Abstract
This study investigated the immunogenicity of, and reactogenicity to, the ChAdOx1 nCoV-19 vaccine according to pre-existing adenovirus immunity. Individuals scheduled for COVID-19 vaccination were prospectively enrolled in a tertiary hospital with 2400 beds from March 2020 onwards. Pre-existing adenovirus immunity data was obtained before ChAdOx1 nCoV-19 vaccination. A total of 68 adult patients administered two doses of the ChAdOx1 nCoV-19 vaccine were enrolled. Pre-existing adenovirus immunity was identified in 49 patients (72.1%), but not in the remaining 19 patients (27.9%). The geometric mean titer of S-specific IgG antibodies was statistically higher in individuals without pre-existing adenovirus immunity at several time points: before the second ChAdOx1 nCoV-19 dose (56.4 (36.6–125.0) vs. 51.0 (17.9–122.3), p = 0.024), 2–3 weeks after the second ChAdOx1 nCoV-19 dose (629.5 (451.5–926.5) vs. 555.0 (287.3–926.0), p = 0.049), and 3 months after the second ChAdOx1 nCoV-19 dose (274.5 (160.5–655.3) vs. 176.0 (94.3–255.3), p = 0.033). In the absence of pre-existing adenovirus immunity, systemic events were observed with higher frequency, especially chills (73.7% vs. 31.9%, p = 0.002). In conclusion, individuals without pre-existing adenovirus immunity showed a higher immune response to ChAdOx1 nCoV-19 vaccination and a higher frequency of reactogenicity to ChAdOx1 nCoV-19 vaccination was observed.
1. Introduction
Adenovirus (Ad)-derived vectors are currently the most commonly used vectors in gene therapy, cancer, and vaccine clinical trials worldwide [1]. Ad-derived vectors have several advantages, such as efficient transduction of many proliferating and quiescent cell types, packaging large amounts of foreign deoxyribonucleic acid (DNA), large scale production while meeting clinical good manufacturing practice standards, and rare integration into the host chromosome [2,3].
However, due to the high incidence of Ad infections in the general population, pre-existing Ad immunity may have an impact on therapeutic efficiency and safety [4,5]. Antibodies bound to the viral capsid mediate sequestration by Fc receptor-positive cells and may lead to vector clearance and poor tissue transduction [2,6,7]. Zak et al. report that responses of volunteers with pre-existing Ad5 neutralizing antibodies show drastically reduced inflammatory responses to the vaccine [8]. On the other hand, Varnavski et al. show that the presence of pre-existing anti-vector immunity is associated with increased mortality following systemic vector infusion [9].
Several approaches are being tested to overcome pre-existing Ad immunity, such as generation of chemically modified Ad5 capsids, generation of chimeric Ads, complete replacement of Ad5-based vaccine platforms with alternative (human and non-human origin) Ad serotypes, and Ad5 genome modification [10]. Nevertheless, in the case of the Janssen (Ad26) or ChAdOx1 nCoV-19 vaccine, the antibody response rate of the Ad vector vaccine is lower than that of the mRNA vaccine [11,12]. In the case of pre-existing Ad immunity, the immune response may be reduced when the Ad vector vaccine is administered compared with that of the control group.
Therefore, this study investigated the immunogenicity of and reactogenicity to the ChAdOx1 nCoV-19 vaccine according to pre-existing Ad immunity.
2. Materials and Methods
2.1. Study Design
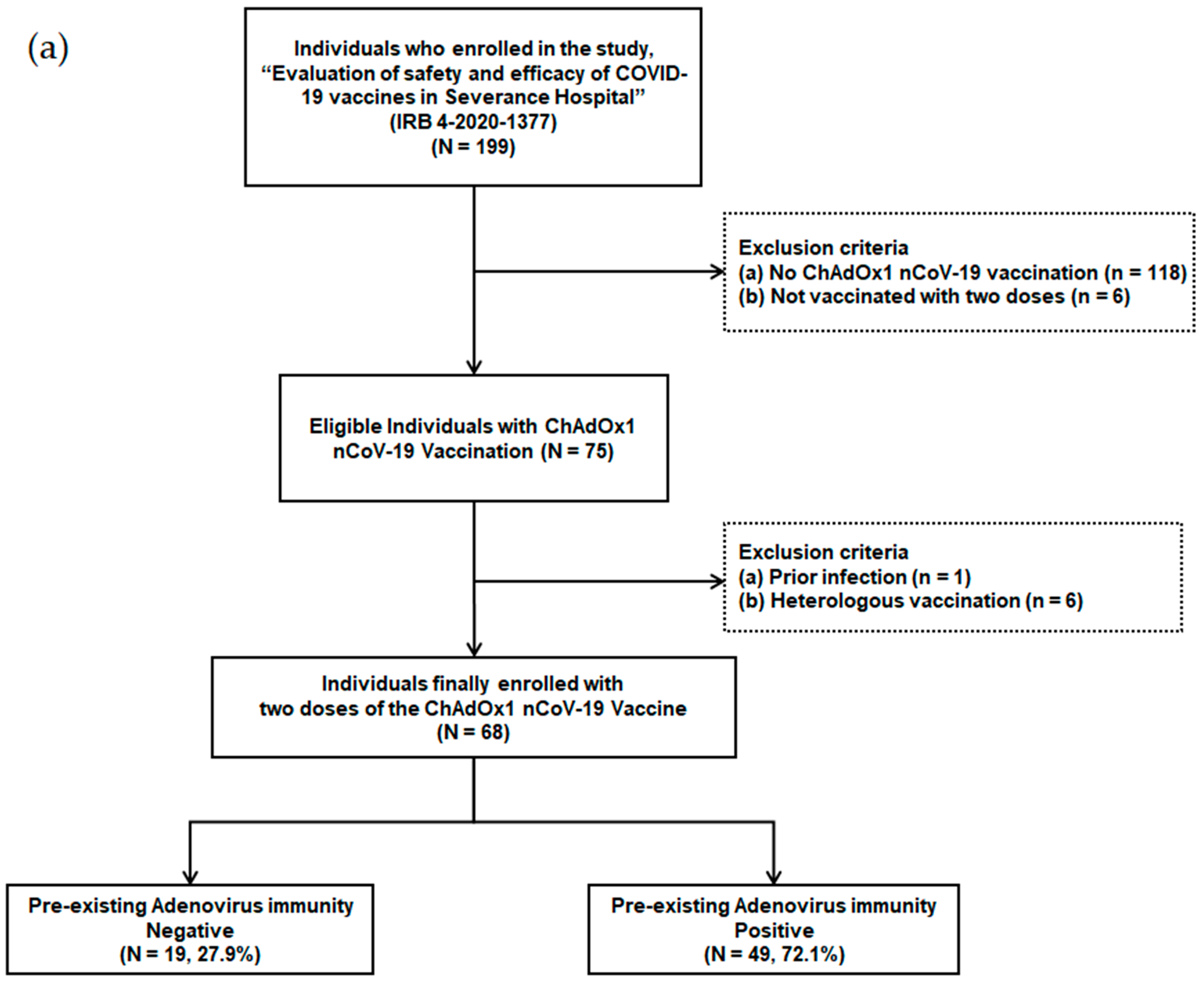
Individuals scheduled for COVID-19 vaccination among patients and health care workers were prospectively enrolled in a tertiary hospital with 2400 beds starting from March 2020. The ChAdOx1 nCoV-19 vaccination was administered from 8 March 2021. Individuals who were not vaccinated with two doses (n = 6), individuals who received heterologous vaccination (n = 6), individuals who were not vaccinated with the ChAdOx1 nCoV-19 vaccine (n = 118), and individuals with a history of previous infection (n = 1) were excluded from the study (Figure 1a). A total of 68 adult individuals that received two doses of the ChAdOx1 nCoV-19 vaccine were finally enrolled.
