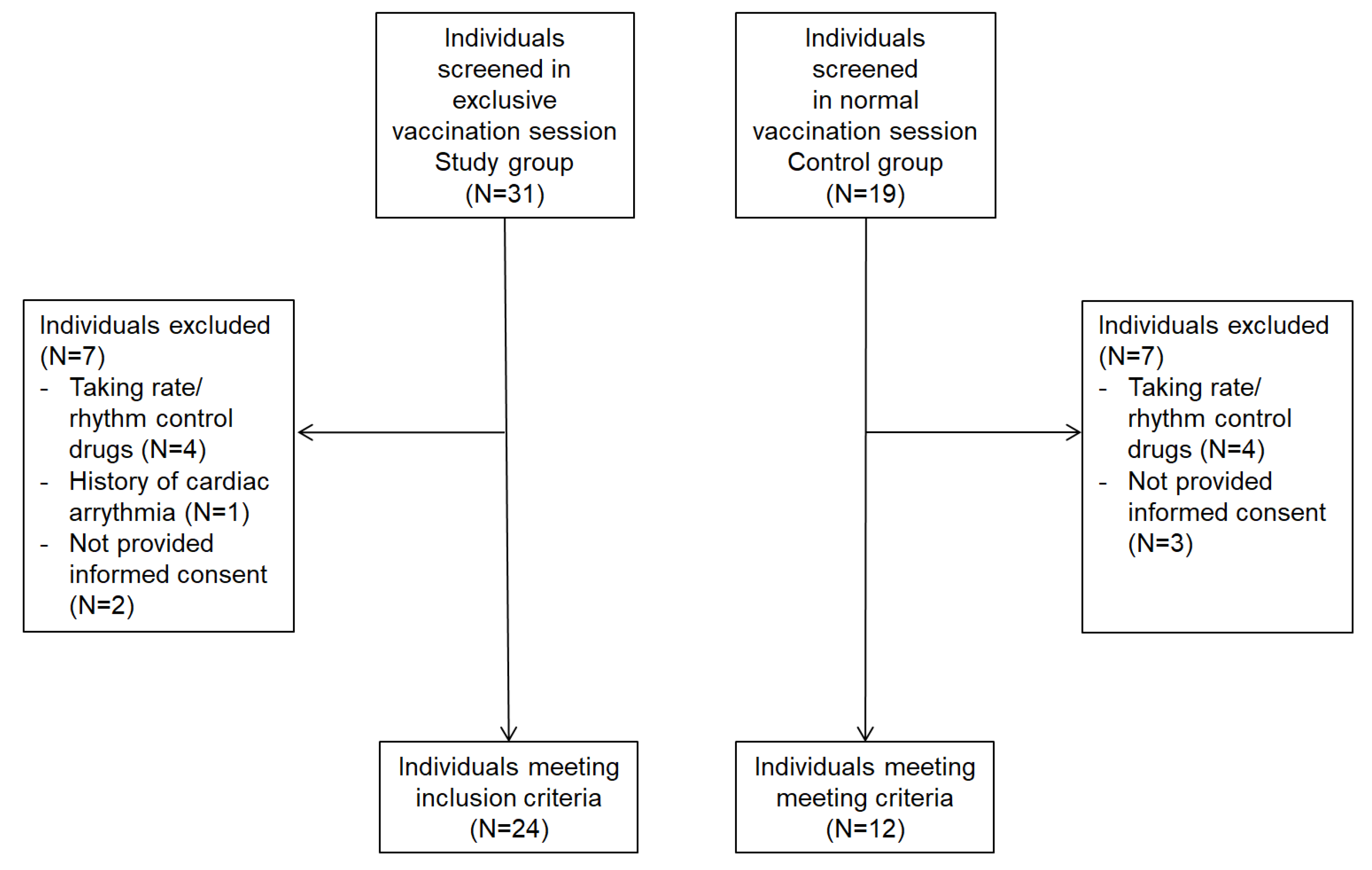
2.2. Enrollment
2.3. Sample Size
2.4. Outcome Measurement
2.5. Statistical Analysis
3. Results
3.1. Study Population
3.2. Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stasi, C.; Fallani, S.; Voller, F.; Silvestri, C. Treatment for COVID-19: An overview. Eur. J. Pharmacol. 2020, 889, 173644. [Google Scholar] [CrossRef] [PubMed]
- Klompas, M. Understanding Breakthrough Infections Following mRNA SARS-CoV-2 Vaccination. JAMA 2021, 326, 2018–2020. [Google Scholar] [CrossRef]
- Rutten, L.J.F.; Zhu, X.; Leppin, A.L.; Ridgeway, J.L.; Swift, M.D.; Griffin, J.M.; Sauver, J.L.S.; Virk, A.; Jacobson, R.M. Evidence-Based Strategies for Clinical Organizations to Address COVID-19 Vaccine Hesitancy. Mayo Clin. Proc. 2021, 96, 699–707. [Google Scholar] [CrossRef]
- Ahamad, M.M.; Aktar, S.; Uddin, M.J.; Rashed-Al-Mahfuz, M.; Azad, A.K.M.; Uddin, S.; Alyami, S.A.; Sarker, I.H.; Khan, A.; Liò, P.; et al. Adverse effects of COVID-19 vaccination: Machine learning and statistical approach to identify and classify incidences of morbidity and post-vaccination reactogenicity. Healthcare 2021, 11, 31. [Google Scholar] [CrossRef]
- Sampath, V.; Rabinowitz, G.; Shah, M.; Jain, S.; Diamant, Z.; Jesenak, M.; Rabin, R.; Vieths, S.; Agache, I.; Akdis, M.; et al. Vaccines and allergic reactions: The past, the current COVID-19 pandemic, and future perspectives. Allergy 2021, 76, 1640–1660. [Google Scholar] [CrossRef]
- Dispenza, M.C. Classification of hypersensitivity reactions. Allergy Asthma Proc. 2019, 40, 470–473. [Google Scholar] [CrossRef] [PubMed]
- Banerji, A.; Wickner, P.G.; Saff, R.; Stone, C.A., Jr.; Robinson, L.B.; Long, A.A.; Wolfson, A.R.; Williams, P.; Khan, D.A.; Phillips, E.; et al. mRNA Vaccines to Prevent COVID-19 Disease and Reported Allergic Reactions: Current Evidence and Suggested Approach. J. Allergy Clin. Immunol. Pract. 2021, 9, 1423–1437. [Google Scholar] [CrossRef]
- Sternberg, E. Eurosterone meeting. Neuroendocrine regulation of autoimmune/inflammatory disease. J. Endocrinol. 2001, 169, 429–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirotti, L.; Castro, J.; Costa-Pinto, F.A.; Russo, M. Neural Pathways in Allergic Inflammation. J. Allergy 2010, 2010, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zilberstein, J.; McCurdy, M.T.; Winters, M.E. Anaphylaxis. J. Emerg. Med. 2014, 47, 182–187. [Google Scholar] [CrossRef]
