Therapeutic Vaccination in Head and Neck Squamous Cell Carcinoma—A Review
Abstract
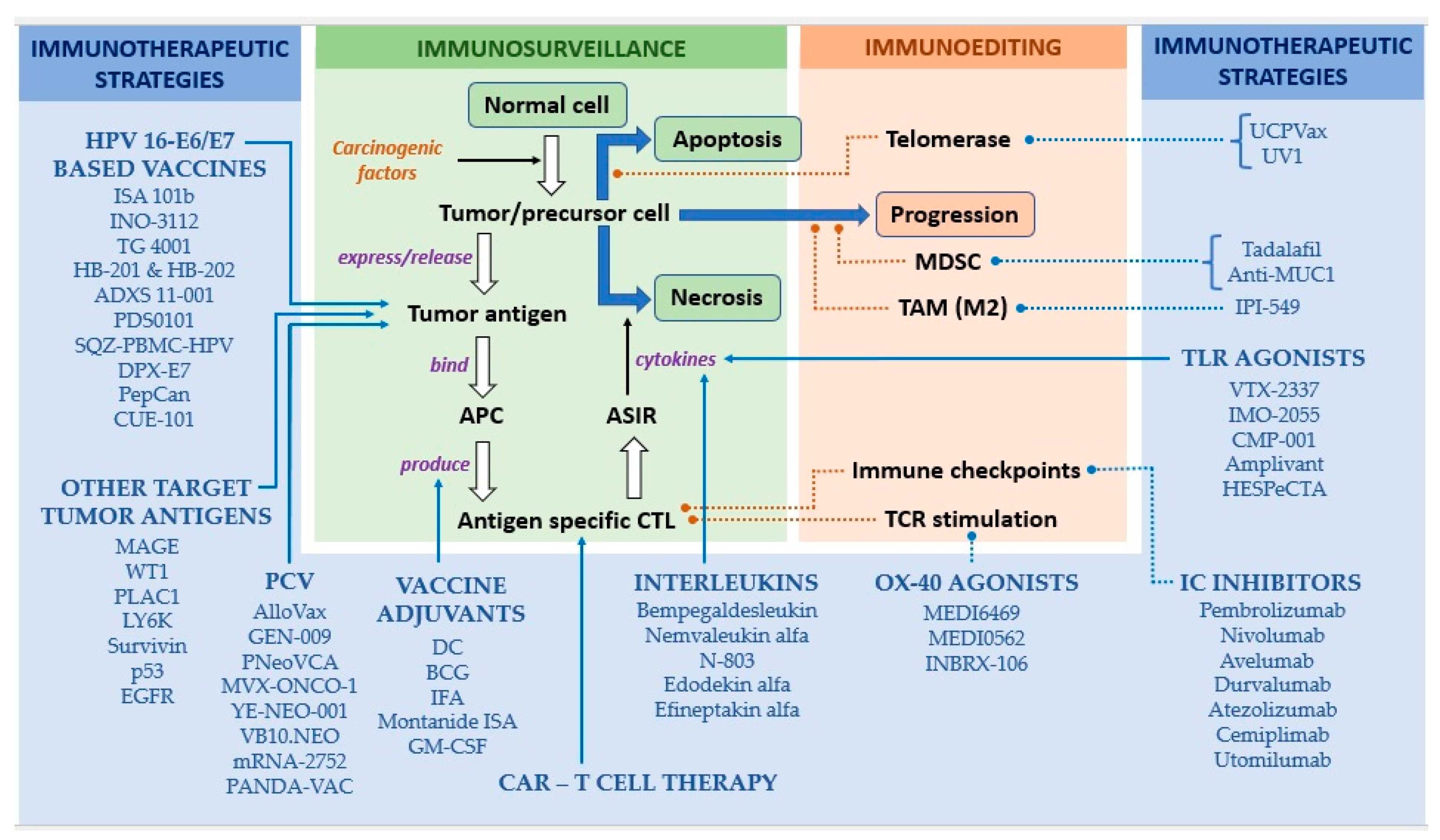
:1. Introduction
2. Basic Principles of Therapeutic Vaccines
2.1. Mechanisms of Action
2.2. Functional Units
2.2.1. Tumor Antigens—Most Widely Used Targets of Therapeutic Vaccines
2.2.2. Vehicles/Platforms of Vaccine Delivery
2.2.3. Vaccine Adjuvants
3. Approaches to and Apparatus of Therapeutic Vaccination in HNSCC
3.1. HPV Infection as the Target for Therapeutic Vaccines
3.1.1. Vaccines Targeting HPV-16 E6/E7
ISI 101b
INO-3112
TG4001
HB-201 and HB-202
ADXS 11-001
PDS0101
SQZ-PBMC-HPV
Other HPV-16 E7/E6 vaccines
3.1.2. Other Targets Related to HPV Infection
p16_37-63
HARE-40
3.2. Other Virus-Related Immunotherapeutic Mechanisms in the Treatment of HNSCC
3.2.1. Vaccination Strategies for NPC
3.2.2. Oncolytic Viruses in HNSCC
3.3. Non-Viral Tumor Antigens in HNSCC
3.3.1. Whole Tumor Cells as a Source of Tumor Antigens
3.3.2. CTA
MAGE
WT1
PLAC1
LY6K
3.3.3. Other TAA in HNSCC
Survivin
p53
EGFR
3.4. Countering the Immunosuppressive TME of HNSCC
3.4.1. Suppression of MDSC
3.4.2. Anti-MUC1 Vaccine
3.4.3. Remodeling the TAM
3.4.4. Telomerase
3.5. Co-Stimulation/Modulation of Anti-Tumor Immunity
3.5.1. OX40 Agonists
3.5.2. TLR Agonists
3.5.3. IL
IL-2
IL-15
3.6. Personalized Medicine in HNSCC
3.6.1. Neoantigen-Based Individualized Therapeutic Vaccines
3.6.2. Personalized T Cell Therapies
4. Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Network Comprehensive Genomic Characterization of Head and Neck Squamous Cell Carcinomas. Nature 2015, 517, 576–582. [CrossRef] [PubMed] [Green Version]
- Devaraja, K.; Aggarwal, S.; Verma, S.S.; Gupta, S.C. Clinico-Pathological Peculiarities of Human Papilloma Virus Driven Head and Neck Squamous Cell Carcinoma: A Comprehensive Update. Life Sci. 2020, 245, 117383. [Google Scholar] [CrossRef] [PubMed]
- Mito, I.; Takahashi, H.; Kawabata-Iwakawa, R.; Ida, S.; Tada, H.; Chikamatsu, K. Comprehensive Analysis of Immune Cell Enrichment in the Tumor Microenvironment of Head and Neck Squamous Cell Carcinoma. Sci. Rep. 2021, 11, 16134. [Google Scholar] [CrossRef]
- Canning, M.; Guo, G.; Yu, M.; Myint, C.; Groves, M.W.; Byrd, J.K.; Cui, Y. Heterogeneity of the Head and Neck Squamous Cell Carcinoma Immune Landscape and Its Impact on Immunotherapy. Front. Cell Dev. Biol. 2019, 7, 52. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Li, S.; Zhang, T.; Cui, F.; Shi, J.-H.; Zhao, F.; Sheng, X. Characterization of Molecular Subtypes in Head and Neck Squamous Cell Carcinoma With Distinct Prognosis and Treatment Responsiveness. Front. Cell Dev. Biol. 2021, 9, 711348. [Google Scholar] [CrossRef]
- Devaraja, K. Current Prospects of Molecular Therapeutics in Head and Neck Squamous Cell Carcinoma. Pharm. Med. 2019, 33, 269–289. [Google Scholar] [CrossRef]
- Duan, Q.; Zhang, H.; Zheng, J.; Zhang, L. Turning Cold into Hot: Firing up the Tumor Microenvironment. Trends Cancer 2020, 6, 605–618. [Google Scholar] [CrossRef]
- Wei, C.; Ma, Y.; Wang, F.; Liao, Y.; Chen, Y.; Zhao, B.; Zhao, Q.; Wang, D.; Tang, D. Igniting Hope for Tumor Immunotherapy: Promoting the “Hot and Cold” Tumor Transition. Clin. Med. Insights Oncol. 2022, 16, 11795549221120708. [Google Scholar] [CrossRef]
- Coley, W.B. The Treatment of Inoperable Sarcoma by Bacterial Toxins (the Mixed Toxins of the Streptococcus Erysipelas and the Bacillus Prodigiosus). Proc. R. Soc. Med. 1910, 3, 1–48. [Google Scholar] [CrossRef] [Green Version]
- Burnet, M. Cancer— A Biological Approach: I. The Processes of Control. Br. Med. J. 1957, 1, 779–786. [Google Scholar] [CrossRef]
- Thomas, L.; Lawrence, H. Cellular and Humoral Aspects of the Hypersensitive States; Hoeber-Harper: New York, NY, USA, 1959; pp. 529–532. [Google Scholar]
- Miller, J.F. Immunological Function of the Thymus. Lancet 1961, 2, 748–749. [Google Scholar] [CrossRef]
- Miller, J.F.; Mitchell, G.F.; Weiss, N.S. Cellular Basis of the Immunological Defects in Thymectomized Mice. Nature 1967, 214, 992–997. [Google Scholar] [CrossRef]
- Burnet, M. Role of the Thymus and Related Organs in Immunity. Br. Med. J. 1962, 2, 807–811. [Google Scholar] [CrossRef] [Green Version]
- Claman, H.N.; Chaperon, E.A.; Triplett, R.F. Thymus-Marrow Cell Combinations. Synergism in Antibody Production. Proc. Soc. Exp. Biol. Med. 1966, 122, 1167–1171. [Google Scholar] [CrossRef]
- Wagner, H.; Röllinghoff, M.; Nossal, G.J. T-Cell-Mediated Immune Responses Induced in Vitro: A Probe for Allograft and Tumor Immunity. Transpl. Rev. 1973, 17, 3–36. [Google Scholar] [CrossRef]
- Song, Q.; Zhang, C.-D.; Wu, X.-H. Therapeutic Cancer Vaccines: From Initial Findings to Prospects. Immunol. Lett. 2018, 196, 11–21. [Google Scholar] [CrossRef]
- Shibata, H.; Xu, N.; Saito, S.; Zhou, L.; Ozgenc, I.; Webb, J.; Fu, C.; Zolkind, P.; Egloff, A.M.; Uppaluri, R. Integrating CD4+ T Cell Help for Therapeutic Cancer Vaccination in a Preclinical Head and Neck Cancer Model. Oncoimmunology 2021, 10, 1958589. [Google Scholar] [CrossRef]
- Brunet, J.F.; Denizot, F.; Luciani, M.F.; Roux-Dosseto, M.; Suzan, M.; Mattei, M.G.; Golstein, P. A New Member of the Immunoglobulin Superfamily--CTLA-4. Nature 1987, 328, 267–270. [Google Scholar] [CrossRef]
- Linsley, P.S.; Wallace, P.M.; Johnson, J.; Gibson, M.G.; Greene, J.L.; Ledbetter, J.A.; Singh, C.; Tepper, M.A. Immunosuppression in Vivo by a Soluble Form of the CTLA-4 T Cell Activation Molecule. Science 1992, 257, 792–795. [Google Scholar] [CrossRef]
- Iwai, Y.; Ishida, M.; Tanaka, Y.; Okazaki, T.; Honjo, T.; Minato, N. Involvement of PD-L1 on Tumor Cells in the Escape from Host Immune System and Tumor Immunotherapy by PD-L1 Blockade. Proc. Natl. Acad. Sci. USA 2002, 99, 12293–12297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, H.; Strome, S.E.; Salomao, D.R.; Tamura, H.; Hirano, F.; Flies, D.B.; Roche, P.C.; Lu, J.; Zhu, G.; Tamada, K.; et al. Tumor-Associated B7-H1 Promotes T-Cell Apoptosis: A Potential Mechanism of Immune Evasion. Nat. Med. 2002, 8, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.S.; Mellman, I. Elements of Cancer Immunity and the Cancer-Immune Set Point. Nature 2017, 541, 321–330. [Google Scholar] [CrossRef]
- Tan, Y.S.; Sansanaphongpricha, K.; Xie, Y.; Donnelly, C.R.; Luo, X.; Heath, B.R.; Zhao, X.; Bellile, E.; Hu, H.; Chen, H.; et al. Mitigating SOX2-Potentiated Immune Escape of Head and Neck Squamous Cell Carcinoma with a STING-Inducing Nanosatellite Vaccine. Clin. Cancer Res. 2018, 24, 4242–4255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kather, J.N.; Suarez-Carmona, M.; Charoentong, P.; Weis, C.-A.; Hirsch, D.; Bankhead, P.; Horning, M.; Ferber, D.; Kel, I.; Herpel, E.; et al. Topography of Cancer-Associated Immune Cells in Human Solid Tumors. eLife 2018, 7, e36967. [Google Scholar] [CrossRef]
- Troiano, G.; Rubini, C.; Togni, L.; Caponio, V.C.A.; Zhurakivska, K.; Santarelli, A.; Cirillo, N.; Lo Muzio, L.; Mascitti, M. The Immune Phenotype of Tongue Squamous Cell Carcinoma Predicts Early Relapse and Poor Prognosis. Cancer Med. 2020, 9, 8333–8344. [Google Scholar] [CrossRef]
- Coulie, P.G.; Van den Eynde, B.J.; van der Bruggen, P.; Boon, T. Tumour Antigens Recognized by T Lymphocytes: At the Core of Cancer Immunotherapy. Nat. Rev. Cancer 2014, 14, 135–146. [Google Scholar] [CrossRef]
