Insights into In Vitro Adaptation of EV71 and Analysis of Reduced Virulence by In Silico Predictions
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. Passage of Virus
2.3. Plaque Assay
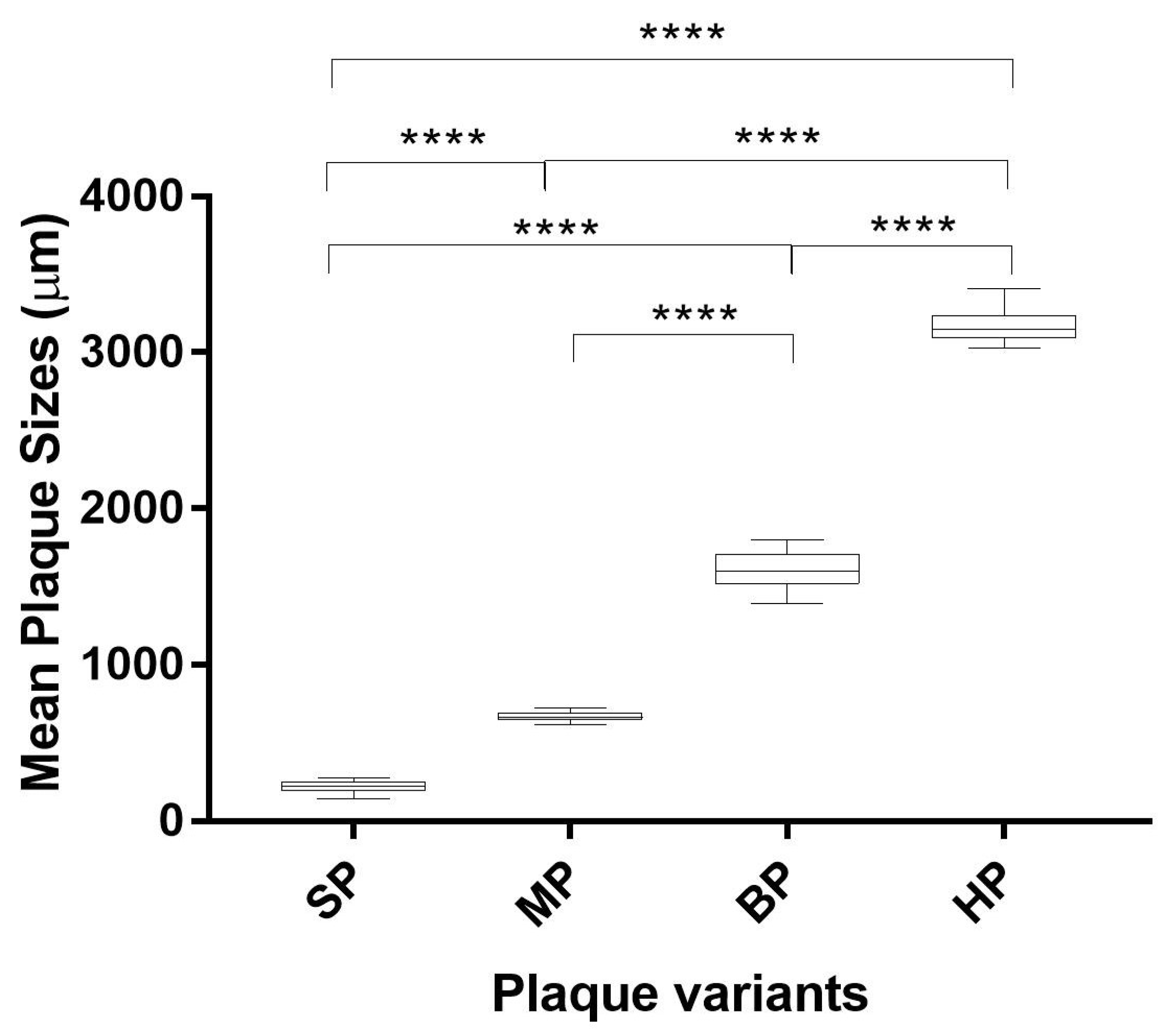
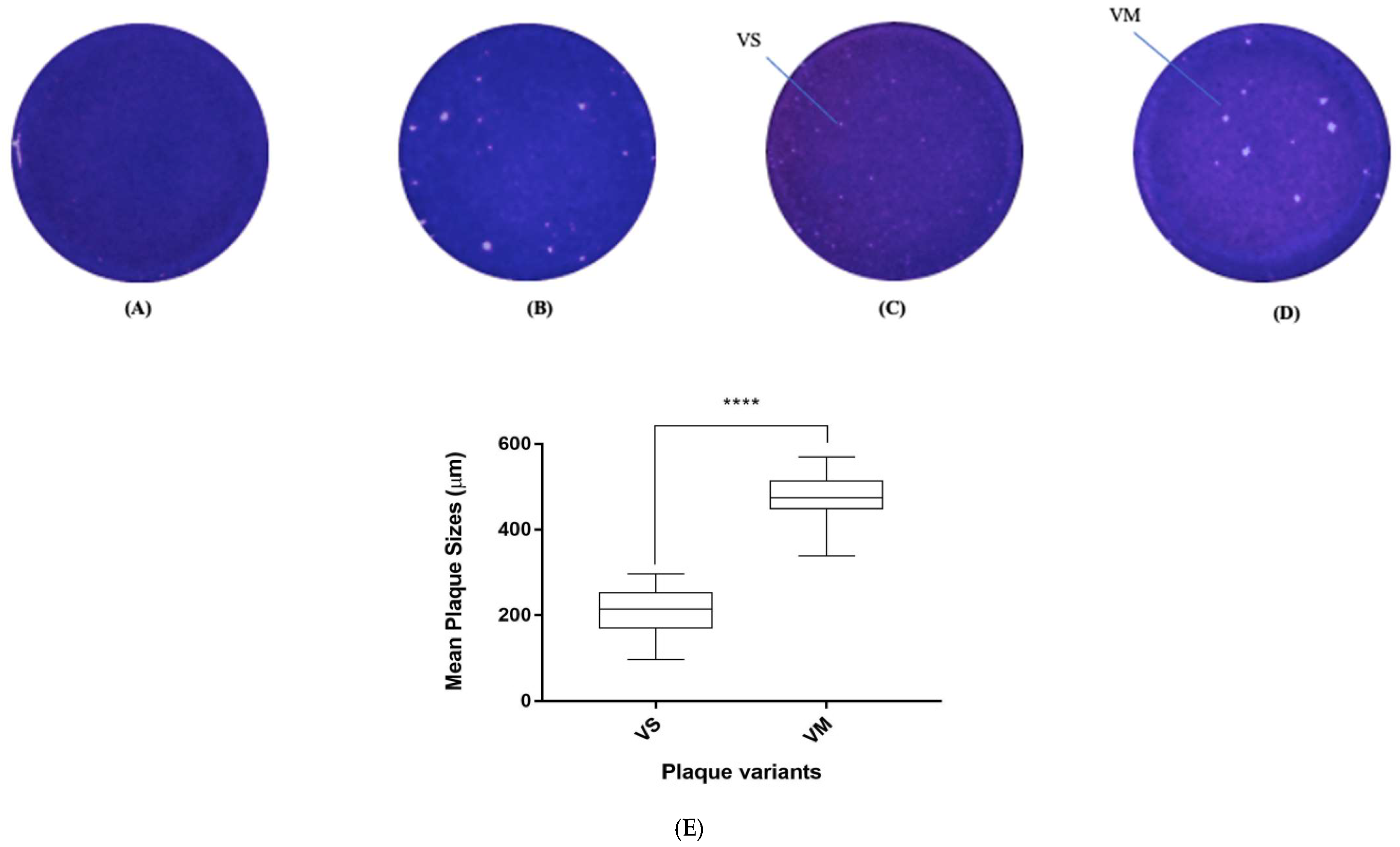
2.4. Isolation of Plaque Variants
2.5. Re-Infection of Cells by Plaque Variants and the Determination of Plaque Sizes
2.6. Growth Kinetics
2.7. Rates of Viral RNA Replication (RT-qPCR)
2.8. Tissue Culture Infectious Dose (TCID50) Assays
2.9. Virus Binding Assay
2.10. Viral Attachment Assay
2.11. Viral Entry Assay
2.12. cDNA Preparation and Amplification
2.13. Genome Sequencing
2.14. Homology Modeling and Structure Analysis
2.15. Next-Generation Sequencing
2.16. Data Analyses
3. Results
3.1. Next-Generation Sequencing (NGS) of EV-A71/WT
3.2. Plaque Morphology of Wildtype EV-A71 and the Plaque Variants
3.3. Comparison of Genome Sequences among Plaque Variants in RD Cells
3.4. Adaptation of EV-A71 in Vero Cells and Isolation of Plaque Variants
3.5. In Vitro Virulence Characteristics of the Small Plaque Variants Isolated from RD Cells
3.5.1. Growth Kinetics and RNA Replication Rate
3.5.2. TCID50
3.5.3. Viral Binding Studies
3.5.4. Viral Attachment and Entry
3.6. Homology Modeling and the Analysis of Mutation in the Small Plaque Variant Isolated from RD Cells
3D Polymerase
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McMinn, P.C. An overview of the evolution of enterovirus 71 and its clinical and public health significance. FEMS Microbiol. Rev. 2002, 26, 91–107. [Google Scholar] [CrossRef] [PubMed]
- Thompson, S.R.; Sarnow, P. Enterovirus 71 contains a type I IRES element that functions when eukaryotic initiation factor eIF4G is cleaved. Virology 2003, 315, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Bedard, K.M.; Semler, B.L. Regulation of picornavirus gene expression. Microbes Infect. 2004, 6, 702–713. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.Y.; Kung, Y.A.; Shih, S.R. Antivirals and vaccines for Enterovirus A71. J. Biomed. Sci. 2019, 26, 65. [Google Scholar] [CrossRef] [PubMed]
- Domingo, E.; Holland, J.J. RNA virus mutations and fitness for survival. Annu. Rev. Microbiol. 1997, 51, 151–178. [Google Scholar] [CrossRef]
- Huang, S.-W.; Huang, Y.-H.; Tsai, H.-P.; Kuo, P.-H.; Wang, S.-M.; Liu, C.-C.; Wang, J.-R. A selective bottleneck shapes the evolutionary mutant spectra of enterovirus A71 during viral dissemination in humans. J. Virol. 2017, 91, e01062-17. [Google Scholar] [CrossRef]
