T-Cell Response and Antibody Production Induced by the COVID-19 Booster Vaccine in Japanese Chronic Kidney Disease Patients Treated with Hemodialysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Anti-S1 Antibody Titers
2.3. Neutralizing Antibodies
2.4. T-SPOT
2.5. Adverse Reactions
2.6. Statistical Analysis
3. Results
3.1. HD Group Had Higher Anti-S1 Antibody Titers after Booster Administration than the Control
3.2. HD Group Had Higher Neutralizing Antibodies after Booster Administration than the Control Group
3.3. HD Group Had Higher T-SPOT Levels after Booster Administration than the Control
3.4. HD Group Had Higher Local and Systemic Adverse Reaction Rates than the Control
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- De Meester, J.; De Bacquer, D.; Naesens, M.; Meijers, B.; Couttenye, M.M.; De Vriese, A.S. Incidence, characteristics, and outcome of COVID-19 in adults on kidney replacement therapy: A regionwide registry study. J. Am. Soc. Nephrol. 2021, 32, 385–396. [Google Scholar] [CrossRef]
- Yen, C.C.; Lin, S.Y.; Chen, S.C.; Chiu, Y.W.; Chang, J.M.; Hwang, S.J. COVID-19 vaccines in patients with maintenance hemodialysis. J. Pers. Med. 2021, 11, 789. [Google Scholar] [CrossRef]
- Yoshifuji, A.; Toda, M.; Ryuzaki, M.; Kikuchi, K.; Kawai, T.; Sakai, K.; Oyama, E.; Koinuma, M.; Katayama, K.; Uehara, Y.; et al. Investigation for the efficacy of COVID-19 vaccine in Japanese CKD patients treated with hemodialysis. Ren Replace Ther. 2022, 8, 39. [Google Scholar] [CrossRef]
- The Japanese Ministry of Health. Available online: https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/vaccine00184.html (accessed on 27 November 2022).
- Bergwerk, M.; Gonen, T.; Lustig, Y.; Amit, S.; Lipsitch, M.; Cohen, C.; Mandelboim, M.; Levin, E.G.; Rubin, C.; Indenbaum, V.; et al. COVID-19 breakthrough infections in vaccinated health care workers. N. Engl. J. Med. 2021, 385, 1474–1484. [Google Scholar] [CrossRef]
- Toda, M.; Yoshifuji, A.; Kikuchi, K.; Koinuma, M.; Komatsu, M.; Fujii, K.; Kato, A.; Kikuchi, T.; Nakazawa, A.; Ryuzaki, M. Factors associated with SARS-CoV-2 antibody titers and prognosis of breakthrough infection in hemodialysis patients. Clin. Exp. Nephrol. 2022, 26, 571–580. [Google Scholar] [CrossRef]
- McMahan, K.; Yu, J.; Mercado, N.B.; Loos, C.; Tostanoski, L.H.; Chandrashekar, A.; Liu, J.; Peter, L.; Atyeo, C.; Zhu, A.; et al. Correlates of protection against SARS-CoV-2 in rhesus macaques. Nature 2021, 590, 630–634. [Google Scholar] [CrossRef]
- Moss, P. The T cell immune response against SARS-CoV-2. Nat. Immunol. 2022, 23, 186–193. [Google Scholar] [CrossRef]
- Kruse, M.; Dark, C.; Aspden, M.; Cochrane, D.; Competiello, R.; Peltz, M.; Torres, L.; Wrighton-Smith, P.; Dudek, M. Performance of the T-SPOT(®).COVID test for detecting SARS-CoV-2-responsive T cells. Int. J. Infect. Dis. 2021, 113, 155–161. [Google Scholar] [CrossRef]
