Quadrivalent Formulation of Intranasal Influenza Vaccine M2SR (M2-Deficient Single Replication) Protects against Drifted Influenza A and B Virus Challenge
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
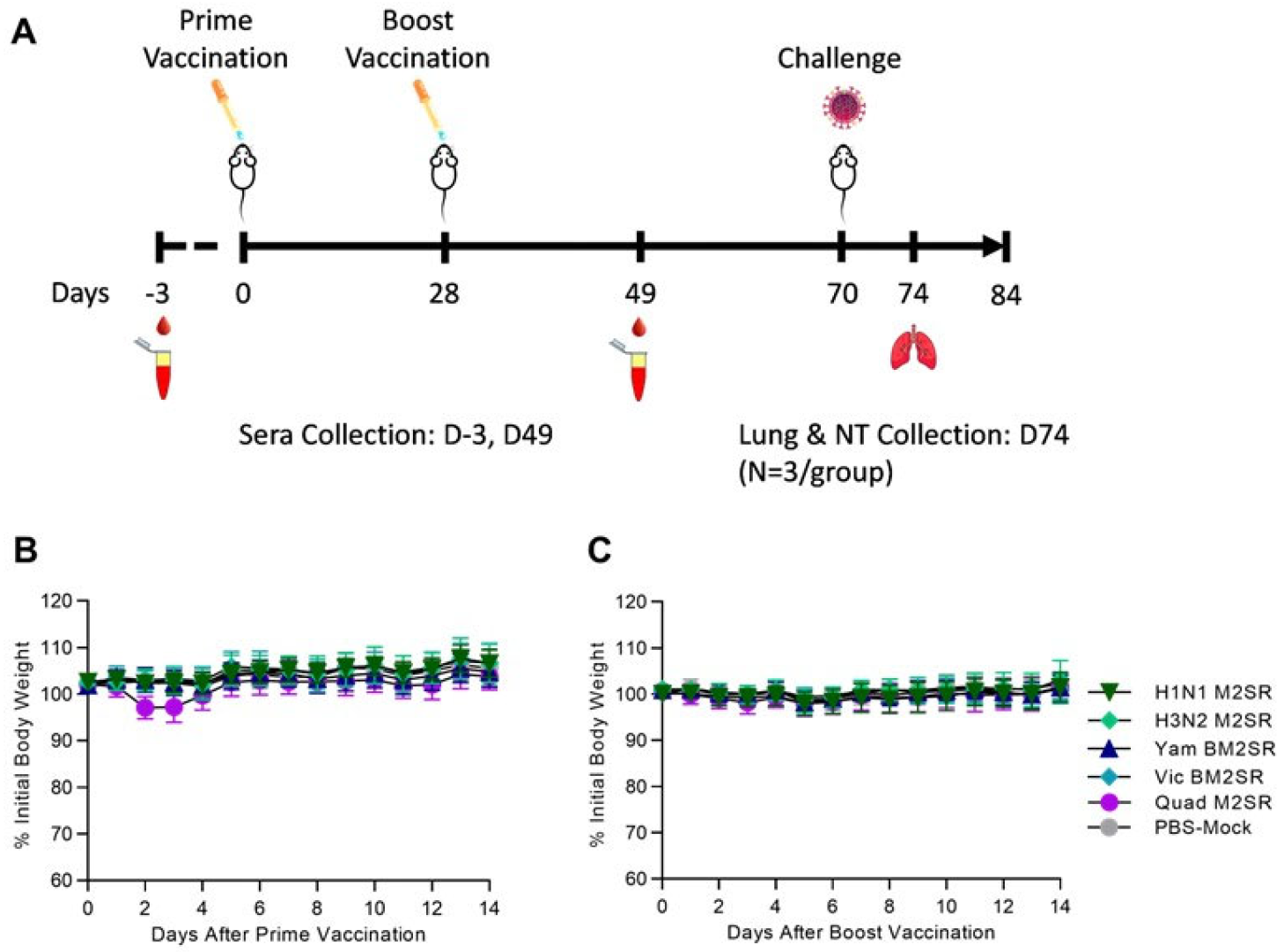
2.2. Mouse Infection and Sample Collection
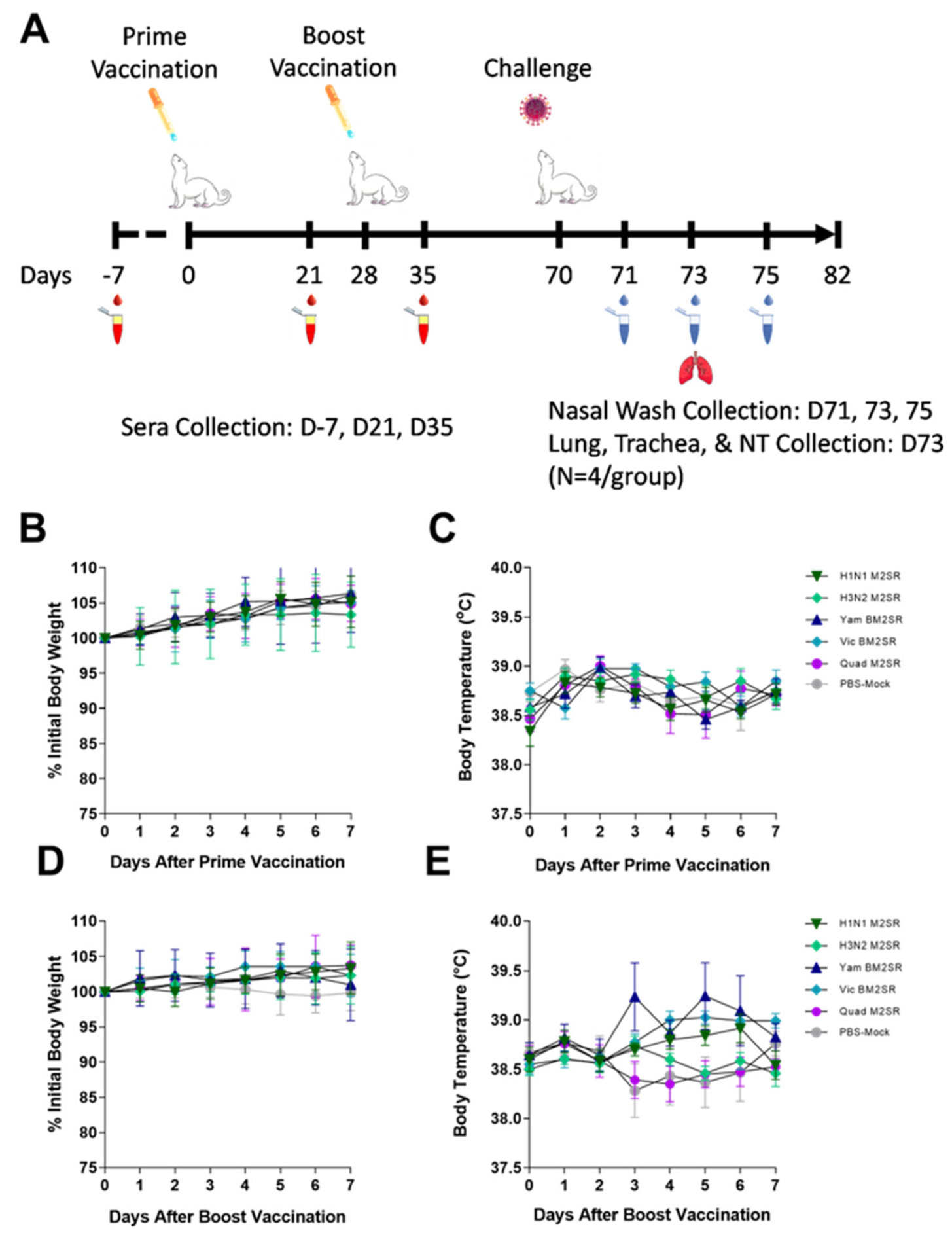
2.3. Ferret Infection and Sample Collection
2.4. Virus Neutralization Assay
3. Results