- Zarour, H.M.; DeLeo, A.; Finn, O.J.; Storkus, W.J. Categories of Tumor Antigens. In Holland-Frei Cancer Medicine, 6th ed.; BC Decker: Hamilton, ON, Canda, 2003. [Google Scholar]
- Kosaka, A.; Yajima, Y.; Hatayama, M.; Ikuta, K.; Sasaki, T.; Hirai, N.; Yasuda, S.; Nagata, M.; Hayashi, R.; Harabuchi, S.; et al. A Stealth Antigen SPESP1, Which Is Epigenetically Silenced in Tumors, Is a Suitable Target for Cancer Immunotherapy. Cancer Sci. 2021, 112, 2705–2713. [Google Scholar] [CrossRef]
- Yajima, Y.; Kosaka, A.; Ishibashi, K.; Yasuda, S.; Komatsuda, H.; Nagato, T.; Oikawa, K.; Kitada, M.; Takekawa, M.; Kumai, T.; et al. A Tumor Metastasis-Associated Molecule TWIST1 Is a Favorable Target for Cancer Immunotherapy Due to Its Immunogenicity. Cancer Sci. 2022, 113, 2526–2535. [Google Scholar] [CrossRef]
- Butterfield, L.H. Lessons Learned from Cancer Vaccine Trials and Target Antigen Choice. Cancer Immunol. Immunother. 2016, 65, 805–812. [Google Scholar] [CrossRef] [Green Version]
- Cheever, M.A.; Allison, J.P.; Ferris, A.S.; Finn, O.J.; Hastings, B.M.; Hecht, T.T.; Mellman, I.; Prindiville, S.A.; Viner, J.L.; Weiner, L.M.; et al. The Prioritization of Cancer Antigens: A National Cancer Institute Pilot Project for the Acceleration of Translational Research. Clin. Cancer Res. 2009, 15, 5323–5337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inaba, K.; Inaba, M.; Romani, N.; Aya, H.; Deguchi, M.; Ikehara, S.; Muramatsu, S.; Steinman, R.M. Generation of Large Numbers of Dendritic Cells from Mouse Bone Marrow Cultures Supplemented with Granulocyte/Macrophage Colony-Stimulating Factor. J. Exp. Med. 1992, 176, 1693–1702. [Google Scholar] [CrossRef] [PubMed]
- Young, J.W.; Szabolcs, P.; Moore, M.A. Identification of Dendritic Cell Colony-Forming Units among Normal Human CD34+ Bone Marrow Progenitors That Are Expanded by c-Kit-Ligand and Yield Pure Dendritic Cell Colonies in the Presence of Granulocyte/Macrophage Colony-Stimulating Factor and Tumor Necrosis Factor Alpha. J. Exp. Med. 1995, 182, 1111–1119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saxena, M.; Bhardwaj, N. Turbocharging Vaccines: Emerging Adjuvants for Dendritic Cell Based Therapeutic Cancer Vaccines. Curr. Opin. Immunol. 2017, 47, 35–43. [Google Scholar] [CrossRef] [PubMed]
- de Souza Apostólico, J.; Lunardelli, V.A.S.; Coirada, F.C.; Boscardin, S.B.; Rosa, D.S. Adjuvants: Classification, Modus Operandi, and Licensing. J. Immunol. Res. 2016, 2016, 1459394. [Google Scholar] [CrossRef] [Green Version]
- Firdaus, F.Z.; Skwarczynski, M.; Toth, I. Developments in Vaccine Adjuvants. Methods Mol. Biol. 2022, 2412, 145–178. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, Z.; Aria, H.; Ghaedrahmati, F.; Bakhtiari, T.; Azizi, M.; Bastan, R.; Hosseini, R.; Eskandari, N. An Update on Human Papilloma Virus Vaccines: History, Types, Protection, and Efficacy. Front. Immunol. 2021, 12, 805695. [Google Scholar] [CrossRef] [PubMed]
- Chabeda, A.; Yanez, R.J.R.; Lamprecht, R.; Meyers, A.E.; Rybicki, E.P.; Hitzeroth, I.I. Therapeutic Vaccines for High-Risk HPV-Associated Diseases. Papillomavirus Res. 2018, 5, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Massarelli, E.; William, W.; Johnson, F.; Kies, M.; Ferrarotto, R.; Guo, M.; Feng, L.; Lee, J.J.; Tran, H.; Kim, Y.U.; et al. Combining Immune Checkpoint Blockade and Tumor-Specific Vaccine for Patients With Incurable Human Papillomavirus 16-Related Cancer: A Phase 2 Clinical Trial. JAMA Oncol. 2019, 5, 67–73. [Google Scholar] [CrossRef] [Green Version]
- Aggarwal, C.; Cohen, R.B.; Morrow, M.P.; Kraynyak, K.A.; Sylvester, A.J.; Knoblock, D.M.; Bauml, J.M.; Weinstein, G.S.; Lin, A.; Boyer, J.; et al. Immunotherapy Targeting HPV16/18 Generates Potent Immune Responses in HPV-Associated Head and Neck Cancer. Clin. Cancer Res. 2019, 25, 110–124. [Google Scholar] [CrossRef] [Green Version]
- Tourneau, C.L.; Delord, J.-P.; Cassier, P.; Loirat, D.; Tavernaro, A.; Bastien, B.; Bendjama, K. Phase Ib/II Trial of TG4001 (Tipapkinogene Sovacivec), a Therapeutic HPV-Vaccine, and Avelumab in Patients with Recurrent/Metastatic (R/M) HPV-16+ Cancers. Ann. Oncol. 2019, 30, v494–v495. [Google Scholar] [CrossRef]
- Fu, S.; Nabell, L.; Pearson, A.T.; Leidner, R.; Adkins, D.; Posner, M.R.; Nieva, J.J.; Richardson, D.L.; Pimentel, A.; Goel, S.; et al. Recommended Phase 2 Dose (RP2D) of HB-200 Arenavirus-Based Cancer Immunotherapies in Patients with HPV16+ Cancers. J. Clin. Oncol. 2022, 40, 2517. [Google Scholar] [CrossRef]
- Krupar, R.; Imai, N.; Miles, B.; Genden, E.; Misiukiewicz, K.; Saenger, Y.; Demicco, E.G.; Patel, J.; Herrera, P.C.; Parikh, F.; et al. Abstract LB-095: HPV E7 Antigen-Expressing Listeria-Based Immunotherapy (ADXS11-001) Prior to Robotic Surgery for HPV-Positive Oropharyngeal Cancer Enhances HPV-Specific T Cell Immunity. Cancer Res. 2016, 76, LB-095. [Google Scholar] [CrossRef]
- Jimeno, A.; Baranda, J.C.; Mita, M.M.; Gordon, M.S.; Taylor, M.H.; Iams, W.T.; Janku, F.; Matulonis, U.A.; Bernstein, H.; Loughhead, S.; et al. Initial Results of a First-in-Human, Dose Escalation Study of a Cell-Based Vaccine in HLA A*02+ Patients (Pts) with Recurrent, Locally Advanced or Metastatic HPV16+ Solid Tumors: SQZ-PBMC-HPV-101. J. Clin. Oncol. 2021, 39, 2536. [Google Scholar] [CrossRef]
- Chung, C.H.; Colevas, A.D.; Adkins, D.; Gibson, M.K.; Rodriguez, C.P.; Sukari, A.; Bauman, J.E.; Wirth, L.J.; Johnson, F.M.; Saba, N.F.; et al. A Phase 1 Dose-Escalation and Expansion Study of CUE-101, a Novel HPV16 E7-PHLA-IL2-Fc Fusion Protein, given Alone and in Combination with Pembrolizumab in Patients with Recurrent/Metastatic HPV16+ Head and Neck Cancer. J. Clin. Oncol. 2022, 40, 6045. [Google Scholar] [CrossRef]
- Reuschenbach, M.; Pauligk, C.; Karbach, J.; Rafiyan, M.-R.; Kloor, M.; Prigge, E.-S.; Sauer, M.; Al-Batran, S.-E.; Kaufmann, A.M.; Schneider, A.; et al. A Phase 1/2a Study to Test the Safety and Immunogenicity of a P16(INK4a) Peptide Vaccine in Patients with Advanced Human Papillomavirus-Associated Cancers. Cancer 2016, 122, 1425–1433. [Google Scholar] [CrossRef]
- Zandberg, D.P.; Rollins, S.; Goloubeva, O.; Morales, R.E.; Tan, M.; Taylor, R.; Wolf, J.S.; Schumaker, L.M.; Cullen, K.J.; Zimrin, A.; et al. A Phase I Dose Escalation Trial of MAGE-A3- and HPV16-Specific Peptide Immunomodulatory Vaccines in Patients with Recurrent/Metastatic (RM) Squamous Cell Carcinoma of the Head and Neck (SCCHN). Cancer Immunol. Immunother. 2015, 64, 367–379. [Google Scholar] [CrossRef]
- Schuler, P.J.; Harasymczuk, M.; Visus, C.; Deleo, A.; Trivedi, S.; Lei, Y.; Argiris, A.; Gooding, W.; Butterfield, L.H.; Whiteside, T.L.; et al. Phase I Dendritic Cell P53 Peptide Vaccine for Head and Neck Cancer. Clin. Cancer Res. 2014, 20, 2433–2444. [Google Scholar] [CrossRef] [Green Version]
- Ferris, R. Adjuvant P53 Peptide Loaded DC-Based Therapy for Subjects with Squamous Cell Cancer of the Head and Neck (A Phase I Safety and Immunogenicity Trial); U.S. National Library of Medicine: Bethesda, MD, USA, 2016. Available online: https://clinicaltrials.gov/ct2/show/NCT00404339 (accessed on 26 November 2022).