- Matsuyama, S.; Nao, N.; Shirato, K.; Kawase, M.; Saito, S.; Takayama, I.; Takeda, M. Enhanced isolation of SARS-CoV-2 by TMPRSS2-expressing cells. Proc. Natl. Acad. Sci. USA 2020, 117, 7001–7003. [Google Scholar] [CrossRef] [Green Version]
- Uwamino, Y.; Yokoyama, T.; Shimura, T.; Nishimura, T.; Sato, Y.; Wakui, M.; Murata, M. The effect of the E484K mutation of SARS-CoV-2 on the neutralizing activity of antibodies from BNT162b2 vaccinated individuals. Vaccine 2022, 40, 1928–1931. [Google Scholar] [CrossRef]
- Kamimaki, C.; Kobayashi, N.; Hirata, M.; Somekawa, K.; Fukuda, N.; Kubo, S.; Katakura, S.; Teranishi, S.; Watanabe, K.; Horita, N.; et al. T-cell response to phytohemagglutinin in the interferon-γ release assay as a potential biomarker for the response to immune checkpoint inhibitors in patients with non-small cell lung cancer. Thorac. Cancer 2021, 12, 1726–1734. [Google Scholar] [CrossRef]
- Wang, F.; Hou, H.Y.; Wu, S.J.; Zhu, Q.; Huang, M.; Yin, B.; Huang, J.; Pan, Y.Y.; Mao, L.; Sun, Z.Y. Using the TBAg/PHA ratio in the T-SPOT(®).TB assay to distinguish TB disease from LTBI in an endemic area. Int. J. Tuberc. Lung Dis. 2016, 20, 487–493. [Google Scholar] [CrossRef]
- Ghadiani, M.H.; Besharati, S.; Mousavinasab, N.; Jalalzadeh, M. Response rates to HB vaccine in CKD stages 3-4 and hemodialysis patients. J. Res. Med. Sci. 2012, 17, 527–533. [Google Scholar]
- Speer, C.; Schaier, M.; Nusshag, C.; Töllner, M.; Buylaert, M.; Kälble, F.; Reichel, P.; Grenz, J.; Süsal, C.; Zeier, M.; et al. Longitudinal humoral responses after COVID-19 vaccination in peritoneal and hemodialysis patients over twelve weeks. Vaccines 2021, 9, 1130. [Google Scholar] [CrossRef]
- Dikow, R.; Eckerle, I.; Ksoll-Rudek, D.; Hampel, H.; Schwenger, V.; Zeier, M.; Schnitzler, P.; Sommerer, C. Immunogenicity and efficacy in hemodialysis patients of an AS03(A)-adjuvanted vaccine for 2009 pandemic influenza A(H1N1): A nonrandomized trial. Am. J. Kidney Dis. 2011, 57, 716–723. [Google Scholar] [CrossRef]
- Jahn, M.; Korth, J.; Dorsch, O.; Anastasiou, O.E.; Sorge-Hädicke, B.; Tyczynski, B.; Gäckler, A.; Witzke, O.; Dittmer, U.; Dolff, S.; et al. Humoral response to SARS-CoV-2-vaccination with BNT162b2 (Pfizer-BioNTech) in patients on hemodialysis. Vaccines 2021, 9, 360. [Google Scholar] [CrossRef]
- Grupper, A.; Sharon, N.; Finn, T.; Cohen, R.; Israel, M.; Agbaria, A.; Rechavi, Y.; Schwartz, I.F.; Schwartz, D.; Lellouch, Y.; et al. Humoral response to the Pfizer BNT162b2 vaccine in patients undergoing maintenance hemodialysis. Clin. J. Am. Soc. Nephrol. 2021, 16, 1037–1042. [Google Scholar] [CrossRef]
- Simon, B.; Rubey, H.; Gromann, M.; Knopf-Völkerer, A.; Hemedi, B.; Zehetmayer, S.; Kirsch, B. SARS-CoV-2 Antibody and T cell response after a third vaccine dose in hemodialysis patients compared with healthy controls. Vaccines 2022, 10, 694. [Google Scholar] [CrossRef]