- Jaimipak, T.; Yoksan, S.; Ubol, S.; Pulmanausahakul, R. Small plaque size variant of chikungunya primary isolate showed reduced virulence in mice. Asian Pac. J. Allergy Immunol. 2018, 36, 201–205. [Google Scholar] [CrossRef]
- Goh, K.C.M.; Tang, C.K.; Norton, D.C.; Gan, E.S.; Tan, H.C.; Sun, B.; Syenina, A.; Yousuf, A.; Ong, X.M.; Kamaraj, U.S.; et al. Molecular determinants of plaque size as an indicator of dengue virus attenuation. Sci. Rep. 2016, 6, 26100. [Google Scholar] [CrossRef]
- Schloer, G.M.; Hanson, R.P. Relationship of plaque size and virulence for chickens in 14 representative Newcastle disease virus strains. J. Virol. 1968, 2, 40–47. [Google Scholar] [CrossRef]
- Kato, F.; Tajima, S.; Nakayama, E.; Kawai, Y.; Taniguchi, S.; Shibasaki, K.; Taira, M.; Maeki, T.; Lim, C.K.; Takasaki, T.; et al. Characterization of large and small-plaque variants in the Zika virus clinical isolate ZIKV/Hu/S36/Chiba/2016. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Jeong, G.K.; Yoon, G.Y.; Moon, H.W.; Lee, W.; Hwang, I.; Kim, H.; Kim, K.-D.; Kim, C.; Ahn, D.-G.; Kim, B.-T.; et al. Comparison of plaque size, thermal stability, and replication rate among SARS-CoV-2 variants of concern. Viruses 2021, 14, 55. [Google Scholar] [CrossRef] [PubMed]
- Mandary, M.B.; Masomian, M.; Ong, S.-K.; Poh, C.L. Characterization of plaque variants and the involvement of quasi-species in a population of EV-A71. Viruses 2020, 12, 651. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Moudy, R.M.; Dupuis, A.P., II; Ngo, K.A.; Maffei, J.G.; Jerzak, G.V.; Franke, M.A.; Kauffman, E.B.; Kramer, L.D. Characterization of a small plaque variant of West Nile virus isolated in New York in 2000. Virology 2007, 367, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.C.; Lian, W.C.; Hsu, L.C.; Liau, M.Y. Japanese encephalitis virus antigenic variants with characteristic differences in neutralization resistance and mouse virulence. Virus Res. 1997, 51, 173–181. [Google Scholar] [CrossRef]
- Lidbury, B.A.; Rulli, N.E.; Musso, C.M.; Cossetto, S.B.; Zaid, A.; Suhrbier, A.; Rothenfluh, H.S.; Rolph, M.S.; Mahalingam, S. Identification and characterization of a ross river virus variant that grows persistently in macrophages, shows altered disease kinetics in a mouse model, and exhibits resistance to type I interferon. J. Virol. 2011, 85, 5651–5663. [Google Scholar] [CrossRef] [PubMed]
