A Comprehensive Review on Bacterial Vaccines Combating Antimicrobial Resistance in Poultry
Abstract
:1. Introduction
2. Overview of Bacterial Diseases in Poultry
- Salmonellosis: A bacterial infection caused by Salmonella, which can lead to severe diarrhea, septicemia, and death in poultry.
- Colibacillosis: A bacterial infection caused by Escherichia coli, which can cause severe diarrhea in young chicks and septicemia in adult chickens.
- Avian Mycoplasmosis: A bacterial infection caused by Mycoplasma gallisepticum, which affects the respiratory and reproductive systems of poultry and can cause decreased egg production and increased mortality.
- Pasteurellosis: A bacterial infection caused by Pasteurella multocida, which affects the respiratory system of poultry and can cause severe pneumonia, septicemia, and death.
- Campylobacteriosis: A bacterial infection caused by Campylobacter jejuni, which can cause severe diarrhea, enteritis, and septicemia in poultry.
- Staphylococcus infection: A bacterial infection caused by Staphylococcus aureus, which can cause skin and wound infections, arthritis, and septicemia in poultry.
- Chlamydiosis: A bacterial infection caused by Chlamydia psittaci, which affects the respiratory and reproductive systems of poultry and can cause decreased egg production and increased mortality.
3. Antibiotics Contribute to Antimicrobial Resistance
- Overuse and misuse of antibiotics: Overuse and misuse of antibiotics are the primary causes of antibiotic resistance. Antibiotics are often prescribed for viral infections that they cannot cure, leading to unnecessary exposure to antibiotics, and making it easier for bacteria to develop resistance. Moreover, people often stop taking antibiotics once they feel better, not realizing that the bacteria may still be present, leading to incomplete treatment and the development of resistance.
- Selection pressure: Antibiotics exert strong selection pressure on bacteria, killing off the susceptible ones and allowing the resistant ones to survive and multiply. The resistant bacteria then go on to spread their resistance genes to other bacteria through horizontal gene transfer, including plasmids, transposons, and integrons. This horizontal transfer of resistance genes can occur within and between different species, making it harder to control the spread of resistance.
- Antibiotic residues in the environment: Antibiotics and their metabolites can persist in the environment for a long time after they are used, even at low concentrations. This can lead to the selection of resistant bacteria in the environment, which can then spread to humans and animals. Antibiotic residues in water bodies can also contribute to the spread of resistance, as they can lead to the selection of resistant bacteria in aquatic environments.
- Agricultural use of antibiotics: Antibiotics are widely used in agriculture, both to treat and prevent infections in animals and as growth promoters. This can lead to the selection of resistant bacteria in animals, which can then spread to humans through the food chain or the environment. Moreover, the use of antibiotics in agriculture contributes to the spread of resistance by releasing antibiotic residues into the environment.
- Lack of new antibiotics: The development of new antibiotics has slowed down in recent years, partly due to the high cost and time required for the development. This means that the antibiotics we have now are becoming less effective against resistant bacteria, which can lead to the further spread of resistance.
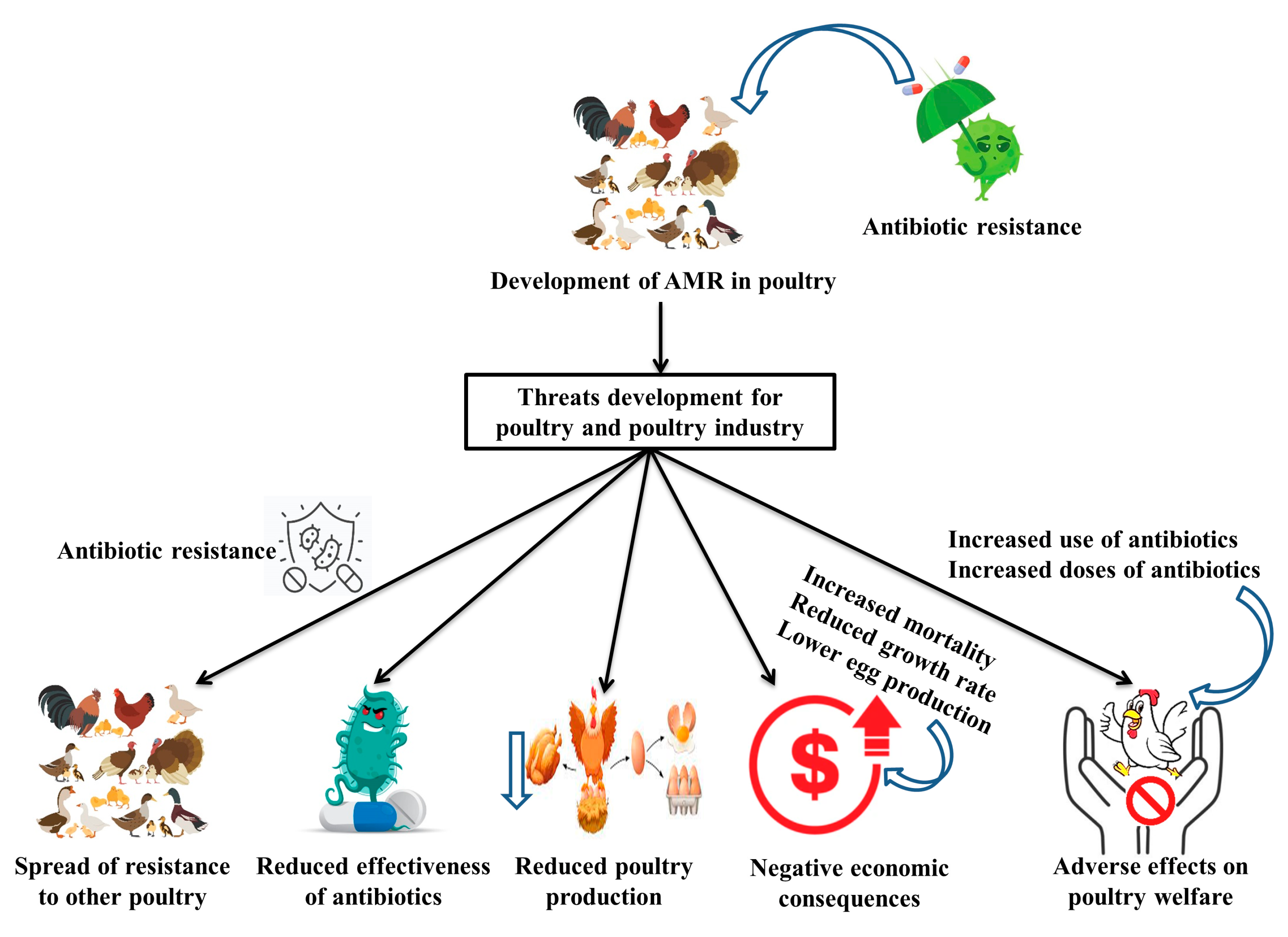
4. Antimicrobial Resistance in Poultry
- Mutations: Bacteria can undergo genetic mutations that alter their structure and make them resistant to antibiotics.
- Horizontal gene transfer: This occurs when bacteria transfer genes that confer resistance to antibiotics to other bacteria through mechanisms such as conjugation, transduction, and transformation.
- Antibiotic pressure: The overuse and misuse of antibiotics can select bacteria that are resistant to these drugs.
- Spread of resistance: Antibiotic-resistant bacteria can spread from poultry to poultry through direct contact or the environment. This can contribute to the spread of resistance and make it more challenging to control bacterial infections in poultry. Moreover, resistance genes can be transferred from bacteria in poultry to bacteria in other poultry through horizontal gene transfer, leading to the further spread of resistance.
- Reduced efficacy of antibiotics: Antibiotic-resistant bacteria are more difficult to treat and can lead to prolonged illness and increased mortality in poultry.
- Impact on animal welfare: The use of antibiotics in poultry can contribute to the development of antibiotic-resistant bacteria, which can lead to the increased use of antibiotics and the use of higher doses, leading to the potential for adverse effects on animal welfare.
- Increased costs: The treatment of antibiotic-resistant infections can be more expensive and time-consuming than the treatment of infections caused by susceptible bacteria.
- Reduced productivity: Antimicrobial-resistant infections can reduce the health and productivity of poultry flocks, leading to decreased egg production and reduced meat quality.
5. Overview of the History of Bacterial Vaccines in Poultry
6. Properties of a Comparative Study of Different Bacterial Vaccines for Poultry
- Efficacy: This refers to the ability of the vaccine to effectively prevent or control the target bacterial infection. The efficacy of a vaccine can be determined through field trials, laboratory experiments, and/or observational studies.
- Safety: The safety of a vaccine is determined by evaluating the potential adverse effects associated with its use, such as local or systemic reactions, toxicity, and interference with other vaccines.
- Cost-effectiveness: The cost-effectiveness of a vaccine is determined by evaluating the cost of the vaccine in relation to the benefits it provides, such as reducing the need for antibiotics, improving productivity, and reducing the risk of AMR.
- Administration: The ease of administration of a vaccine can also be considered, including the route of administration, dosage, and storage requirements.
- Durability: The durability of a vaccine refers to its ability to provide long-lasting protection against the target bacterial infection.
- Spectrum of activity: The spectrum of activity of a vaccine refers to the range of bacterial strains that the vaccine can protect against.
7. Potential Mechanisms of Action of Bacterial Vaccines in Poultry
- The first mechanism of action is the stimulation of the immune response. When a bird is vaccinated, its immune system produces a response against the vaccine, which includes the production of antibodies. These antibodies are specific to the pathogen targeted by the vaccine, and they provide protection against future infections with the same pathogen. This is known as active immunity, and it provides long-lasting protection against bacterial infections.