- Chung, V.; Kos, F.J.; Hardwick, N.; Yuan, Y.; Chao, J.; Li, D.; Waisman, J.; Li, M.; Zurcher, K.; Frankel, P.; et al. Evaluation of Safety and Efficacy of P53MVA Vaccine Combined with Pembrolizumab in Patients with Advanced Solid Cancers. Clin. Transl. Oncol. 2019, 21, 363–372. [Google Scholar] [CrossRef]
- Califano, J.A.; Khan, Z.; Noonan, K.A.; Rudraraju, L.; Zhang, Z.; Wang, H.; Goodman, S.; Gourin, C.G.; Ha, P.K.; Fakhry, C.; et al. Tadalafil Augments Tumor Specific Immunity in Patients with Head and Neck Squamous Cell Carcinoma. Clin. Cancer Res. 2015, 21, 30–38. [Google Scholar] [CrossRef] [Green Version]
- Weed, D.T.; Vella, J.L.; Reis, I.M.; De la Fuente, A.C.; Gomez, C.; Sargi, Z.; Nazarian, R.; Califano, J.; Borrello, I.; Serafini, P. Tadalafil Reduces Myeloid-Derived Suppressor Cells and Regulatory T Cells and Promotes Tumor Immunity in Patients with Head and Neck Squamous Cell Carcinoma. Clin. Cancer Res. 2015, 21, 39–48. [Google Scholar] [CrossRef] [Green Version]
- Weed, D.T.; Zilio, S.; Reis, I.M.; Sargi, Z.; Abouyared, M.; Gomez-Fernandez, C.R.; Civantos, F.J.; Rodriguez, C.P.; Serafini, P. The Reversal of Immune Exclusion Mediated by Tadalafil and an Anti-Tumor Vaccine Also Induces PDL1 Upregulation in Recurrent Head and Neck Squamous Cell Carcinoma: Interim Analysis of a Phase I Clinical Trial. Front. Immunol. 2019, 10, 1206. [Google Scholar] [CrossRef]
- Sullivan, R.J.; Hong, D.S.; Tolcher, A.W.; Patnaik, A.; Shapiro, G.; Chmielowski, B.; Ribas, A.; Brail, L.H.; Roberts, J.; Lee, L.; et al. Initial Results from First-in-Human Study of IPI-549, a Tumor Macrophage-Targeting Agent, Combined with Nivolumab in Advanced Solid Tumors. J. Clin. Oncol. 2018, 36, 3013. [Google Scholar] [CrossRef]
- Duhen, R.; Ballesteros-Merino, C.; Frye, A.K.; Tran, E.; Rajamanickam, V.; Chang, S.-C.; Koguchi, Y.; Bifulco, C.B.; Bernard, B.; Leidner, R.S.; et al. Neoadjuvant Anti-OX40 (MEDI6469) Therapy in Patients with Head and Neck Squamous Cell Carcinoma Activates and Expands Antigen-Specific Tumor-Infiltrating T Cells. Nat. Commun. 2021, 12, 1047. [Google Scholar] [CrossRef] [PubMed]
- Dietsch, G.N.; Lu, H.; Yang, Y.; Morishima, C.; Chow, L.Q.; Disis, M.L.; Hershberg, R.M. Coordinated Activation of Toll-Like Receptor8 (TLR8) and NLRP3 by the TLR8 Agonist, VTX-2337, Ignites Tumoricidal Natural Killer Cell Activity. PLoS ONE 2016, 11, e0148764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chow, L.Q.M.; Morishima, C.; Eaton, K.D.; Baik, C.S.; Goulart, B.H.; Anderson, L.N.; Manjarrez, K.L.; Dietsch, G.N.; Bryan, J.K.; Hershberg, R.M.; et al. Phase Ib Trial of the Toll-like Receptor 8 Agonist, Motolimod (VTX-2337), Combined with Cetuximab in Patients with Recurrent or Metastatic SCCHN. Clin. Cancer Res. 2017, 23, 2442–2450. [Google Scholar] [CrossRef] [Green Version]
- Ferris, R.L.; Saba, N.F.; Gitlitz, B.J.; Haddad, R.; Sukari, A.; Neupane, P.; Morris, J.C.; Misiukiewicz, K.; Bauman, J.E.; Fenton, M.; et al. Effect of Adding Motolimod to Standard Combination Chemotherapy and Cetuximab Treatment of Patients With Squamous Cell Carcinoma of the Head and Neck: The Active8 Randomized Clinical Trial. JAMA Oncol. 2018, 4, 1583–1588. [Google Scholar] [CrossRef] [Green Version]
- Ruzsa, A.; Sen, M.; Evans, M.; Lee, L.W.; Hideghety, K.; Rottey, S.; Klimak, P.; Holeckova, P.; Fayette, J.; Csoszi, T.; et al. Phase 2, Open-Label, 1:1 Randomized Controlled Trial Exploring the Efficacy of EMD 1201081 in Combination with Cetuximab in Second-Line Cetuximab-Naïve Patients with Recurrent or Metastatic Squamous Cell Carcinoma of the Head and Neck (R/M SCCHN). Investig. New Drugs 2014, 32, 1278–1284. [Google Scholar] [CrossRef] [PubMed]
- Speetjens, F.M.; Welters, M.J.P.; Slingerland, M.; van Poelgeest, M.I.E.; de Vos van Steenwijk, P.J.; Roozen, I.; Boekestijn, S.; Loof, N.M.; Zom, G.G.; Valentijn, A.R.P.M.; et al. Intradermal Vaccination of HPV-16 E6 Synthetic Peptides Conjugated to an Optimized Toll-like Receptor 2 Ligand Shows Safety and Potent T Cell Immunogenicity in Patients with HPV-16 Positive (Pre-)Malignant Lesions. J. Immunother. Cancer 2022, 10, e005016. [Google Scholar] [CrossRef] [PubMed]
- Gastman, B.; Cheever, M.; Fling, S.; Perez, C.; Patel, M.; Geiger, J.; Li, Z.; Posner, M.; Steuer, C.; D’Amico, L.; et al. 432 Nemvaleukin Alfa, a Novel Engineered IL-2 Cytokine, in Combination with the Anti-PD-1 Antibody Pembrolizumab in Patients with Recurrent/Metastatic Head and Neck Squamous Cell Carcinoma (ION-01 Study). J. Immunother. Cancer 2021, 9, A462. [Google Scholar] [CrossRef]
- Miller, J.S.; Morishima, C.; McNeel, D.G.; Patel, M.R.; Kohrt, H.E.K.; Thompson, J.A.; Sondel, P.M.; Wakelee, H.A.; Disis, M.L.; Kaiser, J.C.; et al. A First-in-Human Phase I Study of Subcutaneous Outpatient Recombinant Human IL15 (RhIL15) in Adults with Advanced Solid Tumors. Clin. Cancer Res. 2018, 24, 1525–1535. [Google Scholar] [CrossRef] [Green Version]
- Margolin, K.; Morishima, C.; Velcheti, V.; Miller, J.S.; Lee, S.M.; Silk, A.W.; Holtan, S.G.; Lacroix, A.M.; Fling, S.P.; Kaiser, J.C.; et al. Phase I Trial of ALT-803, A Novel Recombinant IL15 Complex, in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2018, 24, 5552–5561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wrangle, J.M.; Awad, M.M.; Badin, F.B.; Rubinstein, M.P.; Bhar, P.; Garner, C.; Reddy, S.K.; Soon-Shiong, P. Preliminary Data from QUILT 3.055: A Phase 2 Multi-Cohort Study of N803 (IL-15 Superagonist) in Combination with Checkpoint Inhibitors (CPI). J. Clin. Oncol. 2021, 39, 2596. [Google Scholar] [CrossRef]
- Wolf, G.T.; Fee, W.E.; Dolan, R.W.; Moyer, J.S.; Kaplan, M.J.; Spring, P.M.; Suen, J.; Kenady, D.E.; Newman, J.G.; Carroll, W.R.; et al. Novel Neoadjuvant Immunotherapy Regimen Safety and Survival in Head and Neck Squamous Cell Cancer. Head Neck 2011, 33, 1666–1674. [Google Scholar] [CrossRef] [Green Version]
- McMichael, E.L.; Benner, B.; Atwal, L.S.; Courtney, N.B.; Mo, X.; Davis, M.E.; Campbell, A.R.; Duggan, M.C.; Williams, K.; Martin, K.; et al. A Phase I/II Trial of Cetuximab in Combination with Interleukin-12 Administered to Patients with Unresectable Primary or Recurrent Head and Neck Squamous Cell Carcinoma. Clin. Cancer Res. 2019, 25, 4955–4965. [Google Scholar] [CrossRef] [PubMed]
- Chindavijak, S.; Har-Noy, M.; Lausoontornsiri, W. Effect of Therapeutic Vaccine on CTLA4 and Tumor Debulking Response in Recurrent and Metastatic HNSCC. J. Clin. Oncol. 2018, 36, 115. [Google Scholar] [CrossRef]
- Gillison, M.L.; Awad, M.M.; Twardowski, P.; Sukari, A.; Johnson, M.L.; Stein, M.N.; Hernandez, R.; Price, J.; Mancini, K.J.; Shainheit, M.; et al. Long Term Results from a Phase 1 Trial of GEN-009, a Personalized Neoantigen Vaccine, Combined with PD-1 Inhibition in Advanced Solid Tumors. J. Clin. Oncol. 2021, 39, 2613. [Google Scholar] [CrossRef]
- Fernandez, E.; Vernet, R.; Charrier, E.; Migliorini, D.; Joerger, M.; Belkouch, M.-C.; Urwyler, M.; Von Rohr, O.; Saingier, V.; Ancrenaz, V.; et al. MVX-ONCO-1 in Advanced Refractory Cancers: Safety, Feasibility, and Preliminary Efficacy Results from All HNSCC Patients Treated in Two Ongoing Clinical Trials. J. Clin. Oncol. 2021, 39, e18005. [Google Scholar] [CrossRef]
- Patel, M.R.; Bauer, T.M.; Jimeno, A.; Wang, D.; LoRusso, P.; Do, K.T.; Stemmer, S.M.; Maurice-Dror, C.; Geva, R.; Zacharek, S.; et al. A Phase I Study of MRNA-2752, a Lipid Nanoparticle Encapsulating MRNAs Encoding Human OX40L, IL-23, and IL-36γ, for Intratumoral (ITu) Injection Alone and in Combination with Durvalumab. J. Clin. Oncol. 2020, 38, 3092. [Google Scholar] [CrossRef]
- De Sousa, L.G.; Rajapakshe, K.; Rodriguez Canales, J.; Chin, R.L.; Feng, L.; Wang, Q.; Barrese, T.Z.; Massarelli, E.; William, W.; Johnson, F.M.; et al. ISA101 and Nivolumab for HPV-16+ Cancer: Updated Clinical Efficacy and Immune Correlates of Response. J. Immunother. Cancer 2022, 10, e004232. [Google Scholar] [CrossRef]
- FDA Gives ISA101b Fast Track Designation for HPV 16+ Oropharyngeal Cancer. Available online: https://www.cancernetwork.com/view/fda-gives-isa101b-fast-track-designation-for-hpv-16-oropharyngeal-cancer (accessed on 18 December 2022).