- Sukkaew, A.; Thanagith, M.; Thongsakulpraset, T.; Mutso, M.; Mahalingam, S.; Smith, D.R.; Ubol, S. Heterogeneity of clinical isolates of chikungunya virus and its impact on the responses of primary human fibroblast-like synoviocytes. J. Gen. Virol. 2018, 99, 525–535. [Google Scholar] [CrossRef]
- Chang, C.-K.; Wu, S.-R.; Chen, Y.-C.; Lee, K.-J.; Chung, N.-H.; Yu, S.-L.; Liu, C.-C.; Chow, Y.-H. Mutations in VP1 and 5′-UTR affect enterovirus 71 virulence. Sci. Rep. 2018, 8, 6688. [Google Scholar] [CrossRef]
- Ciota, A.T.; Lovelace, A.O.; Ngo, K.A.; Le, A.N.; Maffei, J.G.; Franke, M.A.; Payne, A.F.; Jones, S.A.; Kauffman, E.B.; Kramer, L.D. Cell-specific adaptation of two flaviviruses following serial passage in mosquito cell culture. Virology 2007, 357, 165–174. [Google Scholar] [CrossRef]
- Brackney, D.E.; Scott, J.C.; Sagawa, F.; Woodward, J.E.; Miller, N.A.; Schilkey, F.D.; Mudge, J.; Wilusz, J.; Olson, K.E.; Blair, C.D.; et al. C6/36 Aedes albopictus cells have a dysfunctional antiviral RNA interference response. PLoS Negl. Trop. Dis. 2010, 4, e856. [Google Scholar] [CrossRef]
- Yee, P.T.I.; Tan, K.O.; Othman, I.; Poh, C.L. Identification of molecular determinants of cell culture growth characteristics of Enterovirus 71. Virol. J. 2016, 13, 1–12. [Google Scholar] [CrossRef]
- Chen, P.; Song, Z.; Qi, Y.; Feng, X.; Xu, N.; Sun, Y.; Wu, X.; Yao, X.; Mao, Q.; Li, X.; et al. Molecular determinants of enterovirus 71 vira entry: Cleft around Gln-172 on VP1 protein interacts with variable region on scavenge receptor B2. J. Biol. Chem. 2012, 287, 6406–6420. [Google Scholar] [CrossRef]
- Nishimura, Y.; Lee, H.; Hafenstein, S.; Kataoka, C.; Wakita, T.; Bergelson, J.M.; Shimizu, H. Enterovirus 71 binding to PSGL-1 on leukocytes: VP1-145 acts as a molecular switch to control receptor interaction. PLoS Pathog. 2013, 9, e1003511. [Google Scholar] [CrossRef] [PubMed]
- Cordey, S.; Petty, T.J.; Schibler, M.; Martinez, Y.; Gerlach, D.; van Belle, S.; Turin, L.; Zdobnov, E.; Kaiser, L.; Tapparel, C. Identification of site-specific adaptations conferring increased neural cell tropism during human enterovirus 71 infection. PLoS Pathog. 2012, 8, e1002826. [Google Scholar] [CrossRef]
- Ferrer-Orta, C.; Ferrero, D.; Verdaguer, N. RNA-dependent RNA polymerases of picornaviruses: From the structure to regulatory mechanisms. Viruses 2015, 7, 4438–4460. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, Y.; Shan, C.; Chen, C.; Xu, P.; Song, M.; Zhou, H.; Yang, C.; Xu, W.; Shi, P.-Y.; et al. Enterovirus 71 VPg uridylylation uses a two-molecular mechanism of 3D polymerase. J. Virol. 2012, 86, 13662–13671. [Google Scholar] [CrossRef]