- Sekine, T.; Perez-Potti, A.; Rivera-Ballesteros, O.; Strålin, K.; Gorin, J.B.; Olsson, A.; Llewellyn-Lacey, S.; Kamal, H.; Bogdanovic, G.; Muschiol, S.; et al. Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID-19. Cell 2020, 183, 158–168.e114. [Google Scholar] [CrossRef]
- Braun, J.; Loyal, L.; Frentsch, M.; Wendisch, D.; Georg, P.; Kurth, F.; Hippenstiel, S.; Dingeldey, M.; Kruse, B.; Fauchere, F.; et al. SARS-CoV-2-reactive T cells in healthy donors and patients with COVID-19. Nature 2020, 587, 270–274. [Google Scholar] [CrossRef]
- Clarke, C.L.; Martin, P.; Gleeson, S.; Thomson, T.; Edwards, H.; Mortimer, P.; McIntyre, S.; Deborah, J.; Cox, A.; Pickard, G.; et al. Comparison of immunogenicity between BNT162b2 and ChAdOx1 SARS-CoV-2 vaccines in a large haemodialysis population. medRxiv 2021. [Google Scholar] [CrossRef]
- Bertrand, D.; Hamzaoui, M.; Lemee, V.; Lamulle, J.; Hanoy, M.; Laurent, C.; Lebourg, L.; Etienne, I.; Lemoine, M.; Le Roy, F.; et al. Antibody and T cell response to SARS-CoV-2 messenger RNA BNT162b2 vaccine in kidney transplant recipients and hemodialysis patients. J. Am. Soc. Nephrol. 2021, 32, 2147–2152. [Google Scholar] [CrossRef]
- Donati, D.; Degiannis, D.; Combates, N.; Raskova, J.; Raska, K., Jr. Effects of hemodialysis on activation of lymphocytes: Analysis by an in vitro dialysis model. J. Am. Soc. Nephrol. 1992, 2, 1490–1497. [Google Scholar] [CrossRef]
- Bruminhent, J.; Setthaudom, C.; Kitpermkiat, R.; Kiertiburanakul, S.; Malathum, K.; Assanatham, M.; Nongnuch, A.; Phuphuakrat, A.; Chaumdee, P.; Janphram, C.; et al. Immunogenicity of ChAdOx1 nCoV-19 vaccine after a two-dose inactivated SARS-CoV-2 vaccination of dialysis patients and kidney transplant recipients. Sci. Rep. 2022, 12, 3587. [Google Scholar] [CrossRef]
- Shapiro Ben David, S.; Mizrahi, B.; Rahamim-Cohen, D.; Supino-Rosin, L.; Shahar, A.; Hermoni-Alon, S.; Fremder Sacerdote, A.; Irony, A.; Lazar, R.; Kalkstein, N.; et al. Robust antibody response after a third BNT162b2 vaccine compared to the second among immunocompromised and healthy individuals, a prospective longitudinal cohort study. Vaccine 2022, 40, 4038–4045. [Google Scholar] [CrossRef]
- Wyllie, D.; Mulchandani, R.; Jones, H.E.; Taylor-Phillips, S.; Brooks, T.; Charlett, A.; Ades, A.E.; Makin, A.; Oliver, I.; Moore, P.; et al. SARS-CoV-2 responsive T cell numbers are associated with protection from COVID-19: A prospective cohort study in keyworkers. medRxiv 2020. [Google Scholar] [CrossRef]
- Ministry of Health, Labour and Welfare. Available online: https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/0000121431_00348.html (accessed on 7 August 2022).
- The Japanese Society of Nephrology. Available online: https://jsn.or.jp/medic/data/COVID-19number-of-infected_20220603.pdf (accessed on 7 August 2022).