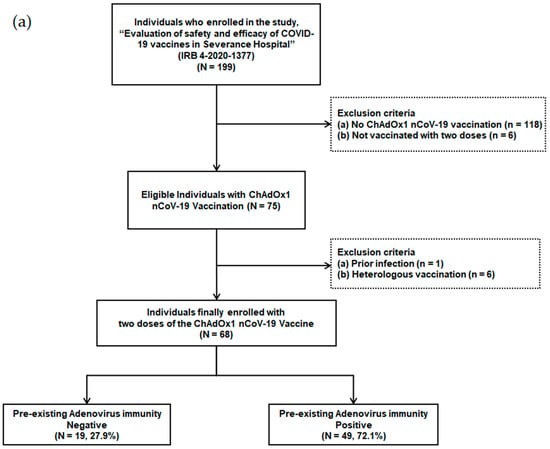
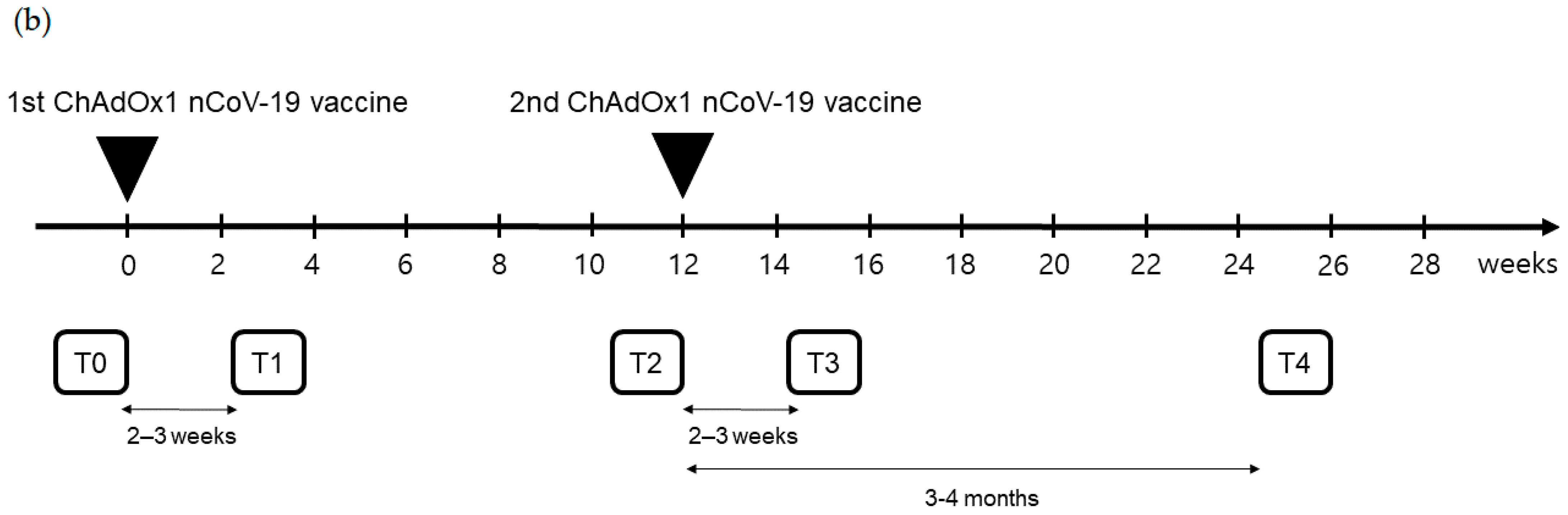
Figure 1.
(a) Flow chart of individuals with ChAdOx1 nCoV-19 vaccination according to pre-existing adenovirus immunity; (b) Timetable of individuals with ChAdOx1 nCoV-19 vaccination.
The Institutional Review Board (IRB) of Yonsei University College of Medicine (IRB no. 4-2020-1377) approved this study. Informed consent was obtained from all participants. This study complied with the Declaration of Helsinki and Good Clinical Practice guidelines.
2.2. Sample Selection
Peripheral blood samples were obtained at five time points: T0, before the first ChAdOx1 nCoV-19 dose; T1, 21.0 (18.0–24.0) days after the first ChAdOx1 nCoV-19 dose; T2, before the second ChAdOx1 nCoV-19 dose [71.0 (68.0–76.0) days after the first ChAdOx1 nCoV-19 dose]; T3, 16.0 (13.0–20.0) days after the second ChAdOx1 nCoV-19 dose; and T4, 103.0 (97.0–109.0) days after the second ChAdOx1 nCoV-19 dose (Figure 1b).
2.3. Adenovirus Titer Immunoassay
Pre-existing Ad immunity data was obtained using the QuickTiter™ Adenovirus Titer enzyme-linked immunosorbent assay Kit (Cell BioLabs, San Diego, CA 92126, USA) at time point T0, before the first ChAdOx1 nCoV-19 dose [13]. HEK 293T cells (5 × 105 cells/mL) were grown as monolayers in 96-well plates and incubated at 37 °C, 5% CO2 for 1 h. HEK 293T cells were infected with 10-fold serial dilution of T0 samples from enrolled patients or a 2-fold serial dilution of an Ad-β-gal positive control. After the immunoassay, the optical density at 450 nm was measured using a VersaMax Microplate Reader (Molecular Devices, LLC, San Jose, CA 95134, USA). Viral titers were calculated based on standard curves from Ad-β-gal positive control titrations.
2.4. Immunogenicity of the ChAdOx1 nCoV-19 Vaccine
Immunogenicity data were obtained at each time point along the primary series of ChAdOx1 nCoV-19 vaccination. The humoral immune response was assessed by testing anti-SARS-CoV-2 spike (S) antibody titers in the serum using the Elecsys® Anti-SARS-CoV-2 S assay Kit (Roche Diagnostics International Ltd., Rotkreuz, Switzerland). Plaque reduction neutralization tests (PRNT) were performed in duplicate using 24-well tissue culture plates (TPP Techno Plastic Products AG, Trasadingen, Switzerland) in a biosafety level 3 facility with Vero E6 TMRESS2 cells. Antibody titers were defined as the highest serum dilution that resulted in >50% (PRNT50) reduction in the number of virus plaques [14,15,16].
2.5. Reactogenicity to the ChAdOx1 nCoV-19 Vaccine
Solicited local and systemic adverse reactions were self-reported over 7 days after each dose, including rash, edema, vomiting, diarrhea, headache, fatigue, chills, muscle pain, joint pain, and fever. Unsolicited adverse events within 28 days after each dose and any adverse reactions leading to discontinuation were also reported. Reactogenicity severity was reported on a scale of 0–4: 0, none; 1, mild; 2, moderate; 3, severe; and 4, very severe. Serious adverse events were collected throughout the entire study period.