- Meng, T.; Kwang, J. Attenuation of human enterovirus 71 high-replication-fidelity variants in AG129 mice. J. Virol. 2014, 88, 5803–5815. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-S.; Liao, C.-C.; Liou, A.-T.; Chou, Y.-C.; Yu, Y.-Y.; Lin, C.-Y.; Lin, J.-S.; Suen, C.-S.; Hwang, M.-J.; Shih, C. Novel naturally occurring mutations of enterovirus 71 associated with disease severity. Fronetiers Microbiol. 2020, 11, 610568. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.W.; Sam, I.-C.; Lee, V.S.; Wong, H.V.; Chan, Y.F. VP1 residues around the five-fold axis of enterovirus A71 mediate heparan sulfate interaction. Virology 2017, 501, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Venkataraman, S.; Prasad, B.V.L.S.; Selvarajan, R. RNA dependent RNA polymerases: Insights into structure, function and evolution. Viruses 2018, 10, 76. [Google Scholar] [CrossRef]
- Chen, C.; Wang, Y.; Shan, C.; Sun, Y.; Xu, P.; Zhou, H.; Yang, C.; Shi, P.-Y.; Rao, Z.; Zhang, B.; et al. Crystal structure of enterovirus 71 RNA-dependent RNA polymerase complexed with its protein primer VPg: Implication for a trans mechanism of VPg uridylylation. J. Virol. 2013, 87, 5755–5768. [Google Scholar] [CrossRef]
- Singh, S.; Poh, C.L.; Chow, V.T.K. Complete sequence analyses of enterovirus 71 strains from fatal and non-fatal of the hand, foot and mouth disease outbreak in Singapore (2000). Microbiol. Immunol. 2002, 46, 801–808. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.W.; Poh, C.L.; Sam, I.-C.; Chan, Y.F. Enterovirus 71 uses cell surface heparan sulfate glycosaminoglycan as an attachment receptor. J. Virol. 2013, 87, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Reed, M.; Muench, H. A simple method of estimating fifty per cent endpoints. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Lei, C.; Yang, J.; Hu, J.; Sun, X. On the calculation of TCID50 for quantification of virus infectivity. Virol. Sin. 2020, 36, 141–144. [Google Scholar] [CrossRef]
- Spurgers, K.B.; Hurt, C.R.; Cohen, J.W.; Eccelston, L.T.; Lind, C.M.; Lingappa, V.R.; Glass, P.J. Validation of a cell-based ELISA as a screening tool identifying anti-alphavirus small-molecule inhibitors. J. Virol. Methods 2013, 193, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.-F.; Wang, K.-C.; Lu, C.-Y.; Chiang, L.-C.; Shieh, D.-E.; Yen, M.-H.; Chang, J.-S. Yakammaoto inhibits enterovirus 71 infection by reducing viral attachment, internalisation, replication, and translation. Kaohsiung J. Med. Sci. 2015, 31, 293–302. [Google Scholar] [CrossRef]