- The second mechanism of action is the competition for nutrients and attachment sites. Some bacterial vaccines work by introducing a benign, or “competitor” bacteria into the poultry’s gut. This competitor bacteria competes with pathogenic bacteria for nutrients and attachment sites in the gut, reducing their ability to colonize and cause disease. This is known as competitive exclusion, and it is a highly effective approach to controlling bacterial infections in poultry.
- The third mechanism of action of bacterial vaccine is a cell-mediated immune response. Bacterial vaccines also stimulate the production of T-cells, which are a type of white blood cell that can directly attack infected cells and help activate other cells in the immune system. T-cells can recognize specific bacterial antigens and release cytokines (small proteins that regulate the immune response) to activate other cells in the immune system.
- Another mechanism of action of bacterial vaccines is the stimulation of phagocytosis. Phagocytosis is the process by which immune cells called phagocytes engulf and destroy bacteria. Bacterial vaccines stimulate the production of phagocytes, such as macrophages and neutrophils, which can recognize and engulf bacteria.
8. Different Types of Bacterial Vaccines Used in Poultry and Their Efficacy
- Inactivated bacterial vaccines: Inactivated bacterial vaccines are made by killing the bacteria and then purifying and inactivating the vaccine. These vaccines stimulate the immune system to produce a response against the specific pathogen, providing long-lasting protection against future infections. Examples of inactivated bacterial vaccines used in poultry include vaccines against Salmonella, Campylobacter, and E. coli.
- Live attenuated bacterial vaccines: Live attenuated bacterial vaccines are made by attenuating or weakening the bacteria, so they are no longer harmful but still able to stimulate an immune response. These vaccines can provide long-lasting protection against future infections with the same pathogen. Examples of live attenuated bacterial vaccines used in poultry include vaccines against Newcastle disease, infectious bronchitis, and fowl pox.
- Subunit bacterial vaccines: Subunit bacterial vaccines are made by purifying and isolating specific proteins or antigens from the bacteria and then using these antigens to stimulate an immune response. These vaccines can be highly effective but may require multiple doses to provide long-lasting protection against future infections. Examples of subunit bacterial vaccines used in poultry include vaccines against Salmonella, Campylobacter, and E. coli.
- Recombinant bacterial vaccines: Recombinant bacterial vaccines are made by using genetic engineering techniques to introduce specific antigens into a harmless vector, such as a bacterium or yeast. These vaccines can provide highly effective protection against specific bacterial pathogens and may only require a single dose to provide long-lasting protection. Examples of recombinant bacterial vaccines used in poultry include vaccines against Salmonella and Campylobacter.
9. Commercially Available Vaccines for Different Common Poultry Bacterial Diseases
- Salmonella vaccine: This vaccine is used to prevent Salmonella infections in poultry. It is composed of killed bacteria or a live attenuated strain that has been modified to reduce its virulence. Vaccinating birds against a particular serovar that is specific to their host, such as Salmonella gallinarum, results in the development of a robust and targeted immune response. The vaccine can be administered through drinking water or injection. The largest selection of available vaccines is designed to target serovar Enteritidis and Typhimurium. These vaccines are typically given through subcutaneous injection when birds are between 10 to 14 weeks old, with two separate doses administered 4 to 6 weeks apart.
- Infectious coryza vaccine: This vaccine is used to protect against infectious coryza (caused by Haemophilus paragallinarum or Avibacterium paragallinarum), a bacterial respiratory disease that affects poultry. The vaccine is typically produced from killed or inactivated bacteria. In the United States and other nations, there are commercial bacterins that typically consist of all serovars of the bacterium. Certain vaccines made by large manufacturers are marketed globally and contain the most common bacterial strains. Nonetheless, there are worries that such vaccines may not protect against locally prevalent variants. These types of vaccines are normally administered through drinking water or injection.
- Avian E. coli vaccine: This vaccine is used to prevent E. coli infections in poultry. It is commonly produced from live attenuated bacteria and administered through drinking water or injection. However, currently, in the United States, there exists only a live attenuated vaccine option. This vaccine features a mutant strain with an aroA deletion. The administration of antibiotics is not allowed when using this vaccine.
- Pasteurella multocida vaccine: Pasteurella multocida vaccines are available in different forms, including bacterins combined with aluminum hydroxide or oil emulsions, or with weakened live organisms. Multivalent P. multocida vaccines usually have serotypes 1, 3, and 4, which are the most common. Inactivated vaccines are typically administered through injection, while attenuated live vaccines (using M9 or PM-1 strains) can be given through the wing web or drinking water. It takes about two weeks for immunity to develop after vaccination.
- Avian mycoplasma vaccine: This vaccine is used to prevent Mycoplasma gallisepticum and Mycoplasma synoviae infections in poultry. The vaccine is typically produced from killed or attenuated bacteria. Live MG vaccines are available in several types, such as the mild F strain, the safer avirulent ts-11 or 6/85 strains, etc. The F strain can be given through intranasal or eye drop methods, while the ts-11 strain is administered through eye drops and the 6/85 strain through fine spray. The use of these attenuated vaccines is considered controlled exposure, which means they cause only mild infection at an age when it is less damaging. The vaccination of pullets is generally employed between 12 to 16 weeks of age, and one dose is enough to make them permanent carriers. Moreover, a live MS vaccine with the MS-H strain is given by eye drop.
10. Advantages and Disadvantages of Using Bacterial Vaccines in Poultry
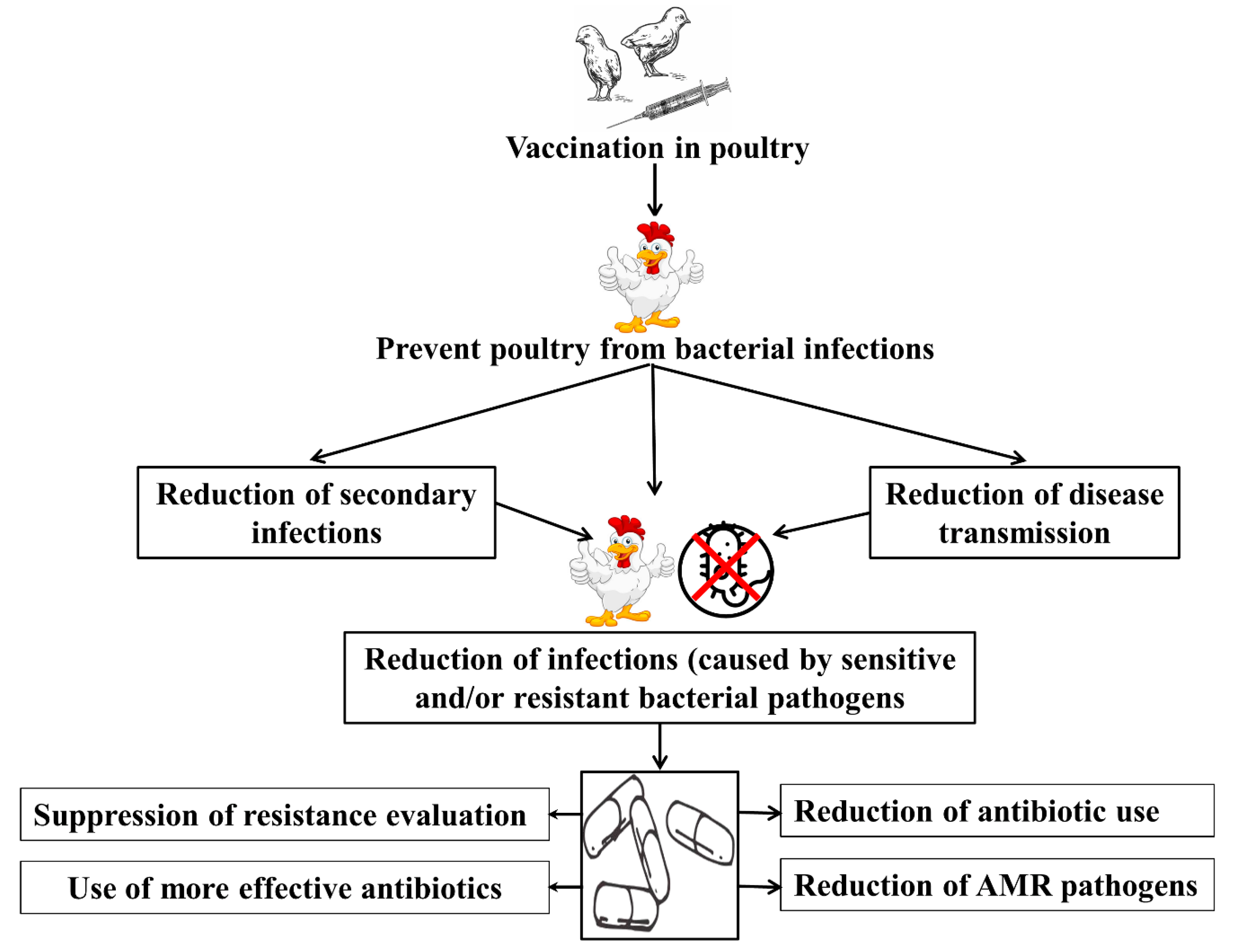
- Reduced need for antibiotics: The primary advantage of bacterial vaccines is that they reduce the need for antibiotics. By stimulating the poultry’s immune system to produce a response against specific pathogens, bacterial vaccines provide protection against future infections with the same pathogen, reducing the need for antibiotics. This is particularly important in the context of AMR, as the overuse of antibiotics in poultry production has contributed to the development of antibiotic-resistant bacteria.
- Increased immunity: Bacterial vaccines can also provide increased immunity against specific bacterial pathogens. By stimulating the poultry’s immune system to produce a response against the pathogen, bacterial vaccines provide long-lasting protection against future infections with the same pathogen. This can improve the health and welfare of the poultry, as well as reduce the need for antibiotics.