- Kim, J.U.; Kim, M.; Kim, S.; Nguyen, T.T.; Kim, E.; Lee, S.; Kim, S.; Kim, H. Dendritic Cell Dysfunction in Patients with End-stage Renal Disease. Immune Netw. 2017, 17, 152–162. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, S.; Fukunaga, A.; Tanaka, A.; Takeuchi, J.S.; Inoue, Y.; Kimura, M.; Maeda, K.; Ueda, G.; Mizoue, T.; Ujiie, M.; et al. Association between reactogenicity and SARS-CoV-2 antibodies after the second dose of the BNT162b2 COVID-19 vaccine. Vaccine 2022, 40, 1924–1927. [Google Scholar] [CrossRef]
- Teijaro, J.R.; Farber, D.L. COVID-19 vaccines: Modes of immune activation and future challenges. Nat. Rev. Immunol. 2021, 21, 195–197. [Google Scholar] [CrossRef]

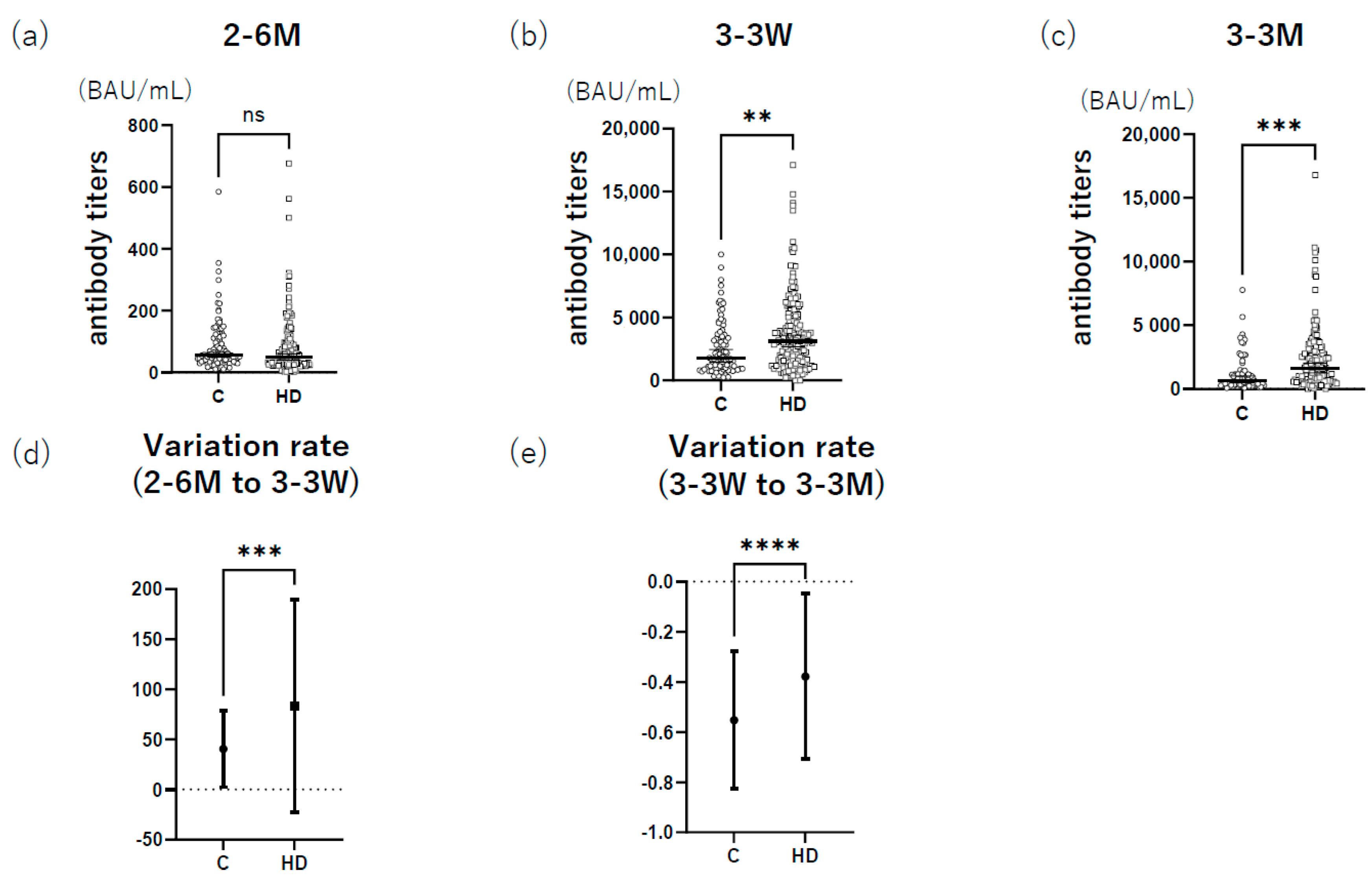
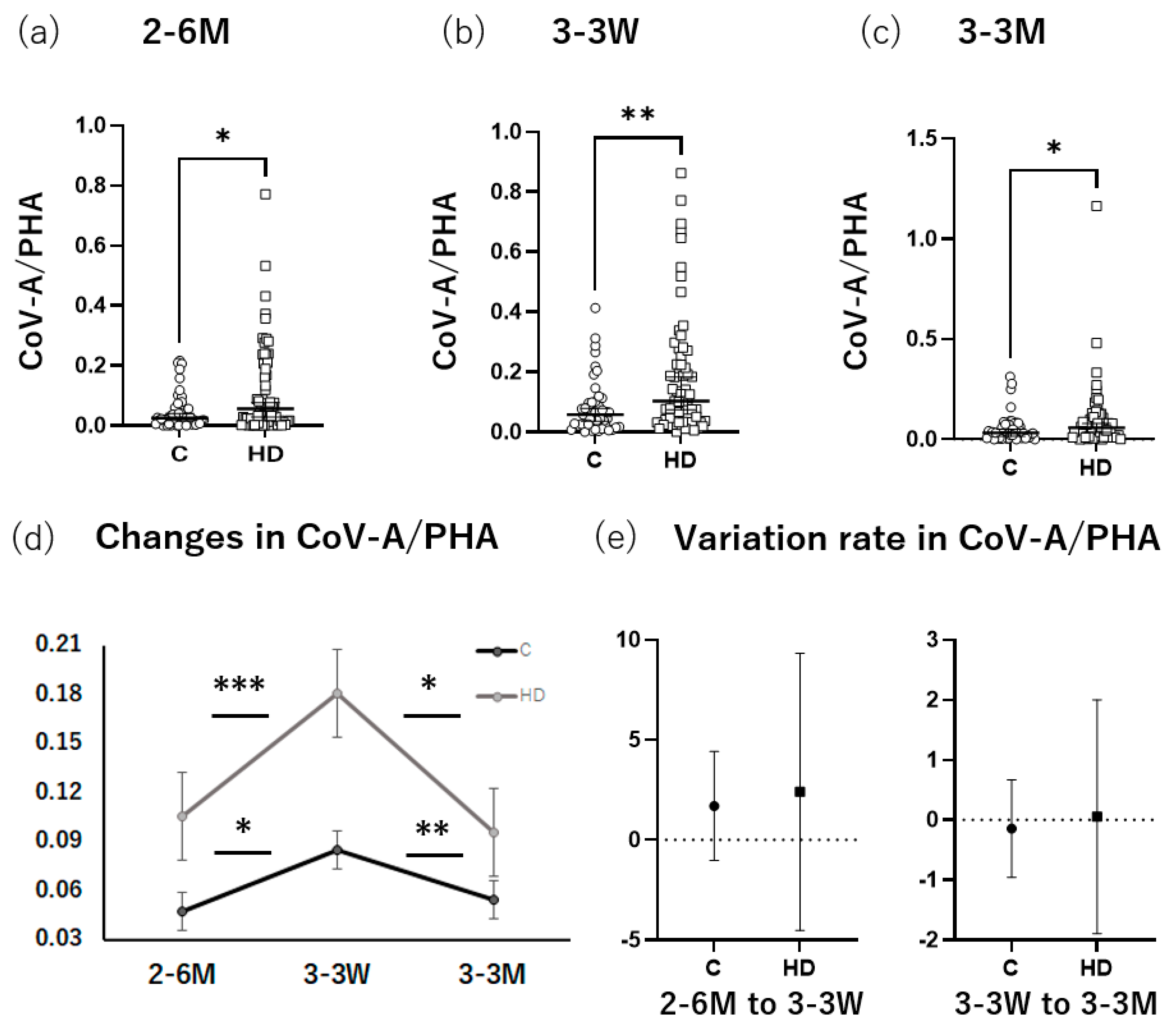

| All | Males | Females | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Control Group (n = 103) | HD Group (n = 194) | p Value | Control Group (n = 62) | HD Group (n = 126) | p Value | Control Group (n = 41) | HD Group (n = 68) | p Value | |
| Males, n (%) | 68 (61.8) | 125 (64.8) | 0.79 | ||||||
| Age, years (±SD) | 65.4 ± 11.6 | 67.0 ± 11.1 | 0.31 | 64.8 ± 11.5 | 67.0 ± 11.5 | 0.78 | 66.5 ± 11.7 | 66.6 ± 10.2 | 0.78 |
| BMI, kg/m2 (±SD) | 23.6 ± 4.2 | 22.5 ± 4.3 | 0.03 | 24.1 ± 4.1 | 22.8 ± 3.9 | 0.01 | 22.8 ± 4.3 | 22.0 ± 4.8 | 0.01 |
| Diabetes mellitus, n (%) | 17 (16.3) * | 79 (40.9) | <0.0001 | 13 (19.7) * | 59 (47.2) | 0.0002 | 4 (10.5) * | 20 (29.4) | 0.03 |