- Garrison, E.; Marth, G. Haplotype-based variant detection from short read sequencing. arXiv 2012, arXiv:1207.3907. [Google Scholar] [CrossRef]
- Knyazev, S.; Tsyvina, V.; Shankar, A.; Melnyk, A.; Artyomenko, A.; Malygina, T.; Porozov, Y.B.; Campbell, E.M.; Switzer, W.M.; Skums, P.; et al. Accurate assembly of minority viral haplotypes from next-generation sequencing through efficient noise reduction. Nucleic Acids Res. 2021, 49, e102. [Google Scholar] [CrossRef]
- Peersen, O.B. Picornaviral polymerase structure, function, and fidelity modulation. Virus Res. 2017, 234, 4–20. [Google Scholar] [CrossRef]
- Domingo, E.; Sheldon, J.; Perales, C. Viral quasispecies evolution. Microbiol. Mol. Biol. Rev. 2012, 76, 159–216. [Google Scholar] [CrossRef]
- Dolan, P.T.; Whitfield, Z.J.; Andino, R. Mapping the evolutionary potential of RNA viruses. Cell Host Microbe 2018, 23, 435–446. [Google Scholar] [CrossRef] [PubMed]
- van der Sanden, S.; Koopmans, M.; Uslu, G.; van der Avoort, H.; Dutch Working Group for Clinical Virology. Epidemiology of enterovirus 71 in the Netherlands, 1963 to 2008. J. Clin. Microbiol. 2009, 47, 2826–2833. [Google Scholar] [CrossRef]
- Zhang, Y.-X.; Huang, Y.-M.; Li, Q.-J.; Li, X.-Y.; Zhou, Y.-D.; Guo, F.; Zhou, J.-M.; Can, S. A highly conserved amino acid in VP1 regulates maturation of enterovirus 71. PLoS Pathog. 2017, 13, e1006625. [Google Scholar] [CrossRef] [PubMed]
- Tee, K.K.; Lam, T.T.; Chan, Y.F.; Bible, J.M.; Kamarulzaman, A.; Tong, C.Y.; Takebe, Y.; Pybus, O.G. Evolutionary genetics of human enterovirus 71: Origin, population dynamics, natural selection, and seasonal periodicity of the VP1 gene. J. Virol. 2010, 84, 3339–3350. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, C.; Suzuki, T.; Kotani, O.; Iwata-Yoshikawa, N.; Nagata, N.; Ami, Y.; Wakita, T.; Nishimura, Y.; Shimizu, H. The role of VP1 amino acid residue 145 of enterovirus 71 in viral fitness and pathogenesis in a cynomolgus monkey model. PLoS Pathog. 2015, 11, e1005033. [Google Scholar] [CrossRef]
- Kobayashi, K.; Sudaka, Y.; Takashino, A.; Imura, A.; Fujii, K.; Koike, S. Amino acid variation at VP1-145 of enterovirus 71 determines attachment receptor usage and neurovirulence in human scavenger receptor B2 transgenic mice. J. Virol. 2018, 92, e00681-18. [Google Scholar] [CrossRef]
- Yang, S.L.; Chou, Y.T.; Wu, C.N.; Ho, M.S. Annexin II binds to casid protein VP1 of enterovirus 71 and enhances viral infectivity. J. Virol. 2011, 85, 11809–11820. [Google Scholar] [CrossRef]