- Iweala, O.I.; Burks, A.W. Food Allergy: Our Evolving Understanding of Its Pathogenesis, Prevention, and Treatment. Curr. Allergy Asthma Rep. 2016, 16, 37. [Google Scholar] [CrossRef] [PubMed]
- Werfel, T.; Kapp, A. 13—Atopic Dermatitis and Allergic Contact Dermatitis, 4th ed.; Church, M.K., Broide, D.H., Mar-tinez, F.D., Eds.; W.B. Saunders: Edinburgh, UK, 2012; p. 263. [Google Scholar]
- Greiner, A.N.; Hellings, P.W.; Rotiroti, G.; Scadding, G.K. Allergic rhinitis. Lancet 2011, 378, 2112–2122. [Google Scholar] [CrossRef]
- Pavlov, V.A.; Chavan, S.S.; Tracey, K.J. Molecular and Functional Neuroscience in Immunity. Annu. Rev. Immunol. 2018, 36, 783–812. [Google Scholar] [CrossRef]
- Voisin, T.; Bouvier, A.; Chiu, I.M. Neuro-immune interactions in allergic diseases: Novel targets for therapeutics. Int. Immunol. 2017, 29, 247–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malik, M. Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation 1996, 93, 1043–1065. [Google Scholar] [CrossRef]
- Sztajzel, J. Heart rate variability: A noninvasive electrocardiographic method to measure the autonomic nervous system. Swiss Med. Wkly. 2004, 134, 514–522. [Google Scholar] [PubMed]
- Thayer, J.F.; Yamamoto, S.S.; Brosschot, J.F. The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int. J. Cardiol. 2010, 141, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Prinsloo, G.E.; Rauch, H.L.; Derman, W.E. A Brief Review and Clinical Application of Heart Rate Variability Biofeedback in Sports, Exercise, and Rehabilitation Medicine. Physician Sportsmed. 2014, 42, 88–99. [Google Scholar] [CrossRef]
- Granero-Gallegos, A.; González-Quílez, A.; Plews, D.; Carrasco-Poyatos, M. HRV-Based Training for Improving VO2max in Endurance Athletes. A Systematic Review with Meta-Analysis. Int. J. Environ. Res. Public Health 2020, 17, 7999. [Google Scholar] [CrossRef]
- Kautzner, J.; Camm, A.J. Clinical relevance of heart rate variability. Clin. Cardiol. 1997, 20, 162–168. [Google Scholar] [CrossRef]
- Matusik, P.S.; Stein, P.K. Heart rate variability in patients with systemic lupus erythematosus: A systematic review and methodological considerations. Lupus 2018, 27, 1225–1239. [Google Scholar] [CrossRef]