- Improved animal performance: Improved immunity against bacterial infections can also result in improved animal performance. Poultry that are protected against bacterial infections are less likely to experience disease, reducing the need for antibiotics and improving overall health. This can result in improved feed conversion, weight gain, and egg production.
- Reduced spread of antibiotic-resistant bacteria: Bacterial vaccines can also help to reduce the spread of antibiotic-resistant bacteria. By controlling bacterial infections in poultry, bacterial vaccines reduce the need for antibiotics, and, in turn, reduce the exposure of bacteria to antibiotics. This reduces the selection pressure for antibiotic-resistant bacteria, reducing their spread to other poultry and to the environment.
- Reduced risk of transmission to humans: Bacterial vaccines can also help to reduce the risk of transmission of bacterial infections from poultry to humans. By controlling bacterial infections in poultry, bacterial vaccines reduce the risk of bacterial pathogens being spread to humans through the food supply, reducing the risk of human infections.
- Cost: One of the main disadvantages of bacterial vaccines is their cost. Bacterial vaccines can be more expensive than antibiotics, particularly in large-scale poultry production systems. This can make them less accessible to some producers, particularly in developing countries.
- Ineffectiveness against some pathogens: Bacterial vaccines may not be effective against all bacterial pathogens, and some may be better suited to certain types of infections than others. This means that producers need to carefully select the most appropriate bacterial vaccine for their needs.
- Time to efficacy: Bacterial vaccines may take several weeks to become effective, during which time the poultry may still be susceptible to infection. This can result in a period of increased risk and may require the use of antibiotics in the meantime.
- Limited availability: Bacterial vaccines may not be widely available in all countries, particularly in developing countries. This can limit their use in some regions, and producers may need to import vaccines from other countries, which can be costly and logistically challenging.
11. The Role of Bacterial Vaccines in Reducing the Use of Antibiotics and the Development of Antimicrobial Resistance in Poultry Top of Form
12. Comparison of Bacterial Vaccines with Other Alternative Strategies for Combating Antimicrobial Resistance in Poultry
- Probiotics are live microorganisms that, when ingested in adequate amounts, have a beneficial effect on the host. They can include beneficial bacteria, such as Lactobacillus and Bifidobacterium, as well as yeast and other microorganisms. In poultry, probiotics can be used to improve gut health and reduce the colonization of pathogenic bacteria. However, probiotics are limited in their ability to target specific pathogens and may have limited efficacy in controlling AMR.
- Prebiotics, on the other hand, are non-digestible food ingredients that promote the growth of beneficial bacteria in the gut. They can include substances such as fructooligosaccharides, inulin, and mannan-oligosaccharides. By promoting the growth of beneficial bacteria, prebiotics can reduce the colonization of pathogenic bacteria and limit the spread of AMR. However, prebiotics are limited in their ability to target specific pathogens and may have limited efficacy in controlling AMR.
- Bacteriophages are viruses that infect and kill bacteria. In poultry, bacteriophages can be used to control specific bacterial pathogens and limit the spread of AMR. However, the development and implementation of bacteriophage-based interventions can be challenging due to the limited availability of specific bacteriophages for different bacterial pathogens and the potential for the development of phage resistance.
- Dietary modifications, such as the inclusion of plant-based compounds, can also be used to combat AMR in poultry. For example, the inclusion of essential oils (e.g., substances such as thyme, cinnamon, and eucalyptus oil) and other plant-based compounds (e.g., garlic extract, turmeric extract, ginger extract, etc.) in the diet of poultry has been shown to have antimicrobial activity and reduce the colonization of pathogenic bacteria. However, the efficacy of dietary modifications in controlling AMR can be limited and may not be as effective as bacterial vaccines.
- Nanoparticles have shown promising potential as antimicrobial agents due to their unique physicochemical properties, which can enhance their efficacy against a broad range of microorganisms. However, the use of nanoparticles as antimicrobial agents also has some limitations that need to be addressed. One of the main concerns is the potential toxicity of nanoparticles to poultry, humans, and the environment. Moreover, the mechanisms of action of nanoparticles as antimicrobial agents are not yet fully understood, which can make it challenging to optimize their efficacy and safety. These limitations highlight the need for further research to better understand the potential benefits and risks of using nanoparticles as antimicrobial agents.
- In comparison, bacterial vaccines offer several advantages over these alternative strategies. Bacterial vaccines are specific to the targeted pathogen and provide a long-lasting immunity that can reduce the colonization of pathogenic bacteria and limit the spread of AMR. Additionally, bacterial vaccines do not have the potential for the development of resistance, as seen with the use of antibiotics and other antimicrobial agents. However, it should be noted that bacterial vaccines are not a cure-all solution for AMR in poultry and may not be effective against all bacterial pathogens. The development and implementation of bacterial vaccines can also be challenging and may require significant investments in research and development.
- Bacterial vaccines offer several advantages over alternative strategies for combating AMR in poultry. However, the relative efficacy of bacterial vaccines in comparison to alternative strategies will depend on the specific bacterial pathogen and the underlying conditions in the poultry production environment. Further research is needed to fully understand the potential of bacterial vaccines and their relative efficacy in comparison to other alternative strategies.
13. Case Studies on Bacterial Vaccines in Poultry Farming
- Disease incidence: This refers to the number of poultry that develop the target bacterial infection after vaccination.
- Mortality rate: This refers to the number of poultry that die as a result of the target bacterial infection.
- Productivity: This refers to measures of the poultry’s performance, such as weight gain, feed efficiency, and egg production.
- Antibiotic use: This refers to the use of antibiotics on the vaccinated poultry and the control group and can provide valuable information on the impact of the vaccine on the need for antibiotics.
- Economic impact: This refers to the costs associated with the use of the vaccine, including the cost of the vaccine itself, the cost of administering the vaccine, and the cost of any associated treatments.
14. A Review of Current Research Studies on the Efficacy of Bacterial Vaccines in Poultry
14.1. Studies on the Efficacy of Bacterial Vaccines in Poultry
14.2. Studies on the Impact of Bacterial Vaccines on Antimicrobial Resistance
15. The global Market for Bacterial Vaccines in Poultry and Current Trends
- Growing concern over AMR: The rise of AMR is a growing concern worldwide, and bacterial vaccines are seen as an effective solution for controlling AMR in poultry.
- Increased demand for meat: With a growing global population, the demand for meat is increasing, leading to an increase in the number of poultry operations. This has resulted in a growing demand for bacterial vaccines to control the spread of AMR in poultry.
- Government initiatives: Governments worldwide are taking steps to reduce the use of antibiotics in animal production and promoting the use of alternatives, including bacterial vaccines.
- Research and development: The bacterial vaccines market in poultry is supported by ongoing research and development initiatives aimed at improving the efficacy of bacterial vaccines and developing new vaccines to target specific bacterial pathogens.
- Increase in poultry production: The growth of the global poultry industry is a significant factor driving the growth of the bacterial vaccines market in poultry. As the number of poultry operations increases, so does the demand for bacterial vaccines to control the spread of AMR.
16. The Future Potential of Bacterial Vaccines in Poultry and their Potential Impact on the Poultry Industry
16.1. The Development of New and Improved Bacterial Vaccines
16.2. The Role of Government and Industry in the Promotion of Bacterial Vaccines
16.3. The Impact of Bacterial Vaccines on the Poultry Industry
17. The impact of Bacterial Vaccines on the Welfare of Poultry and Public Health Top of Form
18. The Economic Benefits of Using Bacterial Vaccines in Poultry and Cost-Effectiveness Analysis
19. The Impact of Bacterial Vaccines on the Environment and Sustainability of the Poultry Industry
20. The Regulatory Landscape for Bacterial Vaccines in Poultry and Challenges to Their Widespread Adoption
21. Challenges in the Development and Implementation of Bacterial Vaccines in Poultry
21.1. Scientific Challenges
21.2. Technical Challenges
21.3. Economic Challenges
22. The Role of Veterinary Clinics and Poultry Farmers in Promoting the Use of Bacterial Vaccines
23. The Role of Technology and Innovation in the Development of New and More Effective Bacterial Vaccines for Poultry
24. The Role of Public–Private Partnerships in Advancing the Development and Implementation of Bacterial Vaccines in Poultry
- PPPs can help to provide the funding and resources needed for research and development of new vaccines. By pooling resources from both the public and private sectors, PPPs can ensure that there is sufficient funding for R&D activities, which is critical for the development of new and more effective vaccines.
- PPPs can help to facilitate collaboration between researchers, industry, and government. Through collaboration, the different stakeholders can share their expertise, knowledge, and resources, which can result in more effective solutions. For example, researchers can benefit from the real-world knowledge and experience of poultry farmers and industry experts, while the industry can benefit from the latest scientific advancements in vaccine development.
- PPPs can help to promote the widespread adoption of bacterial vaccines in poultry. Through joint efforts, public and private sectors can raise awareness about the benefits of using bacterial vaccines and encourage poultry farmers to adopt these vaccines. By working together, PPPs can help to overcome any barriers to the adoption of vaccines, such as the lack of information or lack of access to vaccines.
25. An Overview of Current Global Efforts to Combat Antimicrobial Resistance in Poultry
26. Conclusions and Recommendations
- Development of new and improved bacterial vaccines: Research should be conducted to develop new and improved bacterial vaccines that are specific to the targeted bacterial pathogens and that offer long-lasting immunity.
- Efficacy studies: Further research is needed to fully understand the efficacy of bacterial vaccines in controlling AMR in poultry. This includes large-scale trials to determine the effectiveness of bacterial vaccines in reducing the spread of AMR and the impact of bacterial vaccines on the poultry industry.