| Hypertension, n (%) | 48 (46.2) * | 81 (42.0) | 0.81 | 33 (50.0) * | 55 (44.0) | 0.54 | 15 (39.5) * | 26 (38.2) | >0.99 |
| Malignant tumor, n (%) | 11 (10.6) * | 29 (15.0) | 0.29 | 8 (12.1) * | 19 (15.2) | 0.66 | 3 (7.9) * | 10 (14.7) | 0.37 |
| Cerebrovascular disease, n (%) | 3 (2.9) * | 42 (21.8) | <0.0001 | 2 (3.0) * | 31 (24.8) | 0.0001 | 1 (2.6) * | 11 (16.2) | 0.052 |
| Cardiovascular disease, n (%) | 4 (3.8) * | 37 (19.2) | <0.0001 | 4 (6.1) * | 27 (21.6) | 0.007 | 0 (0) * | 10 (14.7) | 0.013 |
| COPD, n (%) | 8 (7.7) * | 11 (5.7) | 0.63 | 5 (7.6) * | 3 (2.4) | 0.13 | 3 (7.9) * | 8 (11.8) | 0.74 |
| Interval time † (±SD) | 208 ± 18.9 | 213 ± 27.3 | 0.06 | 211 ± 18.9 | 216 ± 30.0 | 0.17 | 205 ± 18.3 | 209 ± 20.5 | 0.17 |
| * n = 104 | * n = 66 | * n = 38 | |||||||
| 2–6 M | Control | HD | p Value | |
|---|---|---|---|---|
| Original | Mean ± SD | 12.3 ± 3.1 | 9.0 ± 2.2 | 0.01 |
| 95% CI | 10.1–14.5 | 6.9–11.1 | ||
| Omicron | Mean ± SD | 5.6 ± 1.7 | 5.1 ± 1.2 | 0.051 |
| 95% CI | 3.5–7.5 | 3.1–7.1 | ||
| 3–3 W | Control | HD | p Value | |
| Original | Mean ± SD | 324.7 ± 3.3 | 717.5 ± 3.4 | <0.0001 |
| 95% CI | 322.5–326.9 | 715.4–719.6 | ||
| Omicron | Mean ± SD | 43.8 ± 3.8 | 54.3 ± 3.2 | 0.18 |
| 95% CI | 38.1–42.5 | 51.9–56.1 | ||
| 3–3 M | Control | HD | p Value | |
| Original | Mean ± SD | 222.7 ± 3.4 | 365.6 ± 3.2 | 0.004 |
| 95% CI | 220.5–224.9 | 363.5–367.7 | ||
| Omicron | Mean ± SD | 14.5 ± 3.2 | 26.7 ± 3.6 | 0.0001 |
| 95% CI | 11.0–15.4 | 24.1–28.5 | ||
| Control (%) | HD (%) | p Value | |