- Boettger, M.; Bär, K.-J.; Dohrmann, A.; Müller, H.; Mertins, L.; Brockmeyer, N.H.; Agelink, M.W. Increased vagal modulation in atopic dermatitis. J. Dermatol. Sci. 2009, 53, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.P.; Koenig, J.; Carnevali, L.; Sgoifo, A.; Jarczok, M.N.; Sternberg, E.M.; Thayer, J.F. Heart rate variability and inflammation: A meta-analysis of human studies. Brain Behav. Immun. 2019, 80, 219–226. [Google Scholar] [CrossRef]
- Schönauer, M.; Thomas, A.; Morbach, S.; Niebauer, J.; Schönauer, U.; Thiele, H. Cardiac autonomic diabetic neuropathy. Diabetes Vasc. Dis. Res. 2008, 5, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.; Baek, H.; Jeong, C.; Yeo, M.; Lee, S.-H.; Cho, J.H.; Baek, K.-H.; Kang, M.I.; Lim, D.-J. Heart Rate Variability in Postoperative Patients with Nonfunctioning Pituitary Adenoma. Endocrinol. Metab. 2021, 36, 678–687. [Google Scholar] [CrossRef]
- Moon, E.; Lee, S.H.; Kim, D.H.; Hwang, B. Comparative study of heart rate variability in patients with schizophrenia, bipolar disorder, post-traumatic stress disorder, or major depressive disorder. Clin. Psychopharmacol. Neurosci. 2013, 11, 137. [Google Scholar] [CrossRef] [Green Version]
- Gandhi, R.A.; Marques, J.L.; Selvarajah, D.; Emery, C.J.; Tesfaye, S. Painful Diabetic Neuropathy Is Associated with Greater Autonomic Dysfunction Than Painless Diabetic Neuropathy. Diabetes Care 2010, 33, 1585–1590. [Google Scholar] [CrossRef] [Green Version]
- Tarvainen, M.P.; Niskanen, J.-P.; Lipponen, J.A.; Ranta-Aho, P.O.; Karjalainen, P.A. Kubios HRV–Heart rate variability analysis software. Comput. Methods Progr. Biomed. 2014, 113, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Cuzick, J. A wilcoxon-type test for trend. Stat. Med. 1985, 4, 87–90. [Google Scholar] [CrossRef]
- Cohen, I.; Huang, Y.; Chen, J.; Benesty, J. Pearson Correlation Coefficient. In Noise Reduction in Speech Processing; Springer: Berlin/Heidelberg, Germany, 2009; pp. 1–4. [Google Scholar]
- Tennant, P.W.; Tomova, G.D.; Arnold, K.F.; Gilthorpe, M.S. OP09 Lord’s ‘Paradox’ Explained: The 50-Year Warning on the Analyses of ‘Change Scores’. 2022, A5. Available online: https://arxiv.org/ftp/arxiv/papers/2302/2302.01822.pdf (accessed on 9 January 2023).