- Regulatory support: Regulators should provide support for the development and implementation of bacterial vaccines in poultry. This includes the establishment of a regulatory framework for the development and commercialization of bacterial vaccines and the provision of technical assistance for the implementation of bacterial vaccines in the field.
- Industry support: The poultry industry should provide support for the development and implementation of bacterial vaccines. This includes the provision of resources for research and development, the promotion of the use of bacterial vaccines in poultry production, and the establishment of incentives for the use of bacterial vaccines in the field.
- Education and outreach: Education and outreach efforts should be conducted to raise awareness of the importance of bacterial vaccines in combating AMR in poultry. This includes the development of educational materials for producers and consumers and the engagement of stakeholders in the poultry industry to promote the use of bacterial vaccines.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Islam, M.S.; Sabuj, A.A.M.; Haque, Z.F.; Pondit, A.; Hossain, M.G.; Saha, S. Seroprevalence and risk factors of avian reovirus in backyard chickens in different areas of Mymensingh district in Bangladesh. J. Adv. Veter. Anim. Res. 2020, 7, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.J.; Attia, Y.; Ballah, F.M.; Islam, M.S.; Sobur, M.A.; Islam, M.A.; Ievy, S.; Rahman, A.; Nishiyama, A.; Islam, M.S.; et al. Zoonotic significance and antimicrobial resistance in Salmonella in poultry in Bangladesh for the period of 2011–2021. Zoonoticdis 2021, 1, 3–24. [Google Scholar] [CrossRef]
- Islam, M.S.; Nayeem, M.M.H.; Sobur, M.A.; Ievy, S.; Islam, M.A.; Rahman, S.; Kafi, M.A.; Ashour, H.M.; Rahman, M.T. Virulence determinants and multidrug resistance of Escherichia coli isolated from migratory birds. Antibiotics 2021, 10, 190. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.; Hossain, M.; Sobur, M.; Punom, S.A.; Rahman, A.M.M.; Rahman, M. A Systematic Review on the Occurrence of Antimicrobial-Resistant Escherichia coli in Poultry and Poultry Environments in Bangladesh between 2010 and 2021. BioMed Res. Int. 2023, 2023, 2425564. [Google Scholar] [CrossRef] [PubMed]
- Ievy, S.; Islam, M.S.; Sobur, M.A.; Talukder, M.; Rahman, M.B.; Khan, M.F.R.; Rahman, M.T. Molecular detection of avian pathogenic Escherichia coli (APEC) for the first time in layer farms in Bangladesh and their antibiotic resistance patterns. Microorganisms 2020, 8, 1021. [Google Scholar] [CrossRef] [PubMed]
- Hoelzer, K.; Bielke, L.; Blake, D.P.; Cox, E.; Cutting, S.M.; Devriendt, B.; Erlacher-Vindel, E.; Goossens, E.; Karaca, K.; Lemiere, S.; et al. Vaccines as alternatives to antibiotics for food producing animals. Part 2: New approaches and potential solutions. Veter. Res. 2018, 49, 70. [Google Scholar] [CrossRef] [Green Version]
- Rabie, N.S.; Amin Girh, Z. Bacterial vaccines in poultry. Bull. Natl. Res. Cent. 2020, 44, 15. [Google Scholar] [CrossRef]
- Birhane, N.; Fesseha, H. Vaccine Failure in Poultry Production and its Control Methods-A Review. Biomed. J. Sci. Tech. Res. 2020, 29, 22588–22596. [Google Scholar]
- Jansen, K.U.; Knirsch, C.; Anderson, A.S. The role of vaccines in preventing bacterial antimicrobial resistance. Nat. Med. 2018, 24, 10–19. [Google Scholar] [CrossRef]
- Excler, J.-L.; Saville, M.; Berkley, S.; Kim, J.H. Vaccine development for emerging infectious diseases. Nat. Med. 2021, 27, 591–600. [Google Scholar] [CrossRef]
- Thøfner, I.; Christensen, J.P. Bacterial diseases in poultry. In Advancements and Technologies in Pig and Poultry Bacterial Disease Control; Academic Press: Cambridge, MA, USA, 2021; pp. 199–227. [Google Scholar]
- MSState. Diseases of Poultry. 2023. Available online: http://extension.msstate.edu/agriculture/livestock/poultry/diseases-poultry (accessed on 26 February 2023).
- Lee, M.D. Avian Campylobacter Infection. 2022. Available online: https://www.msdvetmanual.com/poultry/avian-campylobacter-infection/avian-campylobacter-infection (accessed on 25 February 2023).
- Wettere, A.J.V. Avian Chlamydiosis. 2022. Available online: https://www.msdvetmanual.com/poultry/avian-chlamydiosis/avian-chlamydiosis (accessed on 25 February 2023).
- Sabuj, A.A.M.; Mahmud, T.; Barua, N.; Rahman, M.A.; Islam, M.S.; Bary, M.A. Passive surveillance of clinical poultry diseases in an Upazila Government Veterinary Hospital of Bangladesh. Afr. J. Microbiol. Res. 2019, 13, 632–639. [Google Scholar]
- Islam, M.S.; Sobur, M.A.; Rahman, S.; Ballah, F.M.; Ievy, S.; Siddique, M.P.; Rahman, M.; Kafi, M.A.; Rahman, M.T. Detection of blaTEM, blaCTX-M, blaCMY, and blaSHV Genes Among Extended-Spectrum Beta-Lactamase-Producing Escherichia coli Isolated from Migratory Birds Travelling to Bangladesh. Microb. Ecol. 2022, 83, 942–950. [Google Scholar] [CrossRef]
- Ballah, F.M.; Islam, S.; Rana, L.; Ullah, A.; Ferdous, F.B.; Neloy, F.H.; Ievy, S.; Sobur, A.; Rahman, A.T.; Khatun, M.M.; et al. Virulence Determinants and Methicillin Resistance in Biofilm-Forming Staphylococcus aureus from Various Food Sources in Bangladesh. Antibiotics 2022, 11, 1666. [Google Scholar] [CrossRef]
- Miller, C.P. Development of Bacterial Resistance to Antibiotics. J. Am. Med Assoc. 1947, 135, 749–751. [Google Scholar] [CrossRef]
- Sabtu, N.; Enoch, D.A.; Brown, N.M. Antibiotic resistance: What, why, where, when and how? Br. Med. Bull. 2015, 116, 105–113. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.S.; Paul, A.; Talukder, M.; Roy, K.; Sobur, M.A.; Ievy, S.; Nayeem, M.M.H.; Rahman, S.; Nazir, K.N.H.; Hossain, M.T.; et al. Migratory birds travelling to Bangladesh are potential carriers of multi-drug resistant Enterococcus spp., Salmonella spp. and Vibrio spp. Saudi J. Biol. Sci. 2021, 28, 5963–5970. [Google Scholar] [CrossRef]
- Larsson, D.J.; Flach, C.F. Antibiotic resistance in the environment. Nat. Rev. Microbiol. 2022, 20, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Tawyabur, M.; Islam, M.S.; Sobur, M.A.; Hossain, M.J.; Mahmud, M.M.; Paul, S.; Hossain, M.T.; Ashour, H.M.; Rahman, M.T. Isolation and characterization of multidrug-resistant Escherichia coli and Salmonella spp. from healthy and diseased turkeys. Antibiotics 2020, 9, 770. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.J.; Islam, M.S.; Sobur, M.A.; Zaman, S.B.; Nahar, A.; Rahman, M.; Rahman, M.T. Exploring poultry farm environment for antibiotic resistant Escherichia coli, Salmonella spp. and Staphylococcus spp. having public health significance. J. Bangladesh Agric. Univ. 2020, 18, 615–622. [Google Scholar]
- Reygaert, W.C. An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiol. 2018, 4, 482–501. [Google Scholar] [CrossRef]
- Hedman, H.D.; Vasco, K.A.; Zhang, L. A Review of Antimicrobial Resistance in Poultry Farming within Low-Resource Settings. Animals 2020, 10, 1264. [Google Scholar] [CrossRef]
- Redweik, G.A.; Jochum, J.; Mellata, M. Live Bacterial Prophylactics in Modern Poultry. Front. Veter. Sci. 2020, 7, 592312. [Google Scholar] [CrossRef]
- Plotkin, S. History of vaccination. Proc. Natl. Acad. Sci. USA 2014, 111, 12283–12287. [Google Scholar] [CrossRef] [Green Version]
- Isaacson, R.E. Development of Vaccines for Bacterial Diseases using Recombinant DNA Technology. Avian Dis. 1986, 30, 28. [Google Scholar] [CrossRef]
- Nascimento, I.P.; Leite, L.C.C. Recombinant vaccines and the development of new vaccine strategies. Braz. J. Med. Biol. Res. 2012, 45, 1102–1111. [Google Scholar] [CrossRef] [Green Version]
- Mot, D.; Timbermont, L.; Haesebrouck, F.; Ducatelle, R.; Van Immerseel, F. Progress and problems in vaccination against necrotic enteritis in broiler chickens. Avian Pathol. 2014, 43, 290–300. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.J.; Kang, M.S. Protective efficacy of live Salmonella gallinarum 9R vaccine in commercial layer flocks. Avian Pathol. 2007, 36, 495–498. [Google Scholar] [CrossRef]
- Muniz, E.C.; Verdi, R.; Leão, J.A.; Back, A.; Nascimento, V.P.D. Evaluation of the effectiveness and safety of a genetically modified live vaccine in broilers challenged with Salmonella Heidelberg. Avian Pathol. 2017, 46, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Habibi, P.; Haq, A.N.U.; Saeed, M.; Amjad, N.G.; Khan, I. Seed-Based System for Cost-Effective Production of Vaccine Against Chronic Respiratory Disease in Chickens. Mol. Biotechnol. 2023, 65, 570–580. [Google Scholar] [CrossRef]
- Lin, C.-S.; Lu, T.-L.; Chen, Y.-A.; Yu, H.-Y.; Wu, C.-Y.; Yang, W.-Y. Safety of bivalent live attenuated Salmonella vaccine and its protection against bacterial shedding and tissue invasion in layers challenged with Salmonella. Poult. Sci. 2022, 101, 101943. [Google Scholar] [CrossRef] [PubMed]
- Marouf, S.; Ibrahim, H.M.; El-Naggar, M.S.; Swelum, A.A.; Alqhtani, A.H.; El-Saadony, M.T.; El-Tarabily, K.A.; Salem, H.M. Inactivated pentavalent vaccine against mycopmosis and salmonellosis for chickens. Poult. Sci. 2022, 101, 102139. [Google Scholar] [CrossRef]
- Pulendran, B.; Ahmed, R. Immunological mechanisms of vaccination. Nat. Immunol. 2011, 12, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Sudhakar, P.; Subramani, P. Mechanisms of Bacterial Pathogenesis and Targets for Vaccine Design. Journal of Young Investigators. 2005. Available online: https://www.jyi.org/2005-november/2017/11/5/review-mechanisms-of-bacterial-pathogenesis-and-targets-for-vaccine-design (accessed on 25 December 2022).