- Edwards, D.; Schwendinger, M.; Katchar, K.; Schlienger, K.; Orlinger, K.; Matushansky, I.; Lauterbach, H. Abstract 3284: HB-201 and HB-202, an Arenavirus-Based Immunotherapy, Induces Tumor T Cell Infiltration in Patients with HNSCC and Other HPV16+ Tumors. Cancer Res. 2022, 82, 3284. [Google Scholar] [CrossRef]
- Sikora, A. Window of Opportunity Trial of Neoadjuvant ADXS 11-001 Vaccination Prior to Robot-Assisted Resection of HPV-Positive Oropharyngeal Squamous Cell Carcinoma; U.S. National Library of Medicine: Bethesda, MD, USA, 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT02002182 (accessed on 4 December 2022).
- Schneider, K.; Grønhøj, C.; Hahn, C.H.; von Buchwald, C. Therapeutic Human Papillomavirus Vaccines in Head and Neck Cancer: A Systematic Review of Current Clinical Trials. Vaccine 2018, 36, 6594–6605. [Google Scholar] [CrossRef]
- Maciag, P.C.; Radulovic, S.; Rothman, J. The First Clinical Use of a Live-Attenuated Listeria Monocytogenes Vaccine: A Phase I Safety Study of Lm-LLO-E7 in Patients with Advanced Carcinoma of the Cervix. Vaccine 2009, 27, 3975–3983. [Google Scholar] [CrossRef]
- Miles, B.; Safran, H.P.; Monk, B.J. Therapeutic Options for Treatment of Human Papillomavirus-Associated Cancers—Novel Immunologic Vaccines: ADXS11–001. Gynecol. Oncol. Res. Pr. 2017, 4, 10. [Google Scholar] [CrossRef] [Green Version]
- Smalley Rumfield, C.; Pellom, S.T.; Morillon Ii, Y.M.; Schlom, J.; Jochems, C. Immunomodulation to Enhance the Efficacy of an HPV Therapeutic Vaccine. J. Immunother. Cancer 2020, 8, e000612. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute (NCI). Phase I/II Trial of Combination Immunotherapy in Subjects with Advanced HPV Associated Malignancies; U.S. National Library of Medicine: Bethesda, MD, USA, 2022.
- Wood, L.; Chintakuntlawar, A.V.; Price, K.; Kaczmar, J.; Conn, G.; Bedu-Addo, F.K.; Weiss, J. Preliminary Safety of PDS0101 (Versamune +HPVmix) and Pembrolizumab Combination Therapy in Subjects with Recurrent/Metastatic Human Papillomavirus-16 Positive Oropharyngeal Squamous Cell Carcinoma (OPSCC). Int. J. Radiat. Oncol. Biol. Phys. 2022, 112, e37–e38. [Google Scholar] [CrossRef]
- Weiss, J.; Chintakuntlawar, A.V.; Price, K.A.R.; Kaczmar, J.M.; Riebel, N.; Bedu-Addo, F.K.; Chaney, M.F.; Wood, L.V. PDS0101, a Novel Type I Interferon and CD8 T-Cell Activating Immunotherapy, in Combination with Pembrolizumab in Subjects with Recurrent/Metastatic HPV16-Positive Head and Neck Squamous Cell Carcinoma (HNSCC). J. Clin. Oncol. 2022, 40, 6041. [Google Scholar] [CrossRef]
- Corporation, P.B. PDS Biotechnology Granted FDA Fast Track Designation for Lead Candidate PDS0101. Available online: https://www.globenewswire.com/news-release/2022/06/02/2455153/37149/en/PDS-Biotechnology-Granted-FDA-Fast-Track-Designation-for-Lead-Candidate-PDS0101.html (accessed on 18 December 2022).
- Yang, M.-C.; Yang, A.; Qiu, J.; Yang, B.; He, L.; Tsai, Y.-C.; Jeang, J.; Wu, T.-C.; Hung, C.-F. Buccal Injection of Synthetic HPV Long Peptide Vaccine Induces Local and Systemic Antigen-Specific CD8+ T-Cell Immune Responses and Antitumor Effects without Adjuvant. Cell Biosci. 2016, 6, 17. [Google Scholar] [CrossRef] [Green Version]
- Karkada, M.; Quinton, T.; Blackman, R.; Mansour, M. Tumor Inhibition by DepoVax-Based Cancer Vaccine Is Accompanied by Reduced Regulatory/Suppressor Cell Proliferation and Tumor Infiltration. ISRN Oncol. 2013, 2013, 753427. [Google Scholar] [CrossRef]
- Wang, X.; Coleman, H.N.; Nagarajan, U.; Spencer, H.J.; Nakagawa, M. Candida Skin Test Reagent as a Novel Adjuvant for a Human Papillomavirus Peptide-Based Therapeutic Vaccine. Vaccine 2013, 31, 5806–5813. [Google Scholar] [CrossRef] [Green Version]
- Greenfield, W.W.; Stratton, S.L.; Myrick, R.S.; Vaughn, R.; Donnalley, L.M.; Coleman, H.N.; Mercado, M.; Moerman-Herzog, A.M.; Spencer, H.J.; Andrews-Collins, N.R.; et al. A Phase I Dose-Escalation Clinical Trial of a Peptide-Based Human Papillomavirus Therapeutic Vaccine with Candida Skin Test Reagent as a Novel Vaccine Adjuvant for Treating Women with Biopsy-Proven Cervical Intraepithelial Neoplasia 2/3. Oncoimmunology 2015, 4, e1031439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coleman, H.N.; Greenfield, W.W.; Stratton, S.L.; Vaughn, R.; Kieber, A.; Moerman-Herzog, A.M.; Spencer, H.J.; Hitt, W.C.; Quick, C.M.; Hutchins, L.F.; et al. Human Papillomavirus Type 16 Viral Load Is Decreased Following a Therapeutic Vaccination. Cancer Immunol. Immunother. 2016, 65, 563–573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quayle, S.N.; Girgis, N.; Thapa, D.R.; Merazga, Z.; Kemp, M.M.; Histed, A.; Zhao, F.; Moreta, M.; Ruthardt, P.; Hulot, S.; et al. CUE-101, a Novel E7-PHLA-IL2-Fc Fusion Protein, Enhances Tumor Antigen-Specific T-Cell Activation for the Treatment of HPV16-Driven Malignancies. Clin. Cancer Res. 2020, 26, 1953–1964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, J.; Harris, E.; Lorch, J. Vaccination as a Therapeutic Strategy for Nasopharyngeal Carcinoma. Oral. Oncol. 2022, 135, 106083. [Google Scholar] [CrossRef]
- Taylor, G.S.; Jia, H.; Harrington, K.; Lee, L.W.; Turner, J.; Ladell, K.; Price, D.A.; Tanday, M.; Matthews, J.; Roberts, C.; et al. A Recombinant Modified Vaccinia Ankara Vaccine Encoding Epstein-Barr Virus (EBV) Target Antigens: A Phase I Trial in UK Patients with EBV-Positive Cancer. Clin. Cancer Res. 2014, 20, 5009–5022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nemunaitis, J.; Ganly, I.; Khuri, F.; Arseneau, J.; Kuhn, J.; McCarty, T.; Landers, S.; Maples, P.; Romel, L.; Randlev, B.; et al. Selective Replication and Oncolysis in P53 Mutant Tumors with ONYX-015, an E1B-55kD Gene-Deleted Adenovirus, in Patients with Advanced Head and Neck Cancer: A Phase II Trial. Cancer Res. 2000, 60, 6359–6366. [Google Scholar]
- Khuri, F.R.; Nemunaitis, J.; Ganly, I.; Arseneau, J.; Tannock, I.F.; Romel, L.; Gore, M.; Ironside, J.; MacDougall, R.H.; Heise, C.; et al. A Controlled Trial of Intratumoral ONYX-015, a Selectively-Replicating Adenovirus, in Combination with Cisplatin and 5-Fluorouracil in Patients with Recurrent Head and Neck Cancer. Nat. Med. 2000, 6, 879–885. [Google Scholar] [CrossRef]
- Harrington, K.J.; Kong, A.; Mach, N.; Chesney, J.A.; Fernandez, B.C.; Rischin, D.; Cohen, E.E.W.; Radcliffe, H.-S.; Gumuscu, B.; Cheng, J.; et al. Talimogene Laherparepvec and Pembrolizumab in Recurrent or Metastatic Squamous Cell Carcinoma of the Head and Neck (MASTERKEY-232): A Multicenter, Phase 1b Study. Clin. Cancer Res. 2020, 26, 5153–5161. [Google Scholar] [CrossRef]
- Carr, S.; Allison, K.J.; Van De Velde, L.-A.; Zhang, K.; English, E.Y.; Iverson, A.; Daw, N.C.; Howard, S.C.; Navid, F.; Rodriguez-Galindo, C.; et al. Safety and Immunogenicity of Live Attenuated and Inactivated Influenza Vaccines in Children with Cancer. J. Infect. Dis. 2011, 204, 1475–1482. [Google Scholar] [CrossRef] [Green Version]
- Vandeborne, L.; Pantziarka, P.; Van Nuffel, A.M.T.; Bouche, G. Repurposing Infectious Diseases Vaccines Against Cancer. Front. Oncol. 2021, 11, 688755. [Google Scholar] [CrossRef]
- Newman, J.H.; Chesson, C.B.; Herzog, N.L.; Bommareddy, P.K.; Aspromonte, S.M.; Pepe, R.; Estupinian, R.; Aboelatta, M.M.; Buddhadev, S.; Tarabichi, S.; et al. Intratumoral Injection of the Seasonal Flu Shot Converts Immunologically Cold Tumors to Hot and Serves as an Immunotherapy for Cancer. Proc. Natl. Acad. Sci. USA 2020, 117, 1119–1128. [Google Scholar] [CrossRef] [Green Version]