2.6. Statistical Analysis
Categorical variables were described by numbers and percentages, and continuous variables were expressed as median with interquartile range (IQR). Comparisons between groups were analyzed using the Chi-square and Fisher’s exact tests for categorical variables and the Mann–Whitney U test for continuous variables. p < 0.05 was considered significant. Statistical analysis was performed using GraphPad Prism 5 (GraphPad Software Inc.; San Diego, CA, USA) and IBM SPSS Statistics for Windows version 26 (IBM Corp., Armonk, NY, USA).
3. Results
3.1. Characteristics of Patients Who Received Two Doses of the ChAdOx1 nCoV-19 Vaccine
Out of 199 patients and health care workers, 68 individuals, who received two doses of the ChAdOx1 nCoV-19 vaccine, were finally enrolled (Figure 1a). These individuals were divided into two groups based on their pre-existing Ad immunity. Pre-existing Ad immunity was identified in 49 patients (72.1%), but not in the remaining 19 patients (27.9%).
The median age was 55 years (36–64), and 50.0% of patients were female (Table 1). The median body mass index was 22.1 (20.5–24.6) kg/m2. There were no significant differences in baseline comorbidities between the two groups. Out of the finally enrolled patients, 15 individuals (22.1%) had hypertension, 11 (16.2%) had diabetes mellitus, 2 (2.9%) had coronary artery occlusive disease, 2 (2.9%) had congestive heart failure, 6 (8.8%) had peripheral vascular disease, 3 (4.4%) had chronic kidney disease, 5 (7.4%) had chronic obstructive pulmonary disease, 4 (5.9%) had liver disease, and 1 (1.5%) had connective tissue disease. The median of Charlson comorbidity index was 3 (0–8). The time intervals of blood sampling based on ChAdOx1 nCoV-19 vaccination were not statistically different between the two groups (p = 0.951, p = 0.625, p = 0.143, and p = 0.134, respectively).

Table 1.
Baseline characteristics of individuals with ChAdOx1 nCoV-19 vaccination according to pre-existing adenovirus immunity.
3.2. Immunogenicity of the ChAdOx1 nCoV-19 Vaccine
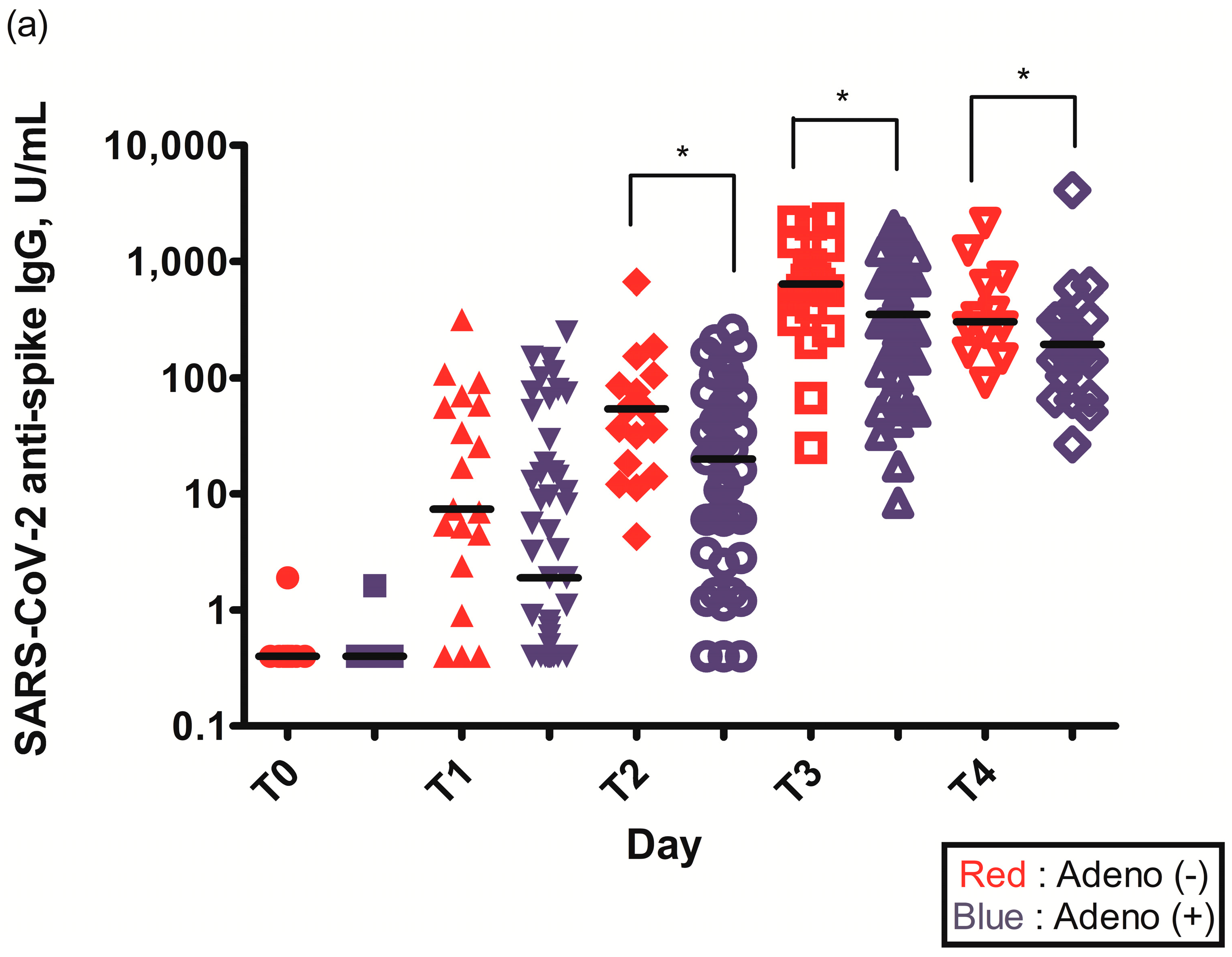
The geometric mean titer (GMT) of S-specific IgG antibodies 2–3 weeks after the first ChAdOx1 nCoV-19 dose (T1) was higher in individuals without pre-existing Ad immunity (29.6 (6.6–75.3) vs. 14.0 (1.6–84.5), p = 0.109) (Table 2). The GMT of S-specific IgG antibodies was statistically higher in individuals without pre-existing Ad immunity before the second ChAdOx1 nCoV-19 dose (T2) (56.4 (36.6–125.0) vs. 51.0 (17.9–122.3), p = 0.034), 2–3 weeks after the second ChAdOx1 nCoV-19 dose (T3) (629.5 (451.5–926.5) vs. 555.0 (287.3–926.0), p = 0.049), and 3 months after the second ChAdOx1 nCoV-19 dose (T4) (274.5 (160.5–655.3) vs. 176.0 (94.3–255.3), p = 0.033) (Figure 2a, Table 2).

Table 2.