- Hajam, I.A.; Dar, P.A.; Won, G.; Lee, J.H. Bacterial ghosts as adjuvants: Mechanisms and potential. Veter. Res. 2017, 48, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, A.; Waris, A.; Khan, M.A.; Asim, M.; Khan, A.U.; Khan, S.; Zeb, J. Recent advancement, immune responses, and mechanism of action of various vaccines against intracellular bacterial infections. Life Sci. 2023, 314, 121332. [Google Scholar] [CrossRef]
- Ike, A.C.; Ononugbo, C.M.; Obi, O.J.; Onu, C.J.; Olovo, C.V.; Muo, S.O.; Chukwu, O.S.; Reward, E.E.; Omeke, O.P. Towards Improved Use of Vaccination in the Control of Infectious Bronchitis and Newcastle Disease in Poultry: Understanding the Immunological Mechanisms. Vaccines 2021, 9, 20. [Google Scholar] [CrossRef]
- Veteriankey. Poultry Vaccines. 2021. Available online: https://veteriankey.com/poultry-vaccines/ (accessed on 27 February 2023).
- Huberman, Y.D.; Velilla, A.V.; Terzolo, H.R. Evaluation of different live Salmonella Enteritidis vaccine schedules administered during layer hen rearing to reduce excretion, organ colonization, and egg contamination. Poult. Sci. 2019, 98, 2422–2431. [Google Scholar] [CrossRef]
- Dal Bérto, L.; Beirão, B.C.; Fernandes Filho, T.; Ingberman, M.; Fávaro, C., Jr.; Tavella, R.; de Mesquita Silva, R.B.; Caron, L.F. Live and inactivated Salmonella Enteritidis vaccines: Immune mechanisms in broiler breeders. World J. Vaccines 2015, 5, 155. [Google Scholar] [CrossRef] [Green Version]
- Jia, S.; McWhorter, A.R.; Andrews, D.M.; Underwood, G.J.; Chousalkar, K.K. Challenges in Vaccinating Layer Hens against Salmonella Typhimurium. Vaccines 2020, 8, 696. [Google Scholar] [CrossRef]
- DVS. List of Approved Veterinary Vaccines Updated February 2022. 2022. Available online: https://www.dvs.gov.my/dvs/resources/user_1/2022/BKAV/VAKSIN/SENARAI_VAKSIN_LULUS_Website_DVS_Mac2022.pdf (accessed on 27 February 2023).
- Poolman, J.T. Expanding the role of bacterial vaccines into life-course vaccination strategies and prevention of antimicrobial-resistant infections. Npj Vaccines 2020, 5, 84. [Google Scholar] [CrossRef]
- Frost, I.; Sati, H.; Garcia-Vello, P.; Hasso-Agopsowicz, M.; Lienhardt, C.; Gigante, V.; Beyer, P. The role of bacterial vaccines in the fight against antimicrobial resistance: An analysis of the preclinical and clinical development pipeline. Lancet Microbe 2022, 4, e113–e125. [Google Scholar] [CrossRef]
- Lee, N.H.; Lee, J.A.; Park, S.Y.; Song, C.S.; Choi, I.S.; Lee, J.B. A review of vaccine development and research for industry animals in Korea. Clin. Exp. Vaccine Res. 2012, 1, 18–34. [Google Scholar] [CrossRef] [Green Version]
- Eskin, S. Vaccines for Poultry Are Crucial for Preventing Salmonella Contamination. 2020. Available online: https://www.pewtrusts.org/en/research-and-analysis/articles/2020/09/24/vaccines-for-poultry-are-crucial-for-preventing-salmonella-contamination (accessed on 11 December 2022).
- Śmiałek, M.; Kowalczyk, J.; Koncicki, A. Influence of vaccination of broiler chickens against Escherichia coli with live attenuated vaccine on general properties of E. coli population, IBV vaccination efficiency, and production parameters—A field experiment. Poult. Sci. 2020, 99, 5452–5460. [Google Scholar] [CrossRef]
- Landman, W.J.M. The downside of broiler vaccination. Vet. Q. 2012, 32, 121–122. [Google Scholar] [CrossRef] [Green Version]
- Poultry World. Preventing Vaccination Failure in Poultry Flocks. 2020. Available online: https://www.poultryworld.net/health-nutrition/preventing-vaccination-failure-in-poultry-flocks/#:~:text=Introducing%20a%20live%20virus%20vaccine,to%20the%20milder%20vaccine%20virus (accessed on 11 December 2022).
- De Paiva, J.B.; Penha Filho, R.A.C.; Argüello, Y.M.S.; da Silva, M.D.; Gardin, Y.; Resende, F.; Berchieri Junior, A.; Sesti, L. Efficacy of several Salmonella vaccination programs against experimental challenge with Salmonella gallinarum in commercial brown layer and broiler breeder hens. Braz. J. Poult. Sci. 2009, 11, 65–72. [Google Scholar] [CrossRef] [Green Version]
- Talukder, M.; Islam, M.S.; Ievy, S.; Sobur, M.A.; Ballah, F.M.; Najibullah, M.; Rahman, M.B.; Rahman, M.T.; Khan, M.F.R. Detection of multidrug resistant Salmonella spp. from healthy and diseased broilers having potential public health significance. J. Adv. Biotechnol. Exp. Ther. 2021, 4, 248. [Google Scholar] [CrossRef]
- Revolledo, L. Vaccines and vaccination against fowl typhoid and pullorum disease: An overview and approaches in developing countries. J. Appl. Poult. Res. 2018, 27, 279–291. [Google Scholar] [CrossRef]
- Micoli, F.; Bagnoli, F.; Rappuoli, R.; Serruto, D. The role of vaccines in combatting antimicrobial resistance. Nat. Rev. Genet. 2021, 19, 287–302. [Google Scholar] [CrossRef]
- Holm, M.; Zellweger, R.M.; Poudyal, N.; Smith, K.H.; Joh, H.S.; Marks, F. Measuring the Link Between Vaccines and Antimicrobial Resistance in Low Resource Settings—Limitations and Opportunities in Direct and Indirect Assessments and Implications for Impact Studies. Front. Trop. Dis. 2022, 3, 2. [Google Scholar] [CrossRef]
- Vekemans, J.; Hasso-Agopsowicz, M.; Kang, G.; Hausdorff, W.P.; Fiore, A.; Tayler, E.; Klemm, E.J.; Laxminarayan, R.; Srikantiah, P.; Friede, M.; et al. Leveraging Vaccines to Reduce Antibiotic Use and Prevent Antimicrobial Resistance: A World Health Organization Action Framework. Clin. Infect. Dis. 2021, 73, e1011–e1017. [Google Scholar] [CrossRef]
- Martelli, P. Vaccination Strategies in the Context of Antibiotic Reduction. 2018. Available online: https://www.thepoultrysite.com/articles/vaccination-strategies-in-the-context-of-antibiotic-reduction (accessed on 30 December 2022).