|---|---|---|---|
| 2–6 M | 66.7 * | 43.1 § | 0.02 |
| 3–3 W | 35.0 † | 29.0 ¶ | 0.66 |
| 3–3 M | 37.2 ‡ | 24.2 ‖ | 0.19 |
| Control: 2–6 M | HD: 2–6 M | ||||||
|---|---|---|---|---|---|---|---|
| SARS-CoV-2 IgG | SARS-CoV-2 IgG | ||||||
| + (%) | − (%) | + (%) | − (%) | ||||
| T-SPOT | + (%) | 34.78 | 0.00 | T-SPOT | + (%) | 50.00 | 6.25 |
| − (%) | 54.35 | 10.87 | − (%) | 39.06 | 4.69 | ||
| Control: 3–3 W | HD: 3–3 W | ||||||
| SARS-CoV-2 IgG | SARS-CoV-2 IgG | ||||||
| + (%) | − (%) | + (%) | − (%) | ||||
| T-SPOT | + (%) | 65.00 | 0.00 | T-SPOT | + (%) | 70.97 | 0.00 |
| − (%) | 35.00 | 0.00 | − (%) | 29.03 | 0.00 | ||
| Control: 3–3 M | HD: 3–3 M | ||||||
| SARS-CoV-2 IgG | SARS-CoV-2 IgG | ||||||
| + (%) | − (%) | + (%) | − (%) | ||||
| T-SPOT | + (%) | 62.79 | 0.00 | T-SPOT | + (%) | 75.81 | 0.00 |
| − (%) | 37.21 | 0.00 | − (%) | 24.19 | 0.00 | ||
| All | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Local n (%) | Systemic n (%) | |||||||||||||
| Pain | Redness | Swelling | Pruritus | Fatigue | Headache | Muscle Pain | Coldness | Fever | Arthralgia | Nausea | Diarrhea | Stomachache | Anaphylaxis | |
| Control (n = 87) | 42(48.3) | 7(8.0) | 5(5.7) | 5(5.7) | 11(12.6) | 6(6.9) | 9(10.3) | 6(6.9) | 10(11.5) | 3(3.4) | 0(0) | 2(2.3) | 0(0) | 0(0) |
| HD (n = 184) | 104(56.5) | 18(9.8) | 24(13.0) | 33(17.9) | 52(28.3) | 18(9.8) | 44(23.9) | 28(15.2) | 45(24.5) | 20(10.9) | 7(3.8) | 2(1.1) | 5(2.7) | 0(0) |