- Chang, A.E.; Li, Q.; Jiang, G.; Teknos, T.N.; Chepeha, D.B.; Bradford, C.R. Generation of Vaccine-Primed Lymphocytes for the Treatment of Head and Neck Cancer. Head Neck 2003, 25, 198–209. [Google Scholar] [CrossRef]
- Karcher, J.; Dyckhoff, G.; Beckhove, P.; Reisser, C.; Brysch, M.; Ziouta, Y.; Helmke, B.H.; Weidauer, H.; Schirrmacher, V.; Herold-Mende, C. Antitumor Vaccination in Patients with Head and Neck Squamous Cell Carcinomas with Autologous Virus-Modified Tumor Cells. Cancer Res. 2004, 64, 8057–8061. [Google Scholar] [CrossRef] [Green Version]
- Herold-Mende, C.; Karcher, J.; Dyckhoff, G.; Schirrmacher, V. Antitumor Immunization of Head and Neck Squamous Cell Carcinoma Patients with a Virus-Modified Autologous Tumor Cell Vaccine. Adv. Otorhinolaryngol. 2005, 62, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Whiteside, T.L. Anti-Tumor Vaccines in Head and Neck Cancer: Targeting Immune Responses to the Tumor. Curr. Cancer Drug Targets 2007, 7, 633–642. [Google Scholar] [CrossRef]
- Chen, X.; Song, Q.; Xia, L.; Xu, X. Synergy of Dendritic Cell Vaccines and Avasimibe in Treatment of Head and Neck Cancer in Mice. Med. Sci. Monit 2017, 23, 4471–4476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuffel, C.; Rivals, J.-P.; Zaugg, Y.; Salvi, S.; Seelentag, W.; Speiser, D.E.; Liénard, D.; Monnier, P.; Romero, P.; Bron, L.; et al. Pattern and Clinical Significance of Cancer-Testis Gene Expression in Head and Neck Squamous Cell Carcinoma. Int. J. Cancer 2011, 128, 2625–2634. [Google Scholar] [CrossRef] [Green Version]
- Cesson, V.; Rivals, J.-P.; Escher, A.; Piotet, E.; Thielemans, K.; Posevitz, V.; Dojcinovic, D.; Monnier, P.; Speiser, D.; Bron, L.; et al. MAGE-A3 and MAGE-A4 Specific CD4(+) T Cells in Head and Neck Cancer Patients: Detection of Naturally Acquired Responses and Identification of New Epitopes. Cancer Immunol. Immunother. 2011, 60, 23–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voskens, C.J.; Sewell, D.; Hertzano, R.; DeSanto, J.; Rollins, S.; Lee, M.; Taylor, R.; Wolf, J.; Suntharalingam, M.; Gastman, B.; et al. Induction of MAGE-A3 and HPV-16 Immunity by Trojan Vaccines in Patients with Head and Neck Carcinoma. Head Neck 2012, 34, 1734–1746. [Google Scholar] [CrossRef]
- Pichichero, M.E. Improving Vaccine Delivery Using Novel Adjuvant Systems. Hum. Vaccin. 2008, 4, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Chong, C.E.; Lim, K.P.; Gan, C.P.; Marsh, C.A.; Zain, R.B.; Abraham, M.T.; Prime, S.S.; Teo, S.-H.; Silvio Gutkind, J.; Patel, V.; et al. Over-Expression of MAGED4B Increases Cell Migration and Growth in Oral Squamous Cell Carcinoma and Is Associated with Poor Disease Outcome. Cancer Lett. 2012, 321, 18–26. [Google Scholar] [CrossRef] [Green Version]
- Lim, K.P.; Chun, N.A.L.; Gan, C.P.; Teo, S.-H.; Rahman, Z.A.A.; Abraham, M.T.; Zain, R.B.; Ponniah, S.; Cheong, S.C. Identification of Immunogenic MAGED4B Peptides for Vaccine Development in Oral Cancer Immunotherapy. Hum. Vaccin. Immunother. 2014, 10, 3214–3223. [Google Scholar] [CrossRef] [Green Version]
- Chai, S.J.; Fong, S.C.Y.; Gan, C.P.; Pua, K.C.; Lim, P.V.H.; Lau, S.H.; Zain, R.B.; Abraham, T.; Ismail, S.M.; Abdul Rahman, Z.A.; et al. In Vitro Evaluation of Dual-Antigenic PV1 Peptide Vaccine in Head and Neck Cancer Patients. Hum. Vaccin. Immunother. 2019, 15, 167–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Zainal, N.S.; Chai, S.J.; Dickie, J.; Gan, C.P.; Zulaziz, N.; Lye, B.K.W.; Sutavani, R.V.; Ottensmeier, C.H.; King, E.V.; et al. DNA Vaccines Targeting Novel Cancer-Associated Antigens Frequently Expressed in Head and Neck Cancer Enhance the Efficacy of Checkpoint Inhibitor. Front. Immunol. 2021, 12, 763086. [Google Scholar] [CrossRef] [PubMed]
- Oka, Y.; Tsuboi, A.; Oji, Y.; Kawase, I.; Sugiyama, H. WT1 Peptide Vaccine for the Treatment of Cancer. Curr. Opin. Immunol. 2008, 20, 211–220. [Google Scholar] [CrossRef]
- Ogasawara, M.; Miyashita, M.; Yamagishi, Y.; Ota, S. Phase I/II Pilot Study of Wilms’ Tumor 1 Peptide-Pulsed Dendritic Cell Vaccination Combined With Conventional Chemotherapy in Patients With Head and Neck Cancer. Apher. Dial. 2019, 23, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhai, M.; Wu, Z.; Qi, Y.; Wu, Y.; Dai, C.; Sun, M.; Li, L.; Gao, Y. Identification of a Novel HLA-A2-Restricted Cytotoxic T Lymphocyte Epitope from Cancer-Testis Antigen PLAC1 in Breast Cancer. Amino Acids 2012, 42, 2257–2265. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudian, J.; Ghods, R.; Nazari, M.; Jeddi-Tehrani, M.; Ghahremani, M.H.; Ghaffari-Tabrizi-Wizsy, N.; Ostad, S.N.; Zarnani, A.-H. PLAC1: Biology and Potential Application in Cancer Immunotherapy. Cancer Immunol. Immunother. 2019, 68, 1039–1058. [Google Scholar] [CrossRef]
- Hayashi, R.; Nagato, T.; Kumai, T.; Ohara, K.; Ohara, M.; Ohkuri, T.; Hirata-Nozaki, Y.; Harabuchi, S.; Kosaka, A.; Nagata, M.; et al. Expression of Placenta-Specific 1 and Its Potential for Eliciting Anti-Tumor Helper T-Cell Responses in Head and Neck Squamous Cell Carcinoma. Oncoimmunology 2020, 10, 1856545. [Google Scholar] [CrossRef] [PubMed]
- de Nooij-van Dalen, A.G.; van Dongen, G.A.M.S.; Smeets, S.J.; Nieuwenhuis, E.J.C.; Stigter-van Walsum, M.; Snow, G.B.; Brakenhoff, R.H. Characterization of the Human Ly-6 Antigens, the Newly Annotated Member Ly-6K Included, as Molecular Markers for Head-and-Neck Squamous Cell Carcinoma. Int. J. Cancer 2003, 103, 768–774. [Google Scholar] [CrossRef]
- Guo, D.; Liu, Y.; Jiang, Y.; Zheng, S.; Xu, T.; Zhu, J.; Chen, P.; Huang, P.; Zhang, Y. A Narrative Review of the Emerging Role of Lymphocyte Antigen 6 Complex Locus K in Cancer: From Basic Research to Clinical Practice. Ann. Transl. Med. 2022, 10, 26. [Google Scholar] [CrossRef]
- Tomita, Y.; Yuno, A.; Tsukamoto, H.; Senju, S.; Kuroda, Y.; Hirayama, M.; Imamura, Y.; Yatsuda, J.; Sayem, M.A.; Irie, A.; et al. Identification of Immunogenic LY6K Long Peptide Encompassing Both CD4+ and CD8+ T-Cell Epitopes and Eliciting CD4+ T-Cell Immunity in Patients with Malignant Disease. Oncoimmunology 2014, 3, e28100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshitake, Y.; Fukuma, D.; Yuno, A.; Hirayama, M.; Nakayama, H.; Tanaka, T.; Nagata, M.; Takamune, Y.; Kawahara, K.; Nakagawa, Y.; et al. Phase II Clinical Trial of Multiple Peptide Vaccination for Advanced Head and Neck Cancer Patients Revealed Induction of Immune Responses and Improved OS. Clin. Cancer Res. 2015, 21, 312–321. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, J.; Torigoe, T.; Hirohashi, Y.; Idenoue, S.; Miyazaki, A.; Yamaguchi, A.; Hiratsuka, H.; Sato, N. Comparative Study on the Immunogenicity between an HLA-A24-Restricted Cytotoxic T-Cell Epitope Derived from Survivin and That from Its Splice Variant Survivin-2B in Oral Cancer Patients. J. Transl. Med. 2009, 7, 1. [Google Scholar] [CrossRef] [Green Version]
- Miyazaki, A.; Kobayashi, J.; Torigoe, T.; Hirohashi, Y.; Yamamoto, T.; Yamaguchi, A.; Asanuma, H.; Takahashi, A.; Michifuri, Y.; Nakamori, K.; et al. Phase I Clinical Trial of Survivin-Derived Peptide Vaccine Therapy for Patients with Advanced or Recurrent Oral Cancer. Cancer Sci. 2011, 102, 324–329. [Google Scholar] [CrossRef] [PubMed]
- DeLeo, A.B.; Whiteside, T.L. Development of Multi-Epitope Vaccines Targeting Wild-Type Sequence P53 Peptides. Expert Rev. Vaccines 2008, 7, 1031–1040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, S.; Fan, C.; Zeng, Z.; Young, K.H.; Li, Y. Clinical and Immunological Effects of P53-Targeting Vaccines. Front. Cell Dev. Biol. 2021, 9, 762796. [Google Scholar] [CrossRef]