- Murugaiyan, J.; Kumar, P.A.; Rao, G.S.; Iskandar, K.; Hawser, S.; Hays, J.P.; Mohsen, Y.; Adukkadukkam, S.; Awuah, W.A.; Jose, R.A.M.; et al. Progress in Alternative Strategies to Combat Antimicrobial Resistance: Focus on Antibiotics. Antibiotics 2022, 11, 200. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Chen, X. Current Promising Strategies against Antibiotic-Resistant Bacterial Infections. Antibiotics 2022, 12, 67. [Google Scholar] [CrossRef] [PubMed]
- Rios, A.C.; Moutinho, C.G.; Pinto, F.C.; Del Fiol, F.S.; Jozala, A.; Chaud, M.V.; Vila, M.M.; Teixeira, J.A.; Balcão, V.M. Alternatives to overcoming bacterial resistances: State-of-the-art. Microbiol. Res. 2016, 191, 51–80. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhang, L.; Lin, W.; Tang, W.; Chan, F.K.; Ng, S.C. Review article: Probiotics, prebiotics and dietary approaches during COVID-19 pandemic. Trends Food Sci. Technol. 2020, 108, 187–196. [Google Scholar] [CrossRef]
- Praharaj, I.; John, S.M.; Bandyopadhyay, R.; Kang, G. Probiotics, antibiotics and the immune responses to vaccines. Philos. Trans. R Soc. B Biol. Sci. 2015, 370, 20140144. [Google Scholar] [CrossRef] [Green Version]
- Salim, H.M.; Huque, K.S.; Kamaruddin, K.M.; Haque Beg, A. Global Restriction of Using Antibiotic Growth Promoters and Alternative Strategies in Poultry Production. Sci. Prog. 2018, 101, 52–75. [Google Scholar] [CrossRef]
- Fancher, C.A.; Zhang, L.; Kiess, A.S.; Adhikari, P.A.; Dinh, T.T.; Sukumaran, A.T. Avian Pathogenic Escherichia coli and Clostridium perfringens: Challenges in No Antibiotics Ever Broiler Production and Potential Solutions. Microorganisms 2020, 8, 1533. [Google Scholar] [CrossRef]
- Mehdi, Y.; Létourneau-Montminy, M.-P.; Gaucher, M.-L.; Chorfi, Y.; Suresh, G.; Rouissi, T.; Brar, S.K.; Côté, C.; Ramirez, A.A.; Godbout, S. Use of antibiotics in broiler production: Global impacts and alternatives. Anim. Nutr. 2018, 4, 170–178. [Google Scholar] [CrossRef]
- Balderrama-González, A.-S.; Piñón-Castillo, H.-A.; Ramírez-Valdespino, C.-A.; Landeros-Martínez, L.-L.; Orrantia-Borunda, E.; Esparza-Ponce, H.-E. Antimicrobial Resistance and Inorganic Nanoparticles. Int. J. Mol. Sci. 2021, 22, 12890. [Google Scholar] [CrossRef]
- Varier, K.M.; Gudeppu, M.; Chinnasamy, A.; Thangarajan, S.; Balasubramanian, J.; Li, Y.; Gajendran, B. Nanoparticles: Antimicrobial Applications and Its Prospects; Springer: Cham, Switzerland, 2019; pp. 321–355. [Google Scholar]
- De Wit, J.S.; Montiel, E. Practical aspects of poultry vaccination. In Avian Immunology; Academic Press: Cambridge, MA, USA, 2022; pp. 469–488. [Google Scholar] [CrossRef]
- Marangon, S.; Busani, L. The use of vaccination in poultry production. Rev. Sci. Tech. Off. Int. Des Epizoot. 2007, 26, 265. [Google Scholar] [CrossRef] [Green Version]
- Dórea, F.C.; Cole, D.J.; Hofacre, C.; Zamperini, K.; Mathis, D.; Doyle, M.P.; Lee, M.D.; Maurer, J.J. Effect of Salmonella Vaccination of Breeder Chickens on Contamination of Broiler Chicken Carcasses in Integrated Poultry Operations. Appl. Environ. Microbiol. 2010, 76, 7820–7825. [Google Scholar] [CrossRef] [Green Version]
- Berghaus, R.D.; Thayer, S.G.; Maurer, J.J.; Hofacre, C.L. Effect of Vaccinating Breeder Chickens with a Killed Salmonella Vaccine on Salmonella Prevalences and Loads in Breeder and Broiler Chicken Flocks. J. Food Prot. 2011, 74, 727–734. [Google Scholar] [CrossRef]
- Hassan, J.O.; Curtiss, R., 3rd. Virulent Salmonella Typhimurium-induced lymphocyte depletion and immunosuppression in chickens. Infect. Immun. 1994, 62, 2027–2036. [Google Scholar] [CrossRef] [Green Version]
- Campagnari, E.; Rossi, G.; Franciosi, C.; Girelli, D.; Giovanardi, D.; Ricci, A.; Bianchi, E.; Prandini, F.; Pesente, P.; Torriani, S. In vitro evaluation of live attenuated vaccines against Salmonella Enteritidis: Cell-mediated immune response. Ital. J. Anim. Sci. 2007, 6, 301–304. [Google Scholar] [CrossRef]
- McReynolds, J.L.; Moore, R.W.; McElroy, A.P.; Hargis, B.M.; Caldwell, D.J. Evaluation of a Competitive Exclusion Culture and Megan Vac 1 on Salmonella Typhimurium Colonization in Neonatal Broiler Chickens. J. Appl. Poult. Res. 2007, 16, 456–463. [Google Scholar] [CrossRef]
- Bailey, J.S.; Rolon, A.; Hofacre, C.L.; Holt, P.S.; Wilson, J.L.; Cosby, D.E.; Richardson, L.J.; Cox, N.A. Intestinal Humoral Immune Response and Resistance to Salmonella Challenge of Progeny from Breeders Vaccinated with Killed Antigen. Int. J. Poult. Sci. 2007, 6, 417–423. [Google Scholar] [CrossRef] [Green Version]
- Springer, S.; Lindner, T.; Ahrens, M.; Woitow, G.; Prandini, F.; Selbitz, H.-J. Duration of immunity induced in chickens by an attenuated live Salmonella Enteritidis vaccine and an inactivated Salmonella Enteritidis/Typhimurium vaccine. Berl. Munch. Tierarztl. Wochenschr. 2011, 124, 89–93. [Google Scholar] [CrossRef]
- Hayashi, R.M.; Tujimoto-Silva, A.; Muniz, E.C.; Verdi, R.; Santin, E. Salmonella Typhimurium vaccine to control a Brazilian Salmonella Heidelberg strain in broiler chickens. Ars Veter. 2018, 34, 105–114. [Google Scholar] [CrossRef] [Green Version]
- Lozica, L.; Morteza Gholi, C.S.; Kela, A.; Lošić, I.; Horvatek Tomić, D.; Gottstein, Ž. Autogenous Escherichia coli Vaccine Application as an Innovative Antimicrobial Therapy in Poultry Farming—A Case Report. Vaccines 2022, 10, 1567. [Google Scholar] [CrossRef]
- Sadeyen, J.-R.; Wu, Z.; Davies, H.; van Diemen, P.M.; Milicic, A.; La Ragione, R.M.; Kaiser, P.; Stevens, M.P.; Dziva, F. Immune responses associated with homologous protection conferred by commercial vaccines for control of avian pathogenic Escherichia coli in turkeys. Veter. Res. 2015, 46, 5. [Google Scholar] [CrossRef] [Green Version]
- Sadeghi, M.; Tavakkoli, H.; Golchin, M.; Ghanbarpour, R.; Amanollahi, S. Efficacy and safety of Poulvac E. coli vaccine in broiler chickens challenged with E. coli serotype O78 and an acute field isolate. Comp. Clin. Pathol. 2018, 27, 1629–1636. [Google Scholar] [CrossRef]
- Mombarg, M.; Bouzoubaa, K.; Andrews, S.; Vanimisetti, H.B.; Rodenberg, J.; Karaca, K. Safety and efficacy of an aroA-deleted live vaccine against avian colibacillosis in a multicentre field trial in broilers in Morocco. Avian Pathol. 2014, 43, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Galal, H.M.; Abdrabou, M.I.; Faraag, A.H.; Mah, C.K.; Tawfek, A.M. Evaluation of commercially available aroA delated gene E. coli O78 vaccine in commercial broiler chickens under Middle East simulating field conditions. Sci. Rep. 2021, 11, 1938. [Google Scholar] [CrossRef]
- Mohamed, M.A.; Bakhit, B.M.; Ibrahim, A.A.; Saleh, M. Evaluation of the living Escherichia coli-O78 deleted aroA vaccine against homologous and heterologous E. coli challenge in broiler chickens. J. Adv. Vet. Res. 2016, 6, 89–92. [Google Scholar]
- Elbestawy, A.R.; Ellakany, H.F.; Abd El-Hamid, H.S.; Ibrahim, M.S.; Gado, A.R.; Mustafa, N.S.; Moussa, I.M.; Al-Maary, K.S.; Al-Sarar, D.S.; Alshammari, H.O.; et al. Comparative evaluation of a live E. coli vaccine and cefotaxime treatment against three E. coli serotypes in broilers. J. King Saud Univ. Sci. 2021, 33, 101353. [Google Scholar] [CrossRef]
- Clark, J.D.; Oakes, R.D.; Redhead, K.; Crouch, C.F.; Francis, M.J.; Tomley, F.M.; Blake, D.P. Eimeria species parasites as novel vaccine delivery vectors: Anti-Campylobacter jejuni protective immunity induced by Eimeria tenella-delivered CjaA. Vaccine 2012, 30, 2683–2688. [Google Scholar] [CrossRef]
- Theoret, J.R.; Cooper, K.K.; Zekarias, B.; Roland, K.L.; Law, B.F.; Curtiss, R., III; Joens, L.A. The Campylobacter jejuni Dps homologue is important for in vitro biofilm formation and cecal colonization of poultry and may serve as a protective antigen for vaccination. Clin. Vaccine Immunol. 2012, 19, 1426–1431. [Google Scholar] [CrossRef] [Green Version]
- Buckley, A.M.; Wang, J.; Hudson, D.L.; Grant, A.J.; Jones, M.A.; Maskell, D.J.; Stevens, M.P. Evaluation of live-attenuated Salmonella vaccines expressing Campylobacter antigens for control of C. jejuni in poultry. Vaccine 2010, 28, 1094–1105. [Google Scholar] [CrossRef]