- Alhumaid, S.; Al Mutair, A.; Al Alawi, Z.; Rabaan, A.A.; Tirupathi, R.; Alomari, M.A.; Alshakhes, A.S.; Alshawi, A.M.; Ahmed, G.Y.; Almusabeh, H.M.; et al. Anaphylactic and nonanaphylactic reactions to SARS-CoV-2 vaccines: A systematic review and me-ta-analysis. Allergy Asthma Clin. Immunol. 2021, 17, 1–24. [Google Scholar] [CrossRef]
- Asperti, C.; Benanti, G.; Ramirez, G.A.; Russo, M.; Vai, B.; Bramé, B.; Viapiana, N.; Nannipieri, S.; Cilona, M.B.; Mazzetti, M.; et al. Interactions between Severe Allergy and Anxiety in Anti-SARS-CoV-2 Vaccinees. Vaccines 2022, 10, 2047. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, J.A.; Quintana, D.S.; Abbott, M.J.-A.; Kemp, A.H. Anxiety Disorders are Associated with Reduced Heart Rate Variability: A Meta-Analysis. Front. Psychiatry 2014, 5, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yokusoglu, M.; Ozturk, S.; Uzun, M.; Baysan, O.; Demirkol, S.; Caliskaner, Z.; Dundaroz, R.; Sag, C.; Karaayvaz, M.; Isik, E. Heart Rate Variability in Patients with Allergic Rhinitis. Mil. Med. 2007, 172, 98–101. [Google Scholar] [CrossRef] [Green Version]
- Lemanske, R.F., Jr.; Kaliner, M.A. Autonomic nervous system abnormalities and asthma. Am. Rev. Respir. Dis. 1990, 141 Pt 2, S157–S161. [Google Scholar] [CrossRef]
- Garcia-Araújo, A.; Di Lorenzo, V.A.P.; Labadessa, I.; Jürgensen, S.P.; Di Thommazo-Luporini, L.; Garbim, C.L.; Borghi-Silva, A. Increased sympathetic modulation and decreased response of the heart rate variability in controlled asthma. J. Asthma 2014, 52, 246–253. [Google Scholar] [CrossRef]
- Lutfi, M.F. Patterns of heart rate variability and cardiac autonomic modulations in controlled and uncontrolled asthmatic patients. BMC Pulm. Med. 2015, 15, 119. [Google Scholar] [CrossRef] [Green Version]
- Lewis, M.; Short, A.; Lewis, K. Autonomic nervous system control of the cardiovascular and respiratory systems in asthma. Respir. Med. 2006, 100, 1688–1705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marshall, G.D. Neuroendocrine mechanisms of immune dysregulation: Applications to allergy and asthma. Ann. Allergy Asthma Immunol. 2004, 93 (Suppl. S1), S11–S17. [Google Scholar] [CrossRef] [PubMed]
- Kenney, M.J.; Ganta, C.K. Autonomic Nervous System and Immune System Interactions. Compr. Physiol. 2014, 4, 1177–1200. [Google Scholar] [CrossRef] [Green Version]
- Van der Kleij, H.P.; Bienenstock, J. Significance of Conversation between Mast Cells and Nerves. Allergy Asthma Clin. Immunol. 2005, 1, 65–80. [Google Scholar] [CrossRef] [Green Version]
- Kazuma, N.; Otsuka, K.; Matsuoka, I.; Murata, M. Heart Rate Variability During 24 Hours in Asthmatic Children. Chrono. Int. 1997, 14, 597–606. [Google Scholar] [CrossRef]
- Garavaglia, L.; Gulich, D.; Defeo, M.M.; Mailland, J.T.; Irurzun, I.M. The effect of age on the heart rate variability of healthy subjects. PLoS ONE 2021, 16, e0255894. [Google Scholar] [CrossRef]
- Tsuji, H.; Venditti, F.J.; Manders, E.S.; Evans, J.C.; Larson, M.G.; Feldman, C.L.; Levy, D. Determinants of heart rate variability. J. Am. Coll. Cardiol. 1996, 28, 1539–1546. [Google Scholar] [CrossRef] [Green Version]