- Zhou, D.; Zhao, Y.; Kotecha, A.; Fry, E.E.; Kelly, J.T.; Wang, X.; Rao, Z.; Rowlands, D.J.; Ren, J.; Stuart, D.I. Unexpected mode of engagement between enterovirus 71 and its receptor SCARB2. Nat. Microbiol. 2019, 4, 414–419. [Google Scholar] [CrossRef]
- Vignuzzi, M.; López, C.B. Defective viral genomes are key drivers of the virus-host interaction. Nat. Microbiol. 2019, 4, 1075–1087. [Google Scholar] [CrossRef]
- Gonçalves-Carneiro, D.; Mastrocola, E.; Lei, X.; DaSilva, J.; Chan, Y.F.; Bieniasz, P.D. Rational attenuation of RNA viruses with zinc finger antiviral protein. Nat. Microbiol. 2022, 7, 1558–1567. [Google Scholar] [CrossRef]
- Tang, W.-F.; Yang, S.-Y.; Wu, B.-W.; Jheng, J.-R.; Chen, Y.-L.; Shih, C.-H.; Lin, K.-H.; Lai, H.-C.; Tang, P.; Horng, J.-T. Reticulon 3 binds the 2C protein of enterovirus 71 and is required for viral replication. J. Biol. Chem. 2017, 282, 5888–5898. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-H.; Wang, K.; Zhao, K.; Hua, S.-C.; Du, J. The structure, function, and mechanisms of action of enterovirus non-structural protein 2C. Front. Microbiol. 2020, 11, 615965. [Google Scholar] [CrossRef] [PubMed]
- Gong, P.; Peersen, O.B. Structural basis for active site closure by the poliovirus RNA-dependent RNA polymerase. Proc. Natl. Acad. Sci. USA 2010, 107, 22505–22510. [Google Scholar] [CrossRef]
- Ambros, V.; Baltimore, D. Protein is linked to the 5′ end of poliovirus RNA bt a phosphodiester linkage to tyrosine. J. Biol. Chem. 1980, 253, 5263–5266. [Google Scholar] [CrossRef]
- Mhaindarkar, D.; Raphael, G.; Lupilov, N.; Hofmann, E.; Leichert, L.I. Loss of a conserved slat bridge in bacterial glycosyl hydrolase BgIM-G1 improves substrate binding in temperate environments. Commun. Biol. 2018, 1, 171. [Google Scholar] [CrossRef]
- Harrus, D.; Ahmed-El-Sayed, N.; Simister, P.C.; Miller, S.; Triconnet, M.; Hagedorn, C.H.; Mahias, K.; Rey, F.A.; Astier-Gin, T.; Bressanelli, S. Further insights into the roles of GTP and the C terminus of the Hepatitis C virus polymerase in the initiation of RNA synthesis. J. Biol. Chem. 2010, 285, 32906–32918. [Google Scholar] [CrossRef]
- Richards, O.C.; Baker, S.; Ehrenfeld, E. Mutation of lysine residues in the nucleotide binding segments of the poliovirus RNA-dependent RNA polymerase. J. Virol. 1996, 70, 8564–8570. [Google Scholar] [CrossRef] [PubMed]
- Sholders, A.J.; Peersen, O.B. Distinct conformations of a putative translocation element in poliovirus polymerase. J. Mol. Biol. 2014, 426, 1407–1419. [Google Scholar] [CrossRef]