Humoral responses of individuals with ChAdOx1 nCoV-19 vaccination according to pre-existing adenovirus immunity.
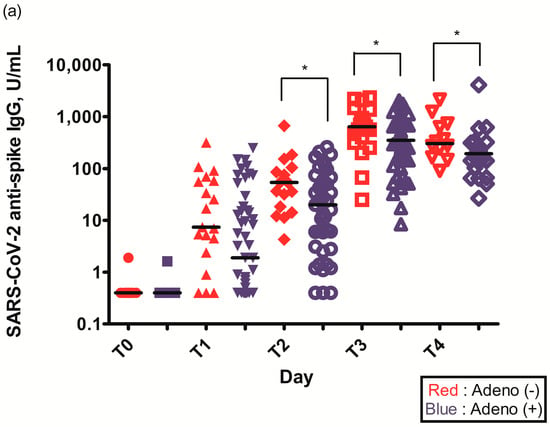
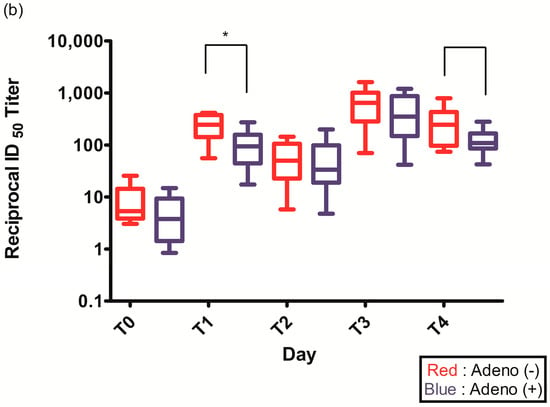
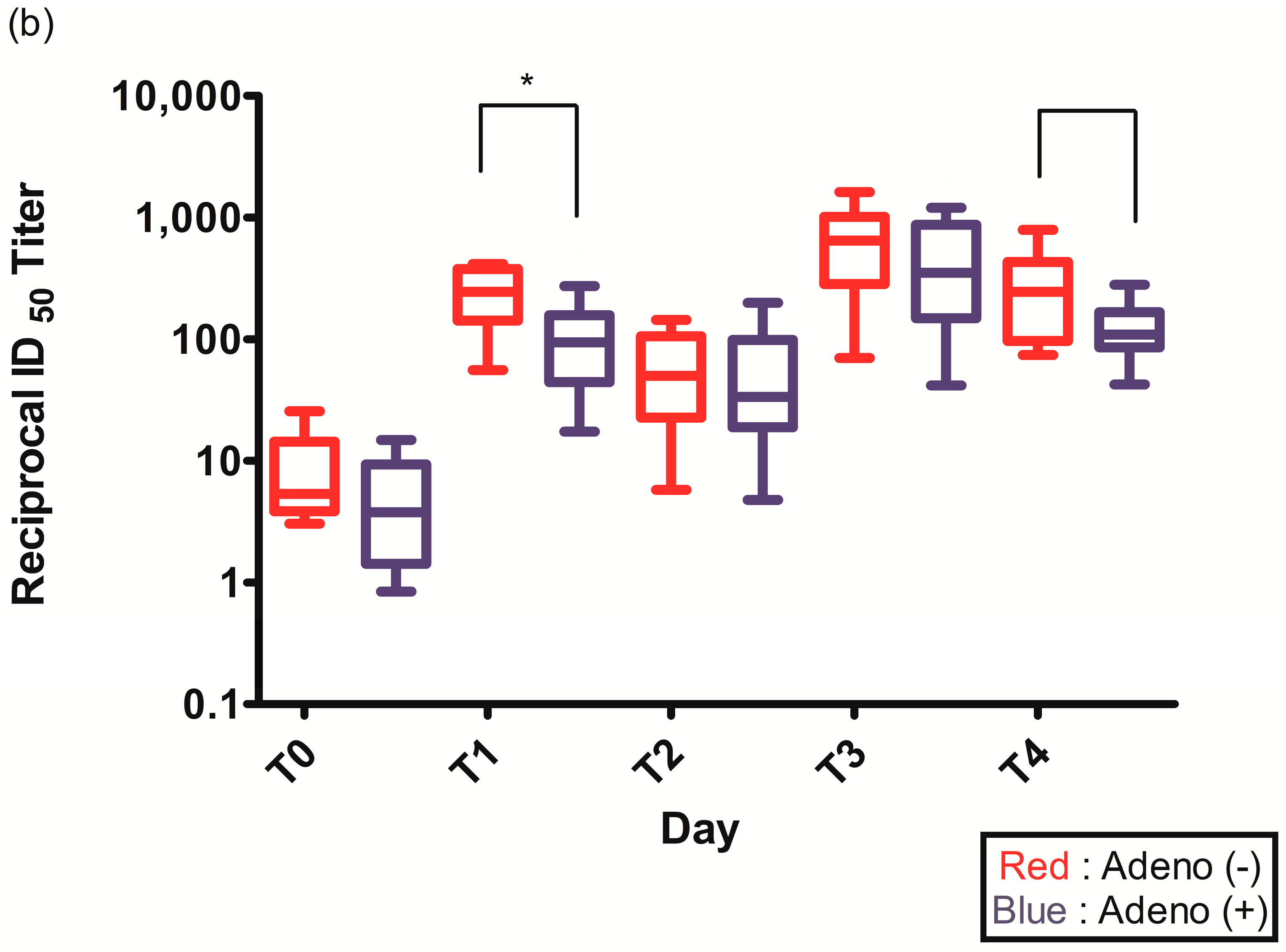
Figure 2.
(a) Spike (S)-specific IgG titers after ChAdOx1 nCoV-19 vaccination according to pre-existing adenovirus immunity; (b) Plaque reduction neutralization test (PRNT50) data after ChAdOx1 nCoV-19 vaccination according to pre-existing adenovirus immunity; *, p < 0.05.
The PRNT50 2–3 weeks after the first ChAdOx1 nCoV-19 dose (T1) was statistically higher in individuals without pre-existing Ad immunity (245.2 (142.6–377.1) vs. 94.1 (44.4–157.5), p = 0.021) (Figure 2b, Table 2). The PRNT50 of other time points after ChAdOx1 nCoV-19 vaccination were higher in individuals without pre-existing Ad immunity but were not statistically different: before the second ChAdOx1 nCoV-19 dose (T2) (50.0 (22.7–105.4) vs. 33.6 (18.9–98.4), p = 0.774), 2–3 weeks after the second ChAdOx1 nCoV-19 dose (T3) (647.5 (283.2–1014.3) vs. 352.4 (149.2–867.9), p = 0.292), and 3 months after the second ChAdOx1 nCoV-19 dose (T4) (245.1 (96.5–432.2) vs. 108.8 (85.8–166.5), p = 0.065) (Figure 2b, Table 2).
The seroconversion rate was 75% after the first dose of ChAdOx1 nCoV-19 vaccine (84.2% vs. 71.4%, p = 0.359), but 100% after the second dose (Table 2).