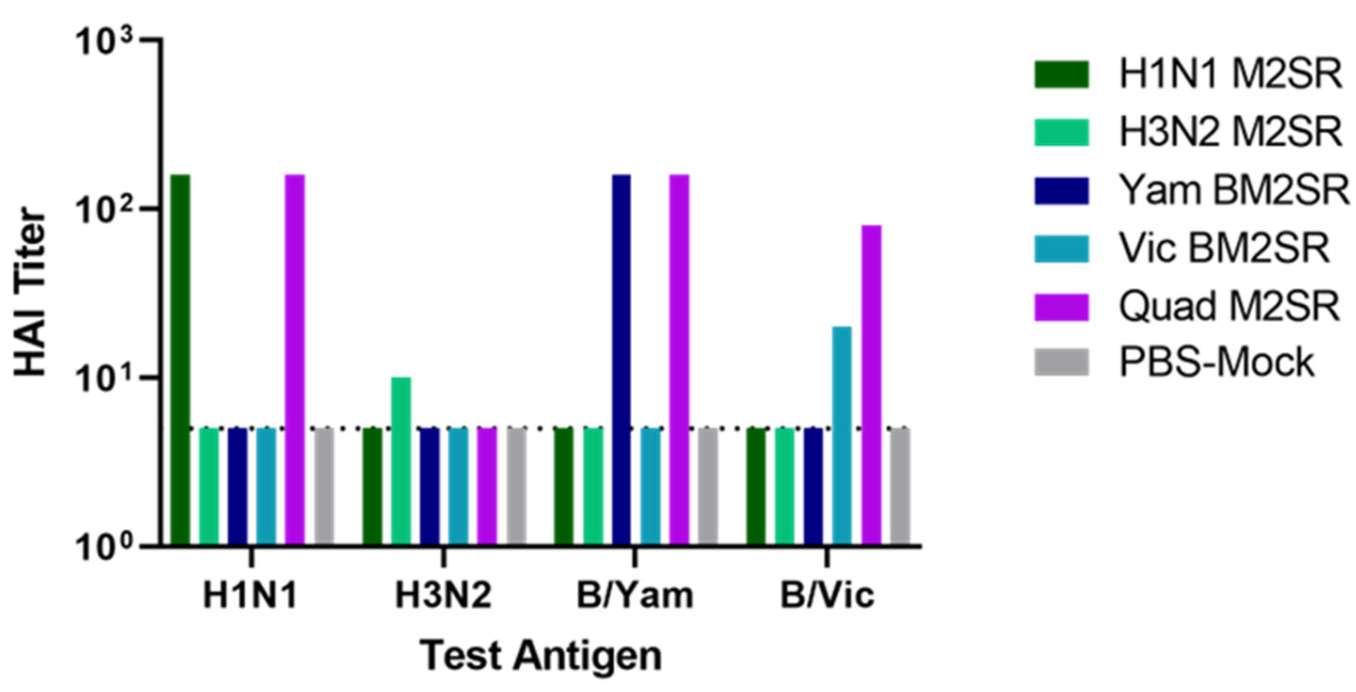
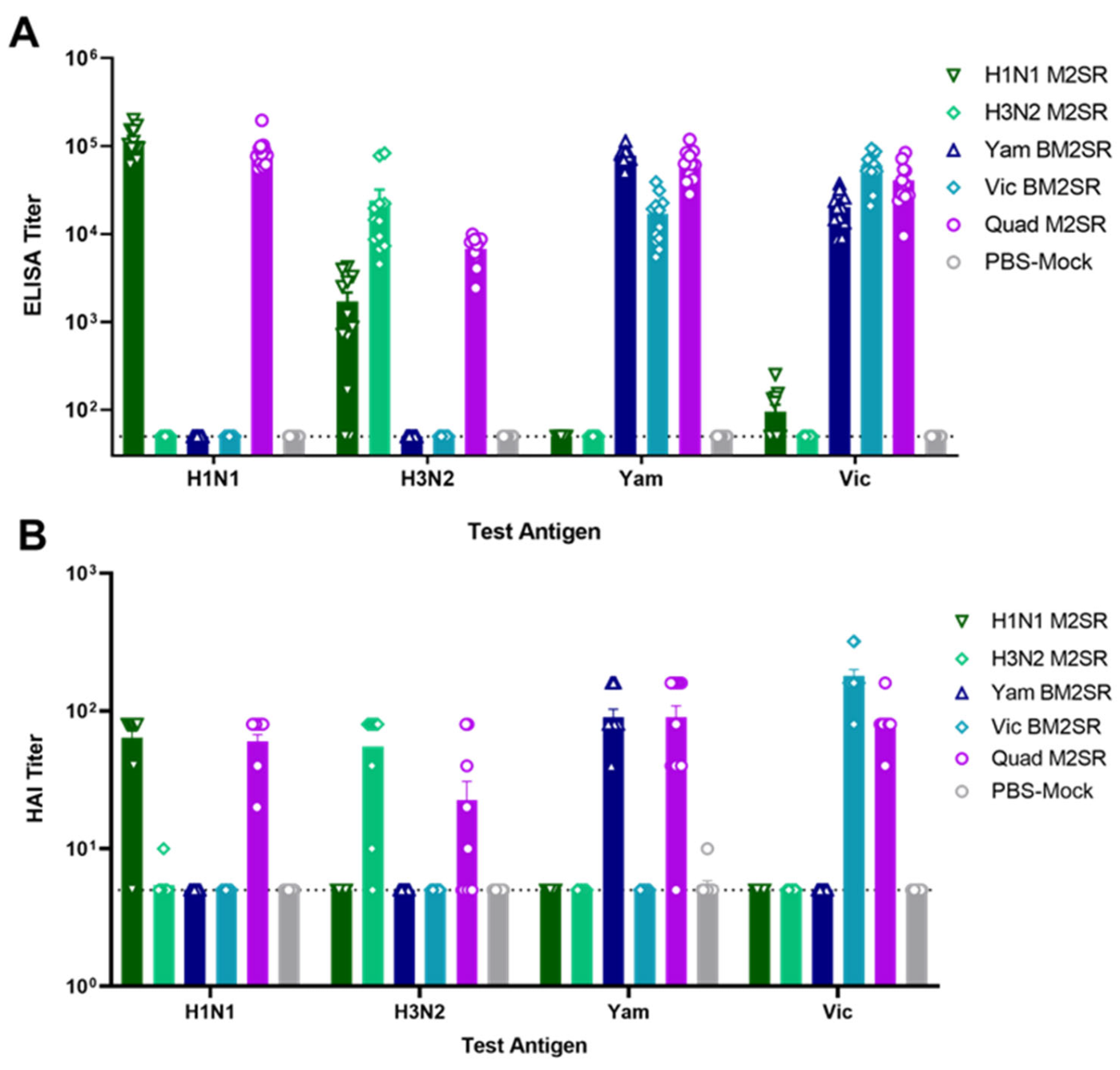
3.1. Quadrivalent M2SR Is Safe In Vivo and Elicits Functional Antibody Responses to Each Component within the Multivalent Formulation