- Yuan, J.; Shen, L.; Wu, J.; Zou, X.; Gu, J.; Chen, J.; Mao, L. Enterovirus A71 proteins: Structure and function. Front. Microbiol. 2018, 9, 286. [Google Scholar] [CrossRef]
- Yeh, M.T.; Wang, S.W.; Yu, C.K.; Lin, K.H.; Lei, H.Y.; Su, I.J.; Wang, J.R. A single nucleotide in stem loop II of 5′-untranslated region contributes to virulence of enterovirus 71 in mice. PLoS ONE 2011, 6, e27082. [Google Scholar] [CrossRef]
- Li, P.; Yue, Y.; Song, N.; Li, B.; Meng, H.; Yang, G.; Li, Z.; An, L.; Qin, L. Genome analysis of enterovirus 71 strains differing in mouse pathogenicity. Virus Genes 2016, 52, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Lyu, K.; Ding, J.; Han, J.F.; Zhang, Y.; Wu, X.Y.; He, Y.L.; Qin, C.F.; Chen, R. Human enterovirus 71 uncoating captured at atomic resolution. J. Virol. 2014, 88, 3114–3126. [Google Scholar] [CrossRef] [PubMed]
- Vignuzzi, M.; Stone, J.K.; Arnold, J.J.; Cameron, C.E.; Andino, R. Quasispecies diversity determines pathogenesis through cooperative interactions in a viral population. Nature 2006, 439, 344–348. [Google Scholar] [CrossRef]



| EV-A71/WT | Hap0 | Hap1 | Hap2 | Hap3 | Hap4 | Hap5 | |
|---|---|---|---|---|---|---|---|
| EV-A71/WT | 100.00% | 99.76% | 99.78% | 99.80% | 99.78% | 99.80% | 99.76% |
| Hap0 | 99.76% | 100.00% | 99.95% | 99.88% | 99.95% | 99.93% | 99.95% |
| Hap1 | 99.78% | 99.95% | 100.00% | 99.88% | 99.97% | 99.93% | 99.95% |
| Hap2 | 99.80% | 99.88% | 99.88% | 100.00% | 99.88% | 99.92% | 99.88% |
| Hap3 | 99.78% | 99.95% | 99.97% | 99.88% | 100.00% | 99.93% | 99.95% |
| Hap4 | 99.80% | 99.93% | 99.93% | 99.92% | 99.93% | 100.00% | 99.93% |
| Hap5 | 99.76% | 99.95% | 99.95% | 99.88% | 99.95% | 99.93% | 100.00% |
| Wild-Type/ Variants | Frequency | Nucleotide Differences (Amino Acid Differences) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5′-UTR | VP4 | VP2 | VP1 | 2A | 3C | |||||||||||
| 290 | 309 | 800 (aa.18) | 1375 (aa.141) | 1664 (aa.237) | 2730 (aa.97) | 2731 (aa.97) | 2752 (aa.104) | 3151 (aa.237) | 3177 (aa.246) | 3285 (aa.282) | 3315 (aa.292) | 3543 (aa.71) | 5825 (aa.144) | 5939 (aa.182) | ||
| EV-A71/WT | - | U | C | A (Ser) | C (Thr) | U (Thr) | C (Leu) | U (Leu) | A (Asn) | C (Thr) | C (Pro) | A (Asn) | A (Thr) | C (His) | A (Gly) | A (Gln) |
| Hap0 | 36.78% | • | • | G (Ser) | U (Ile) | A (Thr) | A (Thr) | C (Thr) | • | • | U (Ser) | • | G (Ala) | • | • | • |
| Hap1 | 17.07% | • | U | • | • | • | A (Thr) | C (Thr) | • | • | U (Ser) | • | G (Ala) | • | • | • |