3.3. Reactogenicity to the ChAdOx1 nCoV-19 Vaccine
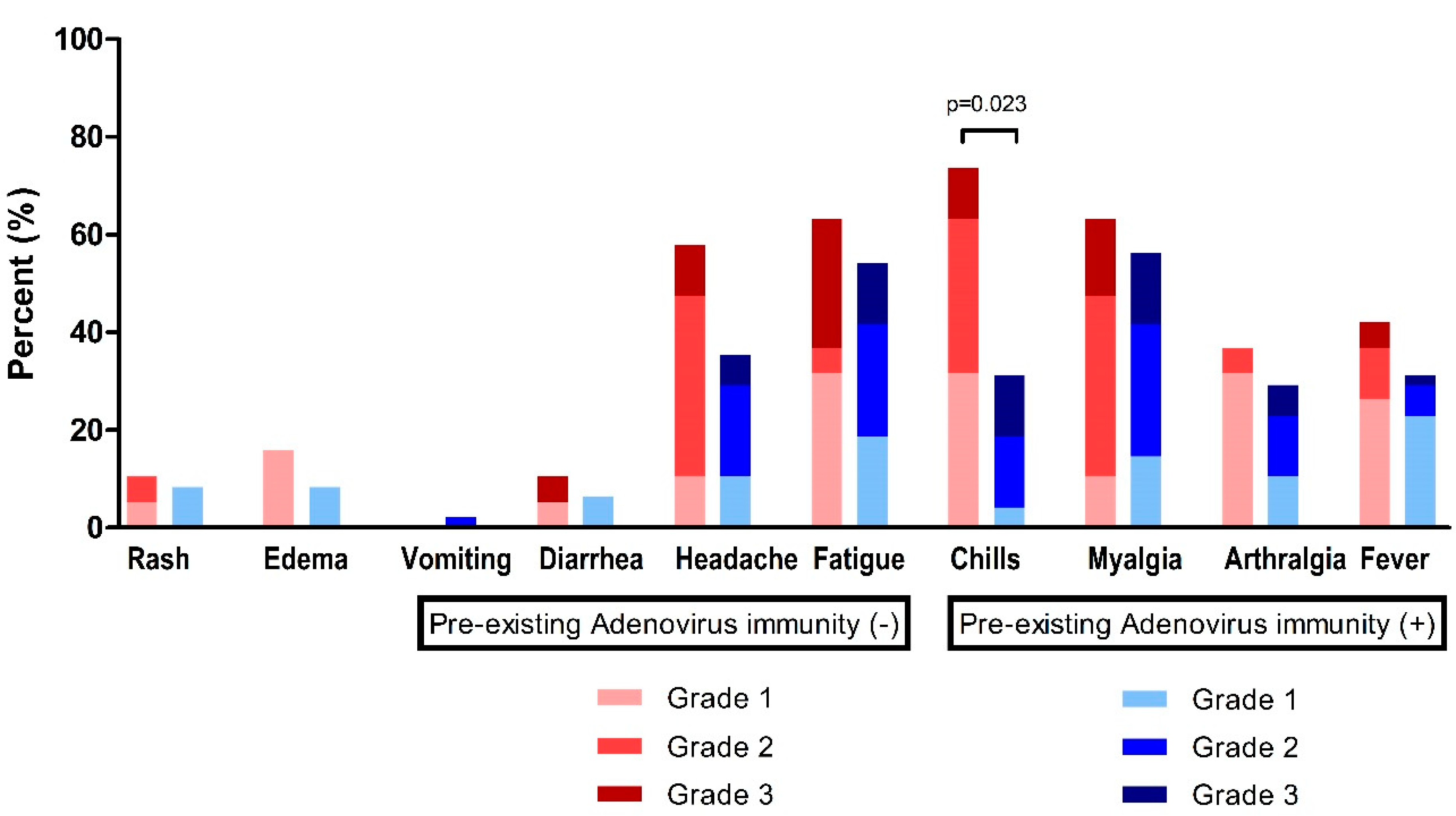
At time point T1 (2–3 weeks after the first ChAdOx1 nCoV-19 dose), fatigue (n = 38, 57.6%) and myalgia (n = 39, 59.1%) were the most frequently observed systemic events, followed by headache (n = 28, 42.4%), chills (n = 29, 43.9%), fever (n = 23, 34.8%), and arthralgia (n = 21, 31.8%) (Figure 3, Table 3). For these systemic adverse reactions, 48 patients (72.7%) took antipyretic medication (Table 3).
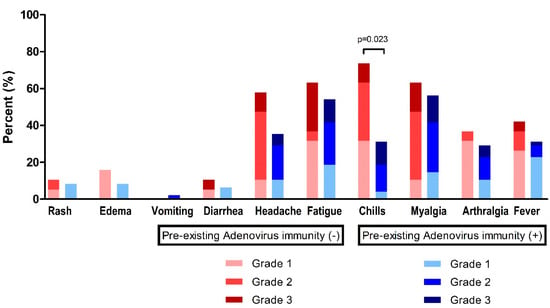
Figure 3.
Reactogenicity to ChAdOx1 nCoV-19 vaccination according to pre-existing adenovirus immunity.

Table 3.
Reactogenicity to the first dose of ChAdOx1 nCoV-19 vaccine according to pre-existing adenovirus immunity.
In the absence of pre-existing Ad immunity, systemic events were observed with a higher frequency, but were not statistically different: fever, 42.1% vs. 31.9%, p = 0.431; headache, 57.9% vs. 36.2%, p = 0.106; and fatigue, 63.2% vs. 55.3%, p = 0.560 (Table 3). However, chills were significantly higher in individuals without pre-existing Ad immunity (73.7% vs. 31.9%, p = 0.002).
At time point T3 (2–3 weeks after the second ChAdOx1 nCoV-19 dose), there was no significant difference in the systemic reactions of both groups according to pre-existing Ad immunity (Supplemental Table S1).
4. Discussion
In this study, individuals without pre-existing Ad immunity had a higher immune response and increased reactogenicity to the ChAdOx1 nCoV-19 vaccine. In addition, the humoral response to the ChAdOx1 nCoV-19 vaccine lasted longer in individuals without pre-existing Ad immunity.
Adenovirus infection is a common cause of upper respiratory tract infections and can manifest in a range of clinical symptoms such as gastroenteritis, pneumonia, conjunctivitis, hepatitis, nephritis, and meningoencephalitis [17]. Adenoviruses have been identified in more than 100 genotypes and 50 serotypes, with the predominant serotypes varying by geographic regions and changing over time periods [18]. Most individuals have been exposed to one or more adenoviruses during their lifetime, and it typically leads to the development of long-lasting immunity against the specific serotypes that were encountered [19]. Among the adenoviruses one distinguishes to date more than 50 serotypes and >100 genotypes, the seroprevalence of neutralizing antibodies produced during the convalescent period after Ad infection varies depending on the subtype but is reported to be approximately 30–60% [20]. Kolavic-Gray SA et al. report that pre-existing serum neutralizing antibody has a protective effect for subsequent infection [20]. These findings are supported by clinical studies showing Ad seroprevalence increases with age and that more than 80% of Ad infections are observed in children under 4 years of age [21,22].