- Layton, S.L.; Morgan, M.J.; Cole, K.; Kwon, Y.M.; Donoghue, D.J.; Hargis, B.M.; Pumford, N.R. Evaluation of Salmonella-Vectored Campylobacter Peptide Epitopes for Reduction of Campylobacter jejuni in Broiler Chickens. Clin. Vaccine Immunol. 2011, 18, 449–454. [Google Scholar] [CrossRef] [Green Version]
- Annamalai, T.; Pina-Mimbela, R.; Kumar, A.; Binjawadagi, B.; Liu, Z.; Renukaradhya, G.J.; Rajashekara, G. Evaluation of nanoparticle-encapsulated outer membrane proteins for the control of Campylobacter jejuni colonization in chickens. Poult. Sci. 2013, 92, 2201–2211. [Google Scholar] [CrossRef]
- Neal-McKinney, J.M.; Samuelson, D.; Eucker, T.P.; Nissen, M.S.; Crespo, R.; Konkel, M.E. Reducing Campylobacter jejuni Colonization of Poultry via Vaccination. PLoS ONE 2014, 9, e114254. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.L.; Yin, Y.X.; Pan, Z.M.; Zhang, G.; Zhu, A.P.; Liu, X.F.; Jiao, X.A. Intranasal immunization with chitosan/pCAGGS-flaA nanoparticles inhibits Campylobacter jejuni in a White Leghorn model. J. Biomed. Biotechnol. 2010, 2010, 589476. [Google Scholar] [CrossRef] [Green Version]
- Rice, B.E.; Rollins, D.M.; Mallinson, E.T.; Carr, L.; Joseph, S.W. Campylobacter jejuni in broiler chickens: Colonization and humoral immunity following oral vaccination and experimental infection. Vaccine 1997, 15, 1922–1932. [Google Scholar] [CrossRef]
- Ziprin, R.L.; Hume, M.E.; Young, C.R.; Harvey, R.B. Inoculation of Chicks with Viable Non-Colonizing Strains of Campylobacter jejuni: Evaluation of Protection Against a Colonizing Strain. Curr. Microbiol. 2002, 44, 221–223. [Google Scholar] [CrossRef]
- Noor, S.M.; Husband, A.J.; Widders, P.R. In ovo oral vaccination with Campylobacter jejuni establishes early development of intestinal immunity in chickens. Br. Poult. Sci. 1995, 36, 563–573. [Google Scholar] [CrossRef]
- Harper, M.; John, M.; Edmunds, M.; Wright, A.; Ford, M.; Turni, C.; Blackall, P.J.; Cox, A.; Adler, B.; Boyce, J.D. Protective efficacy afforded by live Pasteurella multocida vaccines in chickens is independent of lipopolysaccharide outer core structure. Vaccine 2016, 34, 1696–1703. [Google Scholar] [CrossRef]
- Gong, Q.; Qu, N.; Niu, M.F.; Qin, C.L. Evaluation of immunogenicity and protective efficacy of recombinant ptfA of avian Pasteurella multocida. Iran. J. Vet. Res. 2016, 17, 84. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, F.; Shen, H.; Liao, Y.; Zhu, D.; Wang, M.; Jia, R.; Chen, S.; Liu, M.; Yang, Q.; et al. Immunogenicity and protection of a Pasteurella multocida strain with a truncated lipopolysaccharide outer core in ducks. Veter. Res. 2022, 53, 17. [Google Scholar] [CrossRef]
- Handijatno, D.; Ahmad, N.-A.; Yusoff, S.; Salleh, A.; Zamri-Saad, M. Efficacy of a Recombinant Vaccine Against Pasteurellosis in Chickens and Ducks. Adv. Anim. Veter. Sci. 2019, 7, 1134–1139. [Google Scholar] [CrossRef]
- Evans, J.D.; Leigh, S.A.; Purswell, J.L.; Jacob, R.; Peebles, E.D.; Collier, S.D.; Branton, S.L. A Comparative Study of Live Attenuated F Strain–Derived Mycoplasma gallisepticum Vaccines. Avian Dis. 2012, 56, 396–401. [Google Scholar] [CrossRef]
- Evans, J.D.; Jacob, R.; Leigh, S.A.; Collier, S.D.; Peebles, E.D.; Branton, S.L. Spray application of live attenuated F strain-derived Mycoplasma gallisepticum vaccines. J. Appl. Poult. Res. 2013, 22, 842–848. [Google Scholar] [CrossRef] [Green Version]
- Leigh, S.A.; Evans, J.D.; Collier, S.D.; Branton, S.L. The impact of vaccination route on Mycoplasma gallisepticum vaccine efficacy. Poult. Sci. 2018, 97, 3072–3075. [Google Scholar] [CrossRef] [PubMed]
- Peebles, E.D.; Jacob, R.; Branton, S.L.; Evans, J.D.; Leigh, S.A.; Gerard, P.D. Effects of different vaccine combinations against Mycoplasma gallisepticum on blood characteristics in commercial layer chickens. Poult. Sci. 2015, 94, 2108–2113. [Google Scholar] [CrossRef] [PubMed]
- Umar, S.; Munir, M.; Ur-Rehman, Z.; Subhan, S.; Azam, T.; Shah, M.A.A. Mycoplasmosis in poultry: Update on diagnosis and preventive measures. World’s Poult. Sci. J. 2017, 73, 17–28. [Google Scholar] [CrossRef] [Green Version]
- Śmiałek, M.; Kowalczyk, J.; Koncicki, A. The influence of vaccination of broiler chickens and turkeys with live E. coli attenuated vaccine on E. coli population properties and TRT. Vaccin. Effic. Anim. 2021, 11, 2068. [Google Scholar] [CrossRef]
- Research and Market. Poultry Vaccines Market—Growth, Trends, and Forecasts (2020–2025). 2020. Available online: https://www.researchandmarkets.com/reports/4515604/poultry-vaccines-market-growth-trends-and (accessed on 25 January 2023).
- Data Bridge. Global Poultry Vaccines Market—Industry Trends and Forecast to 2028. 2021. Available online: https://www.databridgemarketresearch.com/reports/global-poultry-vaccines-market (accessed on 25 January 2023).
- Rosini, R.; Nicchi, S.; Pizza, M.; Rappuoli, R. Vaccines Against Antimicrobial Resistance. Front. Immunol. 2020, 11, 1048. [Google Scholar] [CrossRef]
- Rodrigues, C.M.; Plotkin, S.A. Impact of Vaccines; Health, Economic and Social Perspectives. Front. Microbiol. 2020, 11, 1526. [Google Scholar] [CrossRef]
- Castelo Taboada, A.C.; Pavic, A. Vaccinating Meat Chickens against Campylobacter and Salmonella: A Systematic Review and Meta-Analysis. Vaccines 2022, 10, 1936. [Google Scholar] [CrossRef]
- Orso, C.; Stefanello, T.B.; Franceschi, C.H.; Mann, M.B.; Varela, A.P.M.; Castro, I.M.S.; Frazzon, J.; Frazzon, A.P.G.; Andretta, I.; Ribeiro, A.M.L. Changes in the ceca microbiota of broilers vaccinated for coccidiosis or supplemented with salinomycin. Poult. Sci. 2021, 100, 100969. [Google Scholar] [CrossRef]
- Davies, R.; Breslin, M. Effects of vaccination and other preventive methods for Salmonella Enteritidis on commercial laying chicken farms. Veter- Rec. 2003, 153, 673–677. [Google Scholar] [CrossRef]
- Baylor, N.W.; Marshall, V.B. Regulation and testing of vaccines. Vaccines 2013, 1427–1446. [Google Scholar] [CrossRef]
- Osterloh, A. Vaccination against Bacterial Infections: Challenges, Progress, and New Approaches with a Focus on Intracellular Bacteria. Vaccines 2022, 10, 751. [Google Scholar] [CrossRef]
- Bekeredjian-Ding, I. Challenges for Clinical Development of Vaccines for Prevention of Hospital-Acquired Bacterial Infections. Front. Immunol. 2020, 11, 1755. [Google Scholar] [CrossRef]
- Ulmer, J.B.; Valley, U.; Rappuoli, R. Vaccine manufacturing: Challenges and solutions. Nat. Biotechnol. 2006, 24, 1377–1383. [Google Scholar] [CrossRef]
- DeBrock, L.M. Economic aspects of vaccine innovation and manufacturing. In Vaccine Supply and Innovation; National Academies Press: Cambridge, MA, USA, 1985; pp. 45–64. [Google Scholar]
- Plotkin, S.; Robinson, J.M.; Cunningham, G.; Iqbal, R.; Larsen, S. The complexity and cost of vaccine manufacturing—An overview. Vaccine 2017, 35, 4064–4071. [Google Scholar] [CrossRef]
- Mayer, R.L.; Impens, F. Immunopeptidomics for next-generation bacterial vaccine development. Trends Microbiol. 2021, 29, 1034–1045. [Google Scholar] [CrossRef]
- McCullers, J.A.; Dunn, J.D. Advances in vaccine technology and their impact on managed care. Pharm. Ther. 2008, 33, 35–41. [Google Scholar]
- Brisse, M.; Vrba, S.M.; Kirk, N.; Liang, Y.; Ly, H. Emerging Concepts and Technologies in Vaccine Development. Front. Immunol. 2020, 11, 583077. [Google Scholar] [CrossRef]
- Joudyian, N.; Doshmangir, L.; Mahdavi, M.; Tabrizi, J.S.; Gordeev, V.S. Public-private partnerships in primary health care: A scoping review. BMC Health Serv. Res. 2021, 21, 4. [Google Scholar] [CrossRef]
- Chu, C.F. Exploring the Role of Public-Private Partnerships in Advancing the Development of Health Technology in a Global Market: A Case Study of Gavi, the Vaccine Alliance. Ph.D. Thesis, School of Public Health, The University of Texas, Austin, TX, USA, 2018. Available online: https://digitalcommons.library.tmc.edu/dissertations/AAI10815141 (accessed on 1 January 2023).
- WHO. Stop Using Antibiotics in Healthy Animals to Prevent the Spread of Antibiotic Resistance. 2017. Available online: https://www.who.int/news/item/07-11-2017-stop-using-antibiotics-in-healthy-animals-to-prevent-the-spread-of-antibiotic-resistance (accessed on 25 December 2022).