| Hap2 | 15.83% | • | • | G (Ser) | • | • | • | • | G (Ser) | A (Asn) | • | G (Asp) | • | • | • | • |
| Hap3 | 9.89% | C | • | • | • | • | A (Thr) | C (Thr) | • | • | U (Ser) | • | G (Ala) | • | • | • |
| Hap4 | 9.64% | • | • | G (Ser) | • | • | A (Ile) | U (Ile) | • | • | U (Ser) | • | • | U (Tyr) | • | • |
| Hap5 | 6.86% | • | • | G (Ser) | • | • | A (Thr) | C (Thr) | • | • | U (Ser) | • | G (Ala) | • | G (Gly) | G (Gln) |
| Unaccounted SNV pairs | 3.93% | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Plaque Variants | Haplotypes | |||||
|---|---|---|---|---|---|---|
| EV-A71/Hap0 | EV-A71/Hap1 | EV-A71/Hap2 | EV-A71/Hap3 | EV-A71/Hap4 | EV-A71/Hap5 | |
| EV-A71/SP | 99.80% | 99.82% | 99.92% | 99.85% | 99.84% | 99.80% |
| EV-A71/MP | 99.82% | 99.85% | 99.95% | 99.88% | 99.87% | 99.82% |
| EV-A71/BP | 99.76% | 99.78% | 99.82% | 99.78% | 99.80% | 99.76% |
| EV-A71/HP | 99.74% | 99.77% | 99.81% | 99.77% | 99.78% | 99.74% |
| Plaque Variants | Haplotypes | |||||
|---|---|---|---|---|---|---|
| EV-A71/Hap0 | EV-A71/Hap1 | EV-A71/Hap2 | EV-A71/Hap3 | EV-A71/Hap4 | EV-A71/Hap5 | |
| EV-A71/VS | 99.78% | 99.81% | 99.77% | 99.81% | 99.82% | 99.78% |
| EV-A71/VM | 99.72% | 99.74% | 99.70% | 99.74% | 99.76% | 99.72% |
| EV-A71/WT | EV-A71/SP | |
|---|---|---|
| RMSD/Å | 0.00 | 0.6457 |
| Hydrogen bond interactions | 390 | 414 |
| Helix | 42.0% | 40.5% |
| Sheet | 15.8% | 15.2% |
| Turn | 11.0% | 12.3% |
| Coil | 30.3% | 31.2% |
| 310 helix | 0.9% | 0.9% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koh, J.X.; Masomian, M.; Anasir, M.I.; Ong, S.-K.; Poh, C.L. Insights into In Vitro Adaptation of EV71 and Analysis of Reduced Virulence by In Silico Predictions. Vaccines 2023, 11, 629. https://doi.org/10.3390/vaccines11030629
Koh JX, Masomian M, Anasir MI, Ong S-K, Poh CL. Insights into In Vitro Adaptation of EV71 and Analysis of Reduced Virulence by In Silico Predictions. Vaccines. 2023; 11(3):629. https://doi.org/10.3390/vaccines11030629
Chicago/Turabian StyleKoh, Jia Xuen, Malihe Masomian, Mohd Ishtiaq Anasir, Seng-Kai Ong, and Chit Laa Poh. 2023. "Insights into In Vitro Adaptation of EV71 and Analysis of Reduced Virulence by In Silico Predictions" Vaccines 11, no. 3: 629. https://doi.org/10.3390/vaccines11030629
APA StyleKoh, J. X., Masomian, M., Anasir, M. I., Ong, S.-K., & Poh, C. L. (2023). Insights into In Vitro Adaptation of EV71 and Analysis of Reduced Virulence by In Silico Predictions. Vaccines, 11(3), 629. https://doi.org/10.3390/vaccines11030629