Adenoviral vectors have been employed in numerous applications of gene therapy, anti-cancer therapy, and vaccines due to their many advantages, which include efficient transduction of both dividing and non-dividing cells, the capacity to carry large DNA loads, large scale production capabilities meeting clinical good manufacturing practices, and low risk of integration into the host chromosome [2]. The influence of pre-existing immunity may vary depending on the specific application of adenoviral vectors. Although gene therapy and anti-cancer therapy employing adenoviral vectors aim to introduce therapeutic genes or drugs into cells, vaccines utilizing adenoviral vectors are intended to trigger an immune response against a particular pathogen or antigen [19]. Pre-existing immunity to Ad can take different forms, such as the presence of neutralizing antibodies, Ad-protein-specific T cell responses, and innate immune responses [3,10]. In particular, serum neutralizing antibodies can hinder the desired immune response against the targeted antigen. In some cases, pre-existing Ad immunity is associated with strong innate inflammatory responses within hours or increased mortality following systemic vector infusion [9,21]. However, in other cases, pre-existing Ad immunity shows significantly reduced inflammatory responses to the vaccine [8]. These immune responses affect not only the vector itself, but also transgene products and cascades [3]. Therefore, to overcome pre-existing Ad immunity, various strategies have been attempted.
Numerous methods are currently being explored to overcome pre-existing Ad immunity, including the production of chemically altered Ad5 capsids, the development of chimeric Ads, modification of Ad5 genomes, and substitution of Ad5-based vaccine platforms with alternative Ad serotypes [10]. In the case of the ChAdOx1 nCoV-19 vaccine, chimpanzee adenovirus is used as a vector to overcome pre-existing Ad immunity [23]. Chimpanzee adenovirus serotypes 63 (ChAd-63), ChAd-68, and ChAd-Y25, which are frequently used, exhibit approximately 90% sequence similarity with the human adenovirus species group E [24,25]. Additionally, up to 90% of residues in three-dimensional positions among ChAd-68, fowl adenovirus 1, Ad2, Ad4, and Ad5 were found to be closely matched when comparing their hexon models using crystallographic methods [26]. Therefore, the similarity of both the sequences and three-dimensional structures implies that the presence of pre-existing immunity to different subtypes via a common hexon structure may impact the immune response [24,26]. Xiang Z et al. reported that the immune response to the Ad5 vaccine was abolished in mice with pre-existing immunity to Ad5 and showed a reduced response in mice with pre-existing immunity to other human adenoviruses, such as serotypes 2, 4, 7, and 12 [27]. Furthermore, neutralizing antibodies against ChAd-68, ChAd-6, and ChAd-1 were frequently detected without any prior history of exposure, and their seroprevalence was reported to range from 21% to 44% [28,29,30]. The higher seroprevalence of neutralizing antibodies against chimpanzee adenoviruses might indicate the possibility of cross-species neutralization of these viruses [30,31]. These findings suggest that the host immune response may be affected by the cross-reaction of pre-existing Ad immunity despite the use of ChAdOx1. Specifically, this study found that the host immune response to the ChAdOx1 nCoV-19 vaccine was reduced when pre-existing immunity to adenoviruses was present.
In both groups, the seroconversion rate reached 100% after the second dose, but the overall median titer of S-specific IgG antibodies was lower in the group with pre-existing Ad immunity and was significantly reduced at time points T2–T4. For S-specific IgG titers at time point T2, seroconversion was not achieved in three individuals, all of whom had pre-existing Ad immunity. Seroconversion was achieved in all individuals 2–3 weeks after the second ChAdOx1 nCoV-19 dose (T3). However, at three months after second ChAdOx1 nCoV-19 dose (T4), both the median and minimum titers of S-specific IgG antibodies were reduced in the group with pre-existing Ad immunity. Furthermore, the ratio of S-specific IgG titers at time point T4 to S-specific IgG titers at time point T3 was lower in the group with pre-existing Ad immunity (31.7% (176.0/555.0) vs. 43.6% (274.5/629.5)). The fact that S-specific IgG titers in the group with pre-existing Ad immunity showed a lower peak and greater decline (68.3% vs. 56.4%) over the same period suggested that antibody waning might occur more quickly in these patients. This indicated that antibody response longevity was longer in the group without pre-existing Ad immunity.
In our study, the PRNT50 after ChAdOx1 nCoV-19 vaccination was also higher in individuals without pre-existing Ad immunity, and statistically significantly higher at 2–3 weeks after the first ChAdOx1 nCoV-19 dose (T1). The association between neutralizing antibody titer and clinical protection against COVID-19 is intricate and requires many considerations [32,33]. According to the findings of David S. Khoury and colleagues, neutralization titer serves as a significant predictor of both protective immune responses and the long-term dynamics of SARS-CoV-2 immunity [33]. The study also suggests that a decline in the neutralization titer post-immunization can result in a substantial loss of protection against SARS-CoV-2 infection, while protection against severe disease is expected to remain mostly intact [33].
Reactogenicity to the first ChAdOx1 dose (T1) was observed at a higher frequency in the group without pre-existing Ad immunity. In particular, systemic events, such as chills were significantly higher in individuals without pre-existing Ad immunity. However, reactogenicity to the second ChAdOx1 dose did not show a significant difference. The difference of immunogenicity after the first ChAdOx1 dose (T1) was also reduced after the second ChAdOx1 dose (T3 and T4). These observations suggested that repeated administration of adenoviral vectors reduces subsequent immune response. Successful gene delivery and expression were achieved upon repeated administration of adenoviral vectors, albeit at significantly reduced levels in comparison to the initial vector administration [34,35]. In the case of Ad5-vectored COVID-19 vaccine, pre-existing immunity to the Ad5 vector was associated with reduced humoral immune responses and lower occurrence of fever after vaccination [36].
Ad-derived vectors are used in various trials because of their advantages, such as efficient transduction, packaging of large amounts of foreign DNA, and rare integration into the host chromosome [2,3]. However, according to the results of recent population-based studies, the efficacy of Ad vector-based vaccines has decreased compared to mRNA COVID-19 vaccines, and mRNA COVID-19 vaccines are preferred as a booster vaccine for COVID-19 [11,12]. These results suggest that there are limitations to Ad vector-based vaccines due to the presence of pre-existing Ad immunity or repeated administration of Ad-derived vectors. Nevertheless, the fact that the seroconversion rate is increased by re-administration suggests that there is potential for improvement with a proper understanding of the immune response according to pre-existing Ad immunity.
This study has several limitations. First, because the sample size of this study was small, the statistical analysis was performed using a non-parametric test. To minimize the influence of confounding factors, the baseline characteristics and blood sampling times of the two groups were matched as closely as possible. Second, because the study was only conducted in Korea, various races could not be considered. However, since the study was conducted in a population with equal seroprevalence of Ad, the influence of confounding factors was also reduced. Third, this study only assessed the humoral immune response based on pre-existing Ad immunity. Further research examining the pre-existing and post-vaccination T cell response could offer additional insights into the subsequent immune response.