- Zaravinos, A. An Updated Overview of HPV-Associated Head and Neck Carcinomas. Oncotarget 2014, 5, 3956–3969. [Google Scholar] [CrossRef] [Green Version]
- Yang, B.-B.; Jiang, H.; Chen, J.; Zhang, X.; Ye, J.-J.; Cao, J. Dendritic Cells Pulsed with GST-EGFR Fusion Protein: Effect in Antitumor Immunity against Head and Neck Squamous Cell Carcinoma. Head Neck 2010, 32, 626–635. [Google Scholar] [CrossRef]
- Farlow, J.L.; Brenner, J.C.; Lei, Y.L.; Chinn, S.B. Immune Deserts in Head and Neck Squamous Cell Carcinoma: A Review of Challenges and Opportunities for Modulating the Tumor Immune Microenvironment. Oral. Oncol. 2021, 120, 105420. [Google Scholar] [CrossRef] [PubMed]
- Elmusrati, A.; Wang, J.; Wang, C.-Y. Tumor Microenvironment and Immune Evasion in Head and Neck Squamous Cell Carcinoma. Int. J. Oral. Sci. 2021, 13, 24. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.M.Y.; Krinsky, A.L.; Woolaver, R.A.; Wang, X.; Chen, Z.; Wang, J.H. Tumor Immune Microenvironment in Head and Neck Cancers. Mol. Carcinog. 2020, 59, 766–774. [Google Scholar] [CrossRef]
- Yu, C.; Li, Q.; Zhang, Y.; Wen, Z.-F.; Dong, H.; Mou, Y. Current Status and Perspective of Tumor Immunotherapy for Head and Neck Squamous Cell Carcinoma. Front. Cell Dev. Biol. 2022, 10, 941750. [Google Scholar] [CrossRef] [PubMed]
- Parmar, K.; Mohamed, A.; Vaish, E.; Thawani, R.; Cetnar, J.; Thein, K.Z. Immunotherapy in Head and Neck Squamous Cell Carcinoma: An Updated Review. Cancer Treat. Res. Commun. 2022, 33, 100649. [Google Scholar] [CrossRef] [PubMed]
- Sarfati, M.; Mateo, V.; Baudet, S.; Rubio, M.; Fernandez, C.; Davi, F.; Binet, J.-L.; Delic, J.; Merle-Beral, H. Sildenafil and Vardenafil, Types 5 and 6 Phosphodiesterase Inhibitors, Induce Caspase-Dependent Apoptosis of B-Chronic Lymphocytic Leukemia Cells. Blood 2003, 101, 265–269. [Google Scholar] [CrossRef] [Green Version]
- Serafini, P.; Meckel, K.; Kelso, M.; Noonan, K.; Califano, J.; Koch, W.; Dolcetti, L.; Bronte, V.; Borrello, I. Phosphodiesterase-5 Inhibition Augments Endogenous Antitumor Immunity by Reducing Myeloid-Derived Suppressor Cell Function. J. Exp. Med. 2006, 203, 2691–2702. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Liang, D.; Zhu, Y.; Xu, W.; Zhou, K.; Liu, L.; Liu, S.; Yang, W. Prognostic and Clinicopathological Significance of MUC Expression in Head and Neck Cancer: A Systematic Review and Meta-Analysis. Oncotarget 2017, 8, 96359–96372. [Google Scholar] [CrossRef] [Green Version]
- Han, S.; Wang, W.; Wang, S.; Yang, T.; Zhang, G.; Wang, D.; Ju, R.; Lu, Y.; Wang, H.; Wang, L. Tumor Microenvironment Remodeling and Tumor Therapy Based on M2-like Tumor Associated Macrophage-Targeting Nano-Complexes. Theranostics 2021, 11, 2892–2916. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, X.; Hao, H. Macrophage Phenotype-Switching in Cancer. Eur. J. Pharm. 2022, 931, 175229. [Google Scholar] [CrossRef]
- Chen, J.; Li, M.; Yang, C.; Yin, X.; Duan, K.; Wang, J.; Feng, B. Macrophage Phenotype Switch by Sequential Action of Immunomodulatory Cytokines from Hydrogel Layers on Titania Nanotubes. Colloids Surf. B Biointerfaces 2018, 163, 336–345. [Google Scholar] [CrossRef]
- De Henau, O.; Rausch, M.; Winkler, D.; Campesato, L.F.; Liu, C.; Cymerman, D.H.; Budhu, S.; Ghosh, A.; Pink, M.; Tchaicha, J.; et al. Overcoming Resistance to Checkpoint Blockade Therapy by Targeting PI3Kγ in Myeloid Cells. Nature 2016, 539, 443–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Dakic, A.; Chen, R.; Disbrow, G.L.; Zhang, Y.; Dai, Y.; Schlegel, R. Cell-Restricted Immortalization by Human Papillomavirus Correlates with Telomerase Activation and Engagement of the HTERT Promoter by Myc. J. Virol. 2008, 82, 11568–11576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, X.; Beck, S.; Sohn, Y.-W.; Kim, J.-K.; Kim, S.-H.; Yin, J.; Pian, X.; Kim, S.-C.; Choi, Y.-J.; Kim, H. Human Telomerase Catalytic Subunit (HTERT) Suppresses P53-Mediated Anti-Apoptotic Response via Induction of Basic Fibroblast Growth Factor. Exp. Mol. Med. 2010, 42, 574–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Roberts, J.; Dakic, A.; Zhang, Y.; Schlegel, R. HPV E7 Contributes to the Telomerase Activity of Immortalized and Tumorigenic Cells and Augments E6-Induced HTERT Promoter Function. Virology 2008, 375, 611–623. [Google Scholar] [CrossRef] [Green Version]
- Veldman, T.; Horikawa, I.; Barrett, J.C.; Schlegel, R. Transcriptional Activation of the Telomerase HTERT Gene by Human Papillomavirus Type 16 E6 Oncoprotein. J. Virol. 2001, 75, 4467–4472. [Google Scholar] [CrossRef] [Green Version]
- Godet, Y.; Fabre, E.; Dosset, M.; Lamuraglia, M.; Levionnois, E.; Ravel, P.; Benhamouda, N.; Cazes, A.; Le Pimpec-Barthes, F.; Gaugler, B.; et al. Analysis of Spontaneous Tumor-Specific CD4 T-Cell Immunity in Lung Cancer Using Promiscuous HLA-DR Telomerase-Derived Epitopes: Potential Synergistic Effect with Chemotherapy Response. Clin. Cancer Res. 2012, 18, 2943–2953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rebucci-Peixoto, M.; Vienot, A.; Adotevi, O.; Jacquin, M.; Ghiringhelli, F.; de la Fouchardière, C.; You, B.; Maurina, T.; Kalbacher, E.; Bazan, F.; et al. A Phase II Study Evaluating the Interest to Combine UCPVax, a Telomerase CD4 TH1-Inducer Cancer Vaccine, and Atezolizumab for the Treatment of HPV Positive Cancers: VolATIL Study. Front. Oncol. 2022, 12, 957580. [Google Scholar] [CrossRef]
- Yadav, R.; Redmond, W.L. Current Clinical Trial Landscape of OX40 Agonists. Curr. Oncol. Rep. 2022, 24, 951–960. [Google Scholar] [CrossRef]
- Curti, B.D.; Kovacsovics-Bankowski, M.; Morris, N.; Walker, E.; Chisholm, L.; Floyd, K.; Walker, J.; Gonzalez, I.; Meeuwsen, T.; Fox, B.A.; et al. OX40 Is a Potent Immune-Stimulating Target in Late-Stage Cancer Patients. Cancer Res. 2013, 73, 7189–7198. [Google Scholar] [CrossRef] [Green Version]
- Glisson, B.S.; Leidner, R.S.; Ferris, R.L.; Powderly, J.; Rizvi, N.A.; Keam, B.; Schneider, R.; Goel, S.; Ohr, J.P.; Burton, J.; et al. Safety and Clinical Activity of MEDI0562, a Humanized OX40 Agonist Monoclonal Antibody, in Adult Patients with Advanced Solid Tumors. Clin. Cancer Res. 2020, 26, 5358–5367. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Kawai, T.; Akira, S. Toll-like Receptors and Innate Immunity. Biochem. Biophys. Res. Commun. 2009, 388, 621–625. [Google Scholar] [CrossRef]
- Sabree, S.A.; Voigt, A.P.; Blackwell, S.E.; Vishwakarma, A.; Chimenti, M.S.; Salem, A.K.; Weiner, G.J. Direct and Indirect Immune Effects of CMP-001, a Virus-like Particle Containing a TLR9 Agonist. J. Immunother. Cancer 2021, 9, e002484. [Google Scholar] [CrossRef] [PubMed]
- Zom, G.G.; Khan, S.; Britten, C.M.; Sommandas, V.; Camps, M.G.M.; Loof, N.M.; Budden, C.F.; Meeuwenoord, N.J.; Filippov, D.V.; van der Marel, G.A.; et al. Efficient Induction of Antitumor Immunity by Synthetic Toll-like Receptor Ligand-Peptide Conjugates. Cancer Immunol. Res. 2014, 2, 756–764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zom, G.G.; Willems, M.M.J.H.P.; Khan, S.; van der Sluis, T.C.; Kleinovink, J.W.; Camps, M.G.M.; van der Marel, G.A.; Filippov, D.V.; Melief, C.J.M.; Ossendorp, F. Novel TLR2-Binding Adjuvant Induces Enhanced T Cell Responses and Tumor Eradication. J. Immunother. Cancer 2018, 6, 146. [Google Scholar] [CrossRef] [Green Version]