- OIE. OIE Standards, Guidelines and Resolution on Antimicrobial Resistance and the Use of Antimicrobial Agents. World Organisation for Animal Health. 2015. Available online: https://www.woah.org/fileadmin/Home/eng/Media_Center/docs/pdf/PortailAMR/EN-book-AMR.PDF (accessed on 30 December 2022).
- More, S.J. European perspectives on efforts to reduce antimicrobial usage in food animal production. Ir. Veter. J. 2020, 73, 2. [Google Scholar] [CrossRef] [Green Version]
| Name of Diseases | Common Clinical Signs | Mode of Transmission | Common Treatment/Control Vaccine/Drugs | Affected Bird Types |
|---|---|---|---|---|
| Avian salmonellosis | Depression, poor growth, weakness, severe diarrhea, dehydration, and death. | Mainly egg transmission, others include mechanical transmission, carrier birds, contaminated premises, etc. | Treatment is mainly a salvage operation. However, antibiotics, e.g., furazolidone, gentamycin sulfate, and sulfa drugs can be used. Vaccines against local strain are used to control the disease. | Chickens, turkeys, ducks, pigeons, pheasants, and other game birds. |
| Avian colibacillosis | Ruffled feathers, fever, labored breathing, reduced appetite, poor growth, occasional coughing, rales, diarrhea, and sudden death. | Inhalation of the fecal contaminated dust. | Early treatment is recommended. Antibiotics such as tetracyclines, sulfas, ampicillin, and streptomycin maybe used to control some E. coli. | All types of poultry. |
| Avian Mycoplasmosis | Coughing, sneezing, respiratory rales, ocular and nasal discharge, decreased feed intake and egg production, increased mortality, poor hatchability, and, primarily in turkeys, swelling of the infraorbital sinus. | Vertically within some eggs (transovarian) from infected breeders to progeny, and horizontally via infectious aerosols and through contamination of feed, water, and the environment, and by human activity on fomites (shoes, equipment, etc.). | Can be treated with antibiotics to alleviate clinical symptoms. Tylosin, tilmicosin, and tiamulin are useful to reduce the mycoplasma load in the flock. However, antibiotic therapy cannot completely eliminate mycoplasma from the flock. Moreover, vaccines against local strain are used to control the disease. | Chickens and turkeys. |
| Pasteurellosis | Stupor, loss of appetite, rapid weight loss, lameness resulting from joint infection, swollen wattles, difficult breathing, watery yellowish or green diarrhea, cyanosis or darkening of the head and wattles, and sudden death. | Ingestion, mechanically by arthropod vectors or by inhalation. | Treatment is not practical, but when individual treatment is applicable, chlortetracycline, oxytetracycline, and sulfaquinoxaline can be used. Vaccines against a local strain are used to control the disease. | Chickens, turkeys, pheasants, pigeons, waterfowl, sparrows, and other free-flying birds. |
| Necrotic Enteritis | Severe depression, ruffled feathers, diarrhea, and sudden increased mortality. | Oral contact with the droppings from infected birds. | Bacitracin, penicillin, and lincomycin most often used. | Mainly broiler chickens. Layers and turkeys can also be affected. |
| Campylobacteriosis | Decreased egg production; death can occur rapidly. | Through a contaminated water source or through contact with feces. | Can be treated with antibiotics, e.g., azithromycin. Bacteriocin OR-7 treatment is also useful. | Broilers, layers, turkeys, ducks, and geese. |
| Staphylococcus infection | Affected chicks usually appear drowsy or droopy with the down being "puffed up". Diarrhea sometimes occurs. Mortality usually begins within 24 hours and peaks by 5-7 days. | Transmitted from unsanitary equipment in the hatchery to newly hatched birds having unhealed navels. | Staphylococcosis can be successfully treated with antibiotics, e.g., penicillin, erythromycin, lincomycin, and spectinomycin. | Chickens. |
| Infectious Coryza | Edematous swelling of the face around the eyes and wattles, nasal discharge and swollen sinuses. | By direct contact, airborne infection by dust or respiratory discharge droplets and drinking water contaminated by infective nasal exudate | A number of drugs (e.g., Sulfadimethoxine or sulfathiazole) are effective for treating the symptoms of the disease although the disease is never completely eliminated. | Chickens. |
| Chlamydiosis | Anorexia, ruffled feathers, apathy, drop in egg production, diarrhea, weight loss, ocular discharge, fever, and respiratory distress. | By the fecal-oral route or by inhalation. | Tetracyclines (chlortetracycline, oxytetracycline, doxycycline) are the antibiotics of choice. | Turkeys, ducks, and chickens. |
| Name of Commercial Bacterial Vaccines | Vaccine Types | Name of Manufacturers, Country | Pathogens or Species | Name of Bacterial Diseases |
|---|---|---|---|---|
| AviPro®MEGAN®VAC 1 | Live, attenuated vaccine Δcya/Δcrp mutation | Elanco Animal Health, USA | Salmonella Typhimurium, Salmonella Enteritidis, and Salmonella Heidelberg | Avian salmonellosis |
| Vaxsafe®ST | Live, attenuated vaccine ΔaroA mutation | Bioproperties Pty Ltd., Australia | Salmonella Typhimurium | Avian salmonellosis |
| AviPro®Salmonella Vac E | Live, attenuated vaccine (Sm24/Rif12/Ssq strain) | Elanco Animal Health, USA | Salmonella Enteritidis | Avian salmonellosis |
| AviPro®Megan®Egg | Live, attenuated vaccine ΔaroA mutation | Elanco Animal Health, USA | Salmonella Enteritidis, Salmonella Typhimurium | Avian salmonellosis |
| Poulvac®ST | Live, attenuated vaccine ΔaroA mutation | Zoetis, USA | Salmonella Typhimurium | Avian salmonellosis |
| AviPro 109 SE4 Conc | Inactivated vaccine | Lohmann Animal Health, Germany | Salmonella Enteritidis | Avian salmonellosis |
| Gallivac®SE | Live, attenuated vaccine ΔaroA mutation | Merial Select, Italy | Salmonella Enteritidis | Avian salmonellosis |
| SALMUNE® | Live, attenuated vaccine | Ceva Animal Health, USA | Salmonella Typhimurium | Avian salmonellosis |
| Poulvac®E. coli | Live, attenuated vaccine ΔaroA mutation, O78 serotype | Zoetis, USA | Escherichia coli | Avian colibacillosis |
| Avipro 101 Coryza Gold | Inactivated (serotype A,B,C) | Lohmann Animal Health, Cuxhaven, Germany | Haemophilus paragallinarum | Infectious coryza |
| Coripravac-O | Killed [serotype A (strain 1753) + B (strain 1755) + C (strain 1756) | Hipra, Spain | Avibacterium paragallinarum | Infectious coryza |
| M-NINEVAX®-C | Live vaccine with mild avirulent M-9 strain | Merck, USA | Pasteurella multocida | Fowl cholera |
| Gallimune Cholera/Bio Chlolera | Inactivated (serotypes 1, 3 and 4.) | Merial Select, Italy | Pasteurella multocida | Fowl cholera |
| Multimune K5 | Killed (serotypes 1, 3 & 4 + serotypes 3&4) | Biomune, USA | Pasteurella multocida | Fowl cholera |
| PM-ONEVAX®-C | Live vaccine with mild avirulent PM-1 strain | Merck, USA | Pasteurella multocida | Fowl cholera |
| MyVAC DP | Killed vaccine (serotype 1) | MVP Sdn. Bhd., Malaysia | Pasteurella multocida | Duck Pasteurellosis |
| MG TS-11 | Live attenuated vaccine (TS-11 strain) | Merial Select, Italy | Mycoplasma gallisepticum | Avian mycoplasmosis |
| Gallimune MG/BioMyco/MG Vax | Killed vaccine (S6 strain) | Merial Select, Italy | Mycoplasma gallisepticum | Avian mycoplasmosis |
| AviPro®MG-F | Live attenuated vaccine (F strain) | Elanco Animal Health, USA | Mycoplasma gallisepticum | Avian mycoplasmosis |
| MG BacterinMS Bacterin | Bacterin (F strain) | Zoetis, USA | Mycoplasma gallisepticum, Mycoplasma synoviae | Avian mycoplasmosis |
| MYCOVAC-L® | Live vaccine (Intervet 6/85 strain) | Merck, USA | Mycoplasma gallisepticum | Avian mycoplasmosis |
| Myc-vac | Killed (NEV40 & NEV45 strain) | Fatro S.p.A, Italy | Mycoplasma gallisepticum | Avian mycoplasmosis |
| Poulvac®MycoF | Live attenuated vaccine (F strain) | Zoetis, USA | Mycoplasma gallisepticum | Avian mycoplasmosis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islam, M.S.; Rahman, M.T. A Comprehensive Review on Bacterial Vaccines Combating Antimicrobial Resistance in Poultry. Vaccines 2023, 11, 616. https://doi.org/10.3390/vaccines11030616
Islam MS, Rahman MT. A Comprehensive Review on Bacterial Vaccines Combating Antimicrobial Resistance in Poultry. Vaccines. 2023; 11(3):616. https://doi.org/10.3390/vaccines11030616
Chicago/Turabian StyleIslam, Md. Saiful, and Md. Tanvir Rahman. 2023. "A Comprehensive Review on Bacterial Vaccines Combating Antimicrobial Resistance in Poultry" Vaccines 11, no. 3: 616. https://doi.org/10.3390/vaccines11030616
APA StyleIslam, M. S., & Rahman, M. T. (2023). A Comprehensive Review on Bacterial Vaccines Combating Antimicrobial Resistance in Poultry. Vaccines, 11(3), 616. https://doi.org/10.3390/vaccines11030616