5. Conclusions
In conclusion, in individuals without pre-existing Ad immunity, the immune response to ChAdOx1 nCoV-19 vaccination was higher and reactogenicity to ChAdOx1 nCoV-19 vaccination was observed with a higher frequency. Seroconversion was achieved in all individuals after the second ChAdOx1 nCoV-19 dose; however, the humoral response to ChAdOx1 nCoV-19 vaccination lasted longer in individuals without pre-existing Ad immunity. Thus, for Ad-derived vaccination to have sufficient immune response and longevity, pre-existing Ad immunity must be evaluated.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/vaccines11040784/s1. Table S1. Reactogenicity to the second dose of ChAdOx1 nCoV-19 vaccine according to pre-existing adenovirus immunity.
Author Contributions
Conceptualization, J.K., J.H.K., J.Y.A., N.S.K., J.-S.Y. and J.Y.C.; methodology J.K., N.S.K., J.-S.Y. and J.Y.C.; formal analysis, J.K., S.J.J., J.-S.Y., Y.G.S. and J.Y.C.; investigation, J.K. and J.Y.C.; data curation, J.K., C.K., J.A.L., S.J.L., K.H.L., S.J.J. and Y.G.S.; writing original draft preparation, J.K. and J.Y.C.; writing review and editing, J.K., C.K., J.A.L., S.J.L., K.H.L., J.H.K., J.Y.A., S.J.J., N.S.K., J.-S.Y., Y.G.S. and J.Y.C.; visualization, J.K. and J.Y.C.; supervision, J.Y.C.; funding acquisition, J.Y.C. All authors have read and agreed to the published version of the manuscript.
Funding
J.Y.C. was supported by the Korea National Institute of Infectious Diseases, Korea National Institute of Health, Korea Disease Control and Prevention Agency [grant numbers #2021-ER1902–00, #2021-ER2601-00], and the 2020 Joint Research Project of Institutes of Science and Technology. The funders had no role in study design, data collection, data analysis, data interpretation, in the preparation of the manuscript, and in the decision to submit.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Yonsei University College of Medicine (IRB no. 4-2020-1377).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We thank Jee Hung Kim and Bio Joo from the Yonsei University College of Medicine for discussing the study design. We thank Youn-Jung Lee at the Division of Infectious Diseases in the Yonsei University College of Medicine for discussing the blood sample immunoassays.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Buchbinder, S.P.; Mehrotra, D.V.; Duerr, A.; Fitzgerald, D.W.; Mogg, R.; Li, D.; Gilbert, P.B.; Lama, J.R.; Marmor, M.; Del Rio, C.; et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): A double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet 2008, 372, 1881–1893. [Google Scholar] [CrossRef] [PubMed]
- Kreppel, F.; Hagedorn, C. Capsid and Genome Modification Strategies to Reduce the Immunogenicity of Adenoviral Vectors. Int. J. Mol. Sci. 2021, 22, 2417. [Google Scholar] [CrossRef] [PubMed]
- Zaiss, A.K.; Machado, H.B.; Herschman, H.R. The influence of innate and pre-existing immunity on adenovirus therapy. J. Cell. Biochem. 2009, 108, 778–790. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.P., 3rd; Fishbein, M.; Echavarria, M. Adenovirus. Semin. Respir. Crit. Care Med. 2011, 32, 494–511. [Google Scholar] [CrossRef]
- Harro, C.D.; Robertson, M.N.; Lally, M.A.; O’Neill, L.D.; Edupuganti, S.; Goepfert, P.A.; Mulligan, M.J.; Priddy, F.H.; Dubey, S.A.; Kierstead, L.S.; et al. Safety and immunogenicity of adenovirus-vectored near-consensus HIV type 1 clade B gag vaccines in healthy adults. AIDS Res. Hum. Retrovir. 2009, 25, 103–114. [Google Scholar] [CrossRef]
- Cotter, M.J.; Zaiss, A.K.; Muruve, D.A. Neutrophils interact with adenovirus vectors via Fc receptors and complement receptor 1. J. Virol. 2005, 79, 14622–14631. [Google Scholar] [CrossRef]
- Zaiss, A.K.; Vilaysane, A.; Cotter, M.J.; Clark, S.A.; Meijndert, H.C.; Colarusso, P.; Yates, R.M.; Petrilli, V.; Tschopp, J.; Muruve, D.A. Antiviral antibodies target adenovirus to phagolysosomes and amplify the innate immune response. J. Immunol. 2009, 182, 7058–7068. [Google Scholar] [CrossRef]
- Zak, D.E.; Andersen-Nissen, E.; Peterson, E.R.; Sato, A.; Hamilton, M.K.; Borgerding, J.; Krishnamurty, A.T.; Chang, J.T.; Adams, D.J.; Hensley, T.R.; et al. Merck Ad5/HIV induces broad innate immune activation that predicts CD8+ T-cell responses but is attenuated by preexisting Ad5 immunity. Proc. Natl. Acad. Sci. USA 2012, 109, E3503–E3512. [Google Scholar] [CrossRef]
- Varnavski, A.N.; Calcedo, R.; Bove, M.; Gao, G.; Wilson, J.M. Evaluation of toxicity from high-dose systemic administration of recombinant adenovirus vector in vector-naive and pre-immunized mice. Gene Ther. 2005, 12, 427–436. [Google Scholar] [CrossRef]
- Seregin, S.S.; Amalfitano, A. Overcoming pre-existing adenovirus immunity by genetic engineering of adenovirus-based vectors. Expert Opin. Biol. Ther. 2009, 9, 1521–1531. [Google Scholar] [CrossRef]
- Shrotri, M.; Navaratnam, A.M.D.; Nguyen, V.; Byrne, T.; Geismar, C.; Fragaszy, E.; Beale, S.; Fong, W.L.E.; Patel, P.; Kovar, J.; et al. Spike-antibody waning after second dose of BNT162b2 or ChAdOx1. Lancet 2021, 398, 385–387. [Google Scholar] [CrossRef] [PubMed]
- Naranbhai, V.; Garcia-Beltran, W.F.; Chang, C.C.; Berrios Mairena, C.; Thierauf, J.C.; Kirkpatrick, G.; Onozato, M.L.; Cheng, J.; St Denis, K.J.; Lam, E.C.; et al. Comparative Immunogenicity and Effectiveness of mRNA-1273, BNT162b2, and Ad26.COV2.S COVID-19 Vaccines. J. Infect. Dis. 2022, 225, 1141–1150. [Google Scholar] [CrossRef] [PubMed]
- Bewig, B.; Schmidt, W.E. Accelerated titering of adenoviruses. Biotechniques 2000, 28, 870–873. [Google Scholar] [CrossRef]
- Cheng, S.M.S.; Mok, C.K.P.; Leung, Y.W.Y.; Ng, S.S.; Chan, K.C.K.; Ko, F.W.; Chen, C.; Yiu, K.; Lam, B.H.S.; Lau, E.H.Y.; et al. Neutralizing antibodies against the SARS-CoV-2 Omicron variant BA.1 following homologous and heterologous CoronaVac or BNT162b2 vaccination. Nat. Med. 2022, 28, 486–489. [Google Scholar] [CrossRef] [PubMed]