- Pinette, A.; McMichael, E.; Courtney, N.B.; Duggan, M.; Benner, B.N.; Choueiry, F.; Yu, L.; Abood, D.; Mace, T.A.; Carson, W.E. An IL-15-Based Superagonist ALT-803 Enhances the NK Cell Response to Cetuximab-Treated Squamous Cell Carcinoma of the Head and Neck. Cancer Immunol. Immunother. 2019, 68, 1379–1389. [Google Scholar] [CrossRef]
- Redman, J.M.; Friedman, J.; Robbins, Y.; Sievers, C.; Yang, X.; Lassoued, W.; Sinkoe, A.; Papanicolau-Sengos, A.; Lee, C.-C.; Marte, J.L.; et al. Enhanced Neoepitope-Specific Immunity Following Neoadjuvant PD-L1 and TGF-β Blockade in HPV-Unrelated Head and Neck Cancer. J. Clin. Investig. 2022, 132, e161400. [Google Scholar] [CrossRef] [PubMed]
- Berinstein, N.L.; Wolf, G.T.; Naylor, P.H.; Baltzer, L.; Egan, J.E.; Brandwein, H.J.; Whiteside, T.L.; Goldstein, L.C.; El-Naggar, A.; Badoual, C.; et al. Increased Lymphocyte Infiltration in Patients with Head and Neck Cancer Treated with the IRX-2 Immunotherapy Regimen. Cancer Immunol. Immunother. 2012, 61, 771–782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vormehr, M.; Türeci, Ö.; Sahin, U. Harnessing Tumor Mutations for Truly Individualized Cancer Vaccines. Annu. Rev. Med. 2019, 70, 395–407. [Google Scholar] [CrossRef]
- Srikrishna, D.; Sachsenmeier, K. We Need to Bring R0 < 1 to Treat Cancer Too. Genome Med. 2021, 13, 120. [Google Scholar] [CrossRef]
- Kananathan, R.; Hospet, C.; Kasim, J.; Noy, M.H.; Lim, T.O. 78P Novel Allogeneic Cell Immunotherapy for Advanced Cancers. Ann. Oncol. 2020, 31, S1272. [Google Scholar] [CrossRef]
- Guedan, S.; Ruella, M.; June, C.H. Emerging Cellular Therapies for Cancer. Annu. Rev. Immunol. 2019, 37, 145–171. [Google Scholar] [CrossRef] [PubMed]
- Pan, K.; Farrukh, H.; Chittepu, V.C.S.R.; Xu, H.; Pan, C.-X.; Zhu, Z. CAR Race to Cancer Immunotherapy: From CAR T, CAR NK to CAR Macrophage Therapy. J. Exp. Clin. Cancer Res. 2022, 41, 119. [Google Scholar] [CrossRef]
- Richardson, N.H.; Luttrell, J.B.; Bryant, J.S.; Chamberlain, D.; Khawaja, S.; Neeli, I.; Radic, M. Tuning the Performance of CAR T Cell Immunotherapies. BMC Biotechnol. 2019, 19, 84. [Google Scholar] [CrossRef]
- Figueroa, J.A.; Reidy, A.; Mirandola, L.; Trotter, K.; Suvorava, N.; Figueroa, A.; Konala, V.; Aulakh, A.; Littlefield, L.; Grizzi, F.; et al. Chimeric Antigen Receptor Engineering: A Right Step in the Evolution of Adoptive Cellular Immunotherapy. Int. Rev. Immunol. 2015, 34, 154–187. [Google Scholar] [CrossRef]
- Andersen, R.; Donia, M.; Ellebaek, E.; Borch, T.H.; Kongsted, P.; Iversen, T.Z.; Hölmich, L.R.; Hendel, H.W.; Met, Ö.; Andersen, M.H.; et al. Long-Lasting Complete Responses in Patients with Metastatic Melanoma after Adoptive Cell Therapy with Tumor-Infiltrating Lymphocytes and an Attenuated IL2 Regimen. Clin. Cancer Res. 2016, 22, 3734–3745. [Google Scholar] [CrossRef] [Green Version]
- Damasio, M.P.S.; Nascimento, C.S.; Andrade, L.M.; de Oliveira, V.L.; Calzavara-Silva, C.E. The Role of T-Cells in Head and Neck Squamous Cell Carcinoma: From Immunity to Immunotherapy. Front. Oncol. 2022, 12, 1021609. [Google Scholar] [CrossRef] [PubMed]
- Mei, Z.; Zhang, K.; Lam, A.K.-Y.; Huang, J.; Qiu, F.; Qiao, B.; Zhang, Y. MUC1 as a Target for CAR-T Therapy in Head and Neck Squamous Cell Carinoma. Cancer Med. 2020, 9, 640–652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silveira, C.R.F.; Corveloni, A.C.; Caruso, S.R.; Macêdo, N.A.; Brussolo, N.M.; Haddad, F.; Fernandes, T.R.; de Andrade, P.V.; Orellana, M.D.; Guerino-Cunha, R.L. Cytokines as an Important Player in the Context of CAR-T Cell Therapy for Cancer: Their Role in Tumor Immunomodulation, Manufacture, and Clinical Implications. Front. Immunol. 2022, 13, 947648. [Google Scholar] [CrossRef]
- Tan, Y.S.; Sansanaphongpricha, K.; Prince, M.E.P.; Sun, D.; Wolf, G.T.; Lei, Y.L. Engineering Vaccines to Reprogram Immunity against Head and Neck Cancer. J. Dent. Res. 2018, 97, 627–634. [Google Scholar] [CrossRef]
| Platforms | Peculiar Characteristics |
| Autologous tumor cell vaccines |
|
| Allogenic whole tumor cell vaccine |
|
| DC vaccines |
|
| Peptide vaccines |
|
| DNA vaccines |
|
| RNA vaccines |
|
| Viral vaccines |
|
| Sl No | Vaccine Name | Constituents | Platform | Trial Number | Initiated | Phase | Adjuvant or Combination | Patient Profile | Enrolment | Status |
| 1. Targeting HPV associated antigens | ||||||||||
| 1 | ISA 101b | SLP derived from HPV-16 E6 and E7 | Peptide | NCT02426892 | Dec 2015 | II | Nivolumab | incurable HPV-16+ OPSSC | 22/24 * | Completed [41] |
| NCT03258008 | Apr 2018 | II | Utomilumab | R/M/P checkpoint naïve HPV+ OPSCC | 3/27 | Terminated | ||||
| NCT03669718 | Nov 2018 | II | Cemiplimab | HPV-16+ R/M OPSCC | 194 | Recruiting | ||||
| NCT04369937 | Jul 2020 | II | Pembrolizumab + Cisplatin-based chemoradiotherapy | Treatment naïve HPV-16+ LA HNSCC | 50 (e) | Recruiting | ||||
| NCT04398524 | Jul 2021 | II | Cemiplimab | HPV-16+ R/M OPSCC | 86 (e) | Recruiting | ||||
| 2 | INO-3112 | DNA plasmid against HPV-16 and -18 E6 and E7 antigens | DNA | NCT02163057 | Aug 2014 | I/IIa | (IL-12) | HPV-16/-18 + HNSCC | 22 | Completed [42] |
| NCT03162224 | Jun 2017 | Ib/IIa | Durvalumab | R/M HPV-16/-18 + HNSCC | 35/50 | Terminated | ||||
| NCT04001413 | Sep 2019 | II | Durvalumab | HPV 16+ OPSCC | 0 | Withdrawn | ||||
| 3 | TG 4001 | MVA -express HPV-16 E6 and E7 + cytokine, IL-2 | Viral vector | NCT03260023 | Sep 2017 | Ib/II | Avelumab | HPV-16 + R/M cancers, including HNSCC | 150 (e) | Recruiting (interim report) [43] |
| 4 | HB-201 and HB-202 | TheraT vector(s) expressing HPV16 E7E6 | Viral vector | NCT04180215 | Dec 2019 | I/II | Pembrolizumab | HPV-16+ R/M cancers including HNSCC | 200 (e) | Recruiting (interim report) [44] |
| 5 | ADXS 11-001 | Attenuated Lm -LLO, engineered to secrete an HPV-E7 tumor antigen | Bacterial vector | NCT01598792 | Feb 2012 | I | - | HPV-16+ OPSCC | 2 | Terminated |
| NCT02002182 | Dec 2013 | WOT | Neoadjuvant vaccine before TORS | HPV+ OPSCC | 15/30 | Completed (interim report) [45] | ||||
| NCT02291055 | Apr 2015 | I/II | Durvalumab | LA/M cervical cancers or HPV + HNSCC | 66 (e) | Unknown | ||||
| 6 | PDS0101 | Liposomal-based HPV-16 E6/E7 multipeptide vaccine | Peptide (nano-particle based) | NCT04287868 | Jun 2020 | I/II | NHS-IL12 + Bintrafusp alfa | LA/M HPV+ Cancers including OPSCC | 51 | Active, not recruiting |
| NCT04260126 | Mar 2021 | II | Pembrolizumab | R/M HPV-16+ HNSCC | 95 (e) | Recruiting | ||||
| NCT05232851 | Mar 2022 | I/II | Pembrolizumab | HPV+ LA OPSCC | 24 (e) | Recruiting | ||||
| 7 | SQZ-PBMC-HPV | Generated from PBMC squeezed with HPV16 E6 and E7 antigens | Autologous | NCT04084951 | Jan 2020 | I | Atezolizumab or Other ICI | HPV16+ LA or R/M Solid Tumors | 200 (e) | Recruiting (interim report) [46] |
| 8 | DPX-E7 | HPV16-E711-19 nanomer | Peptide | NCT02865135 | Dec 2016 | Ib/II | - | R/M HPV-16+ HNSCC, cervical Ca, anal Ca | 11 | Active, not recruiting |
| 9 | PepCan | Four synthetic peptides covering HPV16E6 | Peptide | NCT03821272 | Nov 2019 | I/II | Candin | Previously treated HNSCC patients who are in remission | 20 (e) | Recruiting |
| 10 | CUE-101 | HPV16 E7 peptide epitope (E711–20) +IL-2 | Peptide | NCT03978689 | Jul 2019 | I | Pembrolizumab | HPV16+ R/M HNSCC | 85 (e) | Recruiting (interim results) [47] |
| 11 | HARE-40 | HPV Anti-CD40 RNA Vaccine | RNA | NCT03418480 | Apr 2017 | I/II | - | Previously treated disease-free HPV16+ cancers | 44 (e) | Recruiting |
| 12 | p16_37-63 peptide vaccine | 27-amino-acid-long p16(INK4a)-based peptide vaccine | Peptide | NCT01462838 | Aug 2011 | I/II | Montanide ISA-51 | R/M HPV+ cancers including HNSCC | 26 | Prematurely terminated [48] |