- Yacoub, M.-R.; Cucca, V.; Asperti, C.; Ramirez, G.A.; Della-Torre, E.; Moro, M.; Zandalasini, C.; Di Napoli, D.; Ambrosio, A.; Signorelli, C.; et al. Efficacy of a rational algorithm to assess allergy risk in patients receiving the BNT162b2 vaccine. Vaccine 2021, 39, 6464–6469. [Google Scholar] [CrossRef]
- Klimek, L.; Jutel, M.; Akdis, C.A.; Bousquet, J.; Akdis, M.; Torres, M.J.; Agache, I.; Canonica, G.W.; Del Giacco, S.; O’Mahony, L.; et al. ARIA-EAACI statement on severe allergic reactions to COVID-19 vaccines—An EAACI-ARIA Position Paper. Allergy 2021, 76, 1624–1628. [Google Scholar] [CrossRef]
| Study Group (N 24) | Control Group (N 12) | p-Value (<0.05) | |
|---|---|---|---|
| Male sex (%) | 1 (4%) | 3 (25%) | 0.02 |
| Age (years), median (interquartile) | 47 (34.5–53.3) | 59.5(53–66.8) | 0.008 |
| Other medical condition | 12 (50%) | 6 (50%) | 0.99 |
| Previous COVID-19 vaccination dose(s) | |||
| 0 | 6 (25%) | 1 (8%) | 0.2 |
| ≥I | 18 (75%) | 11 (92%) | 0.2 |
| Allergic reaction to COVID-19 vaccination | 1 (4%) | 0 (0%) | 0.5 |
| Drugs allergy | 14 (58%) | 2 (17%) | 0.01 |
| Food allergy | 11 (46%) | 0 (0%) | 0.005 |
| Patients with at least one allergic comorbidity, n (%) | 14 (58%) | 4 (33%) | 0.15 |
| Rhinitis | 11 (46%) | 2 (17%) | 0.09 |
| Asthma | 5 (21%) | 0 (0%) | 0.09 |
| Atopic dermatitis | 3 (13%) | 0 (0%) | 0.2 |
| Chronic itch | 4 (17%) | 0 (0%) | 0.1 |
| Contact dermatitis | 5 (21%) | 1 (8%) | 0.3 |
| Chronic urticaria | 1 (4%) | 0 (0%) | 0.5 |
| Positivity to allergic test | 16 (67%) | 0 (0%) | <0.001 |
| Antiallergic therapies | |||
| Antihistaminic drugs | 11(46%) | 0 (0%) | 0.005 |
| Bronchodilators | 4 (17%) | 1 (8%) | 0.5 |
| Montelukast | 2 (8%) | 0 (0%) | 0.3 |
| Steroid | 0 (0%) | 0 (0%) | - |
| Study Group (N 24) | Control Group (N 12) | p-Value (<0.05) | |
|---|---|---|---|
| RR (ms) median (interquartile) | 687(645–759) | 821(759–902) | 0.02 |
| SDNN (ms) median (interquartile) | 32 (23–36) | 50 (43–54) | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cilona, M.B.; D’Amico, F.; Asperti, C.; Ramirez, G.A.; Turi, S.; Benanti, G.; Bohane, S.M.; Nannipieri, S.; Labanca, R.; Gervasini, M.; et al. Heart Rate Variability in Subjects with Severe Allergic Background Undergoing COVID-19 Vaccination. Vaccines 2023, 11, 567. https://doi.org/10.3390/vaccines11030567
Cilona MB, D’Amico F, Asperti C, Ramirez GA, Turi S, Benanti G, Bohane SM, Nannipieri S, Labanca R, Gervasini M, et al. Heart Rate Variability in Subjects with Severe Allergic Background Undergoing COVID-19 Vaccination. Vaccines. 2023; 11(3):567. https://doi.org/10.3390/vaccines11030567
Chicago/Turabian StyleCilona, Maria Bernadette, Filippo D’Amico, Chiara Asperti, Giuseppe Alvise Ramirez, Stefano Turi, Giovanni Benanti, Shai Marc Bohane, Serena Nannipieri, Rosa Labanca, Matteo Gervasini, and et al. 2023. "Heart Rate Variability in Subjects with Severe Allergic Background Undergoing COVID-19 Vaccination" Vaccines 11, no. 3: 567. https://doi.org/10.3390/vaccines11030567
APA StyleCilona, M. B., D’Amico, F., Asperti, C., Ramirez, G. A., Turi, S., Benanti, G., Bohane, S. M., Nannipieri, S., Labanca, R., Gervasini, M., Russetti, F., Viapiana, N., Lezzi, M., Landoni, G., Dagna, L., & Yacoub, M.-R. (2023). Heart Rate Variability in Subjects with Severe Allergic Background Undergoing COVID-19 Vaccination. Vaccines, 11(3), 567. https://doi.org/10.3390/vaccines11030567