- Lau, E.H.; Hui, D.S.; Tsang, O.T.; Chan, W.H.; Kwan, M.Y.; Chiu, S.S.; Cheng, S.M.; Ko, R.L.; Li, J.K.; Chaothai, S.; et al. Long-term persistence of SARS-CoV-2 neutralizing antibody responses after infection and estimates of the duration of protection. EClinicalMedicine 2021, 41, 101174. [Google Scholar] [CrossRef] [PubMed]
- Perera, R.A.; Mok, C.K.; Tsang, O.T.; Lv, H.; Ko, R.L.; Wu, N.C.; Yuan, M.; Leung, W.S.; Chan, J.M.; Chik, T.S.; et al. Serological assays for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), March 2020. Eurosurveillance 2020, 25, 2000421. [Google Scholar] [CrossRef]
- Lynch, J.P., 3rd; Kajon, A.E. Adenovirus: Epidemiology, Global Spread of Novel Serotypes, and Advances in Treatment and Prevention. Semin. Respir. Crit. Care Med. 2016, 37, 586–602. [Google Scholar] [CrossRef]
- Lynch, J.P., 3rd; Kajon, A.E. Adenovirus: Epidemiology, Global Spread of Novel Types, and Approach to Treatment. Semin. Respir. Crit. Care Med. 2021, 42, 800–821. [Google Scholar] [CrossRef]
- Wold, W.S.; Toth, K. Adenovirus vectors for gene therapy, vaccination and cancer gene therapy. Curr. Gene Ther. 2013, 13, 421–433. [Google Scholar] [CrossRef]
- Kolavic-Gray, S.A.; Binn, L.N.; Sanchez, J.L.; Cersovsky, S.B.; Polyak, C.S.; Mitchell-Raymundo, F.; Asher, L.V.; Vaughn, D.W.; Feighner, B.H.; Innis, B.L. Large epidemic of adenovirus type 4 infection among military trainees: Epidemiological, clinical, and laboratory studies. Clin. Infect. Dis. 2002, 35, 808–818. [Google Scholar] [CrossRef]
- Muruve, D.A.; Barnes, M.J.; Stillman, I.E.; Libermann, T.A. Adenoviral gene therapy leads to rapid induction of multiple chemokines and acute neutrophil-dependent hepatic injury in vivo. Hum. Gene Ther. 1999, 10, 965–976. [Google Scholar] [CrossRef] [PubMed]
- Yeung, R.; Eshaghi, A.; Lombos, E.; Blair, J.; Mazzulli, T.; Burton, L.; Drews, S.J. Characterization of culture-positive adenovirus serotypes from respiratory specimens in Toronto, Ontario, Canada: September 2007–June 2008. Virol. J. 2009, 6, 11. [Google Scholar] [CrossRef] [PubMed]
- Folegatti, P.M.; Ewer, K.J.; Aley, P.K.; Angus, B.; Becker, S.; Belij-Rammerstorfer, S.; Bellamy, D.; Bibi, S.; Bittaye, M.; Clutterbuck, E.A.; et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: A preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 2020, 396, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Farina, S.F.; Gao, G.P.; Xiang, Z.Q.; Rux, J.J.; Burnett, R.M.; Alvira, M.R.; Marsh, J.; Ertl, H.C.; Wilson, J.M. Replication-defective vector based on a chimpanzee adenovirus. J. Virol. 2001, 75, 11603–11613. [Google Scholar] [CrossRef] [PubMed]
- Folegatti, P.M.; Jenkin, D.; Morris, S.; Gilbert, S.; Kim, D.; Robertson, J.S.; Smith, E.R.; Martin, E.; Gurwith, M.; Chen, R.T. Vaccines based on the replication-deficient simian adenoviral vector ChAdOx1: Standardized template with key considerations for a risk/benefit assessment. Vaccine 2022, 40, 5248–5262. [Google Scholar] [CrossRef]
- Rux, J.J.; Kuser, P.R.; Burnett, R.M. Structural and phylogenetic analysis of adenovirus hexons by use of high-resolution X-ray crystallographic, molecular modeling, and sequence-based methods. J. Virol. 2003, 77, 9553–9566. [Google Scholar] [CrossRef]
- Xiang, Z.; Gao, G.; Reyes-Sandoval, A.; Cohen, C.J.; Li, Y.; Bergelson, J.M.; Wilson, J.M.; Ertl, H.C. Novel, chimpanzee serotype 68-based adenoviral vaccine carrier for induction of antibodies to a transgene product. J. Virol. 2002, 76, 2667–2675. [Google Scholar] [CrossRef]
- Dudareva, M.; Andrews, L.; Gilbert, S.C.; Bejon, P.; Marsh, K.; Mwacharo, J.; Kai, O.; Nicosia, A.; Hill, A.V. Prevalence of serum neutralizing antibodies against chimpanzee adenovirus 63 and human adenovirus 5 in Kenyan children, in the context of vaccine vector efficacy. Vaccine 2009, 27, 3501–3504. [Google Scholar] [CrossRef]
- Zhao, H.; Xu, C.; Luo, X.; Wei, F.; Wang, N.; Shi, H.; Ren, X. Seroprevalence of Neutralizing Antibodies against Human Adenovirus Type-5 and Chimpanzee Adenovirus Type-68 in Cancer Patients. Front. Immunol. 2018, 9, 335. [Google Scholar] [CrossRef]
- Ersching, J.; Hernandez, M.I.; Cezarotto, F.S.; Ferreira, J.D.; Martins, A.B.; Switzer, W.M.; Xiang, Z.; Ertl, H.C.; Zanetti, C.R.; Pinto, A.R. Neutralizing antibodies to human and simian adenoviruses in humans and New-World monkeys. Virology 2010, 407, 1–6. [Google Scholar] [CrossRef]
- Xiang, Z.; Li, Y.; Cun, A.; Yang, W.; Ellenberg, S.; Switzer, W.M.; Kalish, M.L.; Ertl, H.C. Chimpanzee adenovirus antibodies in humans, sub-Saharan Africa. Emerg. Infect. Dis. 2006, 12, 1596–1599. [Google Scholar] [CrossRef]
- Sekine, T.; Perez-Potti, A.; Rivera-Ballesteros, O.; Strålin, K.; Gorin, J.B.; Olsson, A.; Llewellyn-Lacey, S.; Kamal, H.; Bogdanovic, G.; Muschiol, S.; et al. Robust T Cell Immunity in Convalescent Individuals with Asymptomatic or Mild COVID-19. Cell 2020, 183, 158–168.e114. [Google Scholar] [CrossRef] [PubMed]
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Abbink, P.; Lemckert, A.A.; Ewald, B.A.; Lynch, D.M.; Denholtz, M.; Smits, S.; Holterman, L.; Damen, I.; Vogels, R.; Thorner, A.R.; et al. Comparative seroprevalence and immunogenicity of six rare serotype recombinant adenovirus vaccine vectors from subgroups B and D. J. Virol. 2007, 81, 4654–4663. [Google Scholar] [CrossRef] [PubMed]
- Lasaro, M.O.; Ertl, H.C. New insights on adenovirus as vaccine vectors. Mol. Ther. 2009, 17, 1333–1339. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.C.; Guan, X.H.; Li, Y.H.; Huang, J.Y.; Jiang, T.; Hou, L.H.; Li, J.X.; Yang, B.F.; Wang, L.; Wang, W.J.; et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 2020, 396, 479–488. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).