| NCT02526316 | Jun 2015 | I | Montanide ISA-51 + Cisplatin based chemotherapy +/-radiotherapy | HPV+ cancers including HNSCC | 11 | Completed | ||||
| 2. Targeting non-viral tumor antigens | ||||||||||
| 13 | Trojan vaccines | MAGE-A3 or HPV-16 derived peptides | Peptide | NCT00257738 | Nov 2005 | I | Montanide ISA 51 and GM-CSF | P/R/M HNSCC | 16/90 * | Completed [49] |
| 14 | p53-specific autologous DC -based vaccine | Peptide | NCT00404339 | Sep 2005 | I | DC | LA HNSCC after treatment | 16/50 * | Completed [50,51] | |
| 15 | p53MVA vaccine | Viral vector | NCT02432963 | Jun 2016 | I | Pembrolizumab | LA or R/M Solid cancers | 11/19 * | Active, not recruiting [52] | |
| 16 | CIMAvax | Recombinant human EGF-rP64K | Peptide | NCT02955290 | Dec 2016 | I/II | Montanide ISA 51 + Nivolumab | Metastatic NSCLC or HNSCC | 193 (e) | Recruiting |
| 3. Targeting TME | ||||||||||
| 17 | Tadalafil | PDE-5 inhibitor | NCT00894413 | May 2007 | II | - | Newly diagnosed or recurrent HNSCC | 45 | Completed [53] | |
| NCT00843635 | Sep 2008 | I | Surgery | OSCC or OPSCC undergoing Surgery | 35 | Completed [54] | ||||
| NCT01697800 | Sep 2012 | II | Conventional therapy | Newly diagnosed or recurrent HNSCC | 40 | Completed | ||||
| NCT02544880 | Apr 2016 | I/II | Anti-MUC1 | Resectable recurrent or second primary HNSCC | 14/16 | Completed [55] | ||||
| 18 | UCPVax | Universal cancer peptides derived from hTERT | NCT03946358 | Feb 2020 | II | Atezolizumab | HPV+ cancers including HNSCC | 47 (e) | Recruiting | |
| 19 | UV1 | Three SLPs from hTERT | NCT05075122 | Aug 2021 | II | Sargramostim | R/M PDL1+ HSNCC | 75 (e) | Recruiting | |
| 20 | IPI-549 | A specific PI3Kγ inhibitor | NCT02637531 | Dec 2015 | I/Ib | Nivolumab | LA or metastatic solid tumors, including HNSCC | 219 | Active, not recruiting (interim report) [56] | |
| NCT03795610 | Mar 2020 | II | Surgery | LAHNSCC undergoing surgical excision | 15 (e) | Recruiting | ||||
| 4. Co-stimulation strategies | ||||||||||
| 21 | MEDI6469 | Murine anti-human OX40 agonist antibody | NCT02274155 | Oct 2014 | Ib | Surgery | Surgically resectable LA HNSCC | 17 | Completed [57] | |
| MEDI0562 | Humanized OX40 agonist | NCT02318394 | Mar 2015 | I | - | Heavily pre-treated solid tumors, including HNSCC | 55 | Completed | ||
| NCT03336606 | Jul 2018 | Ib | Surgery | Surgically resectable HNSCC or melanoma | 35 (e) | Active, not recruiting | ||||
| INBRX-106 | Hexavalent OX40 agonist antibody | NCT04198766 | Dec 2019 | I | Pembrolizumab | LA or metastatic solid tumors, including HNSCC | 200 (e) | Recruiting | ||
| 22 | VTX-2337 | TLR8-agonist | NCT01334177 | Jun 2011 | I | Cetuximab | LA or R/M HNSCC | 13 | Completed [58,59] | |
| NCT01836029 | Oct 2013 | II | EXTREME regimen | R/M HNSCC | 195 | Completed [60] | ||||
| 23 | IMO-2055 | TLR 9-agonist | NCT01040832 | Dec 2009 | II | Cetuximab | Cetuximab-naïve subjects with R/M HNSCC | 107 | Completed [61] | |
| NCT01360827 | Aug 2010 | Ib | 5-FU/Cisplatin and Cetuximab | R/M HNSCC | 13 | Terminated | ||||
| 24 | CMP-001 | VLP-containing TLR 9 agonist | NCT04633278 | Nov 2020 | II | Pembrolizumab | R/M HNSCC | 24 | Active, not recruiting | |
| 25 | HESPeCTA | HPV E six peptides conjugated to amplivant | SLP | NCT02821494 | Mar 2015 | I | - | HPV+ tumors or premalignant conditions | 25 | Completed [62] |
| 26 | NKTR-214 | IL-2 agonist | Protein | NCT04052204 | Dec 2019 | Ib/II | Avelumab plus Talazoparib or Enzalutamide | LA or R/M HNSCC and mCRPC | 3 | Terminated |
| NCT04936841 | Aug 2021 | II | Radiotherapy and pembrolizumab | R/M HNSCC | 5 | Active, not recruiting | ||||
| 27 | ALKS 4230 | IL-2 and extracellular domain of CD25 | NCT04144517 | Feb 2020 | II | Pembrolizumab | Advanced or recurrent HNSCC | 14 | Completed [63] | |
| 28 | ALT-803 | Recombinant human IL15 | NCT01727076 | Feb 2013 | I | - | LA or recurrent solid tumors, including HNSCC | 20 | Completed [64,65] | |
| 29 | N 803 | IL-15 superagonist complex | NCT03228667 | Dec 2018 | IIb | ICI | R/M Solid tumors including HNSCC | 135/145 * | Active, not recruiting (Interim report) [66] | |
| 30 | Irradiated PD-L1 CAR-NK cells | Autologous CAR-T cell therapy | NCT04847466 | Dec 2021 | II | N-803 + Pembrolizumab | R/M gastric or HNSCC | 55 (e) | Recruiting | |
| 31 | M7824 | Anti-PD-L1/TGF-beta Trap | NCT04247282 | Jun 2020 | WOT | TriAd Vaccine + N-803 | HNSCC | 21 | Active, not recruiting | |
| 32 | IRX-2 | Numerous active cytokine components | NCT00210470 | Jul 2005 | II | Cyclophosphamide, indomethacin, and zinc | Treatment-naïve HNSCC | 27 | Completed [67] | |
| 33 | Edodekin alfa | Recombinant interleukin-12 | NCT01468896 | Oct 2011 | I/II | Cetuximab | Unresectable primary or recurrent HNSCC | 23 | Completed [68] | |
| 34 | NT-I7 | Recombinant human IL-7 | NCT04588038 | Mar 2021 | WOT | Surgery | Recurrent HNSCC undergoing salvage surgery | 10 (e) | Recruiting | |
| 5. Personalized vaccines | ||||||||||
| 35 | AlloVax | Chaperone-rich cell lysate | NCT01998542 | Jan 2016 | I | AlloStim | R/M HNSCC | 10/12 * | Completed [69] | |
| 36 | GEN-009 | Up to 20 neoantigens | NCT03633110 | Aug 2018 | I/IIa | Nivolumab or Pembrolizumab | Solid tumors, including HNSCC | 15/24 * | Completed [70] | |
| 37 | PNeoVCA | Personalized neoantigen peptide-based vaccine | NCT05269381 | Mar 2022 | I | Sargramostim plus pembrolizumab | LA and R/M solid tumors, including HNSCC | 36 (e) | Recruiting | |
| 38 | MVX-ONCO-1 | Irradiated, autologous tumor cells | NCT02193503 | Mar 2014 | I | GM-CSF | LA or R/M solid tumors, including HNSCC | 34 | Active, not recruiting | |
| NCT02999646 | Jul 2018 | II | GM-CSF | LA or R/M HNSCC | 21 (e) | Recruiting (Interim report) [71] | ||||
| 39 | YE-NEO-001 | NANT neoepitope yeast-based vaccine | NCT03552718 | Aug 2018 | I | - | Previously treated solid tumors, including HNSCC | 16 (e) | Unknown | |
| 40 | TG4050 | MVA based on the myvac® platform | NCT04183166 | Dec 2019 | I | - | Treatment naïve LA HNSCC | 30 (e) | Recruiting | |
| 41 | VB10.NEO | DNA plasmid vaccine with intrinsic adjuvant effect | NCT03548467 | Apr 2018 | I/IIa | NKTR-214 | LA or metastatic solid tumors, including HNSCC | 65 (e) | Active not recruiting | |
| 42 | mRNA-2752 | Lipid nanoparticle encapsulating mRNAs encoding human OX40L, IL-23, and IL-36γ | NCT03739931 | Nov 2018 | I | Durvalumab | R/M solid tumors or lymphoma, including HNSCC | 264 (e) | Recruiting (Interim report) [72] | |
| 43 | PANDA-VAC | Personalized and adjusted neoantigen peptide vaccine | NCT04266730 | Feb 2023 | I | - | Advanced lung cancer and HNSCC | 6 (e) | Not yet recruiting | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Devaraja, K.; Aggarwal, S.; Singh, M. Therapeutic Vaccination in Head and Neck Squamous Cell Carcinoma—A Review. Vaccines 2023, 11, 634. https://doi.org/10.3390/vaccines11030634
Devaraja K, Aggarwal S, Singh M. Therapeutic Vaccination in Head and Neck Squamous Cell Carcinoma—A Review. Vaccines. 2023; 11(3):634. https://doi.org/10.3390/vaccines11030634
Chicago/Turabian StyleDevaraja, K., Sadhna Aggarwal, and Manisha Singh. 2023. "Therapeutic Vaccination in Head and Neck Squamous Cell Carcinoma—A Review" Vaccines 11, no. 3: 634. https://doi.org/10.3390/vaccines11030634
APA StyleDevaraja, K., Aggarwal, S., & Singh, M. (2023). Therapeutic Vaccination in Head and Neck Squamous Cell Carcinoma—A Review. Vaccines, 11(3), 634. https://doi.org/10.3390/vaccines11030634