| p-value | 0.24 | 0.82 | 0.09 | 0.008 | 0.005 | 0.5 | 0.009 | 0.08 | 0.015 | 0.06 | 0.1 | 0.59 | 0.18 | >0.99 |
| Male | ||||||||||||||
| Local n (%) | Systemic n (%) | |||||||||||||
| Pain | Redness | Swelling | Pruritus | Fatigue | Headache | Muscle Pain | Coldness | Fever | Arthralgia | Nausea | Diarrhea | Stomachache | Anaphylaxis | |
| Control (n = 51) | 23(45.1) | 1(0.2) | 1(0.2) | 1(0.2) | 5(9.8) | 1(0.2) | 3(5.9) | 0(0) | 2(3.9) | 1(0.2) | 0(0) | 0(0) | 0(0) | 0(0) |
| HD (n = 121) | 57(47.1) | 9(7.4) | 11(9.1) | 11(9.1) | 30(24.8) | 6(5.0) | 27(22.3) | 17(14.0) | 29(24.0) | 11(9.1) | 4(3.3) | 2(1.7) | 3(2.5) | 0(0) |
| p-value | 0.87 | 0.29 | 0.11 | 0.11 | 0.04 | 0.68 | 0.008 | 0.004 | 0.001 | 0.11 | 0.32 | >0.99 | 0.56 | >0.99 |
| Female | ||||||||||||||
| Local n (%) | Systemic n (%) | |||||||||||||
| Pain | Redness | Swelling | Pruritus | Fatigue | Headache | Muscle Pain | Coldness | Fever | Arthralgia | Nausea | Diarrhea | Stomachache | Anaphylaxis | |
| Control (n = 36) | 19(52.8) | 6(16.7) | 4(11.1) | 4(11.1) | 6(16.7) | 5(13.9) | 6(16.7) | 6(16.7) | 8(22.2) | 2(5.6) | 0(0) | 2(5.6) | 0(0) | 0(0) |
| HD (n = 63) | 47(74.6) | 10(15.9) | 13(20.6) | 22(34.9) | 22(34.9) | 12(19.0) | 17(27.0) | 11(17.5) | 16(25.4) | 9(14.3) | 3(4.8) | 0(0) | 2(3.2) | 0(0) |
| p-value | 0.05 | >0.99 | 0.28 | 0.01 | 0.07 | 0.59 | 0.32 | >0.99 | 0.81 | 0.32 | 0.55 | 0.13 | 0.53 | >0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshifuji, A.; Toda, M.; Ryuzaki, M.; Oyama, E.; Kikuchi, K.; Kawai, T.; Sakai, K.; Koinuma, M.; Katayama, K.; Yokoyama, T.; et al. T-Cell Response and Antibody Production Induced by the COVID-19 Booster Vaccine in Japanese Chronic Kidney Disease Patients Treated with Hemodialysis. Vaccines 2023, 11, 653. https://doi.org/10.3390/vaccines11030653
Yoshifuji A, Toda M, Ryuzaki M, Oyama E, Kikuchi K, Kawai T, Sakai K, Koinuma M, Katayama K, Yokoyama T, et al. T-Cell Response and Antibody Production Induced by the COVID-19 Booster Vaccine in Japanese Chronic Kidney Disease Patients Treated with Hemodialysis. Vaccines. 2023; 11(3):653. https://doi.org/10.3390/vaccines11030653
Chicago/Turabian StyleYoshifuji, Ayumi, Masataro Toda, Munekazu Ryuzaki, Emi Oyama, Kan Kikuchi, Toru Kawai, Ken Sakai, Masayoshi Koinuma, Kazuhiko Katayama, Takashi Yokoyama, and et al. 2023. "T-Cell Response and Antibody Production Induced by the COVID-19 Booster Vaccine in Japanese Chronic Kidney Disease Patients Treated with Hemodialysis" Vaccines 11, no. 3: 653. https://doi.org/10.3390/vaccines11030653
APA StyleYoshifuji, A., Toda, M., Ryuzaki, M., Oyama, E., Kikuchi, K., Kawai, T., Sakai, K., Koinuma, M., Katayama, K., Yokoyama, T., Uehara, Y., Ohmagari, N., Kanno, Y., Kon, H., Shinoda, T., Takano, Y., Tanaka, J., Hora, K., Nakazawa, Y., ... Takemoto, Y. (2023). T-Cell Response and Antibody Production Induced by the COVID-19 Booster Vaccine in Japanese Chronic Kidney Disease Patients Treated with Hemodialysis. Vaccines, 11(3), 653. https://doi.org/10.3390/vaccines11030653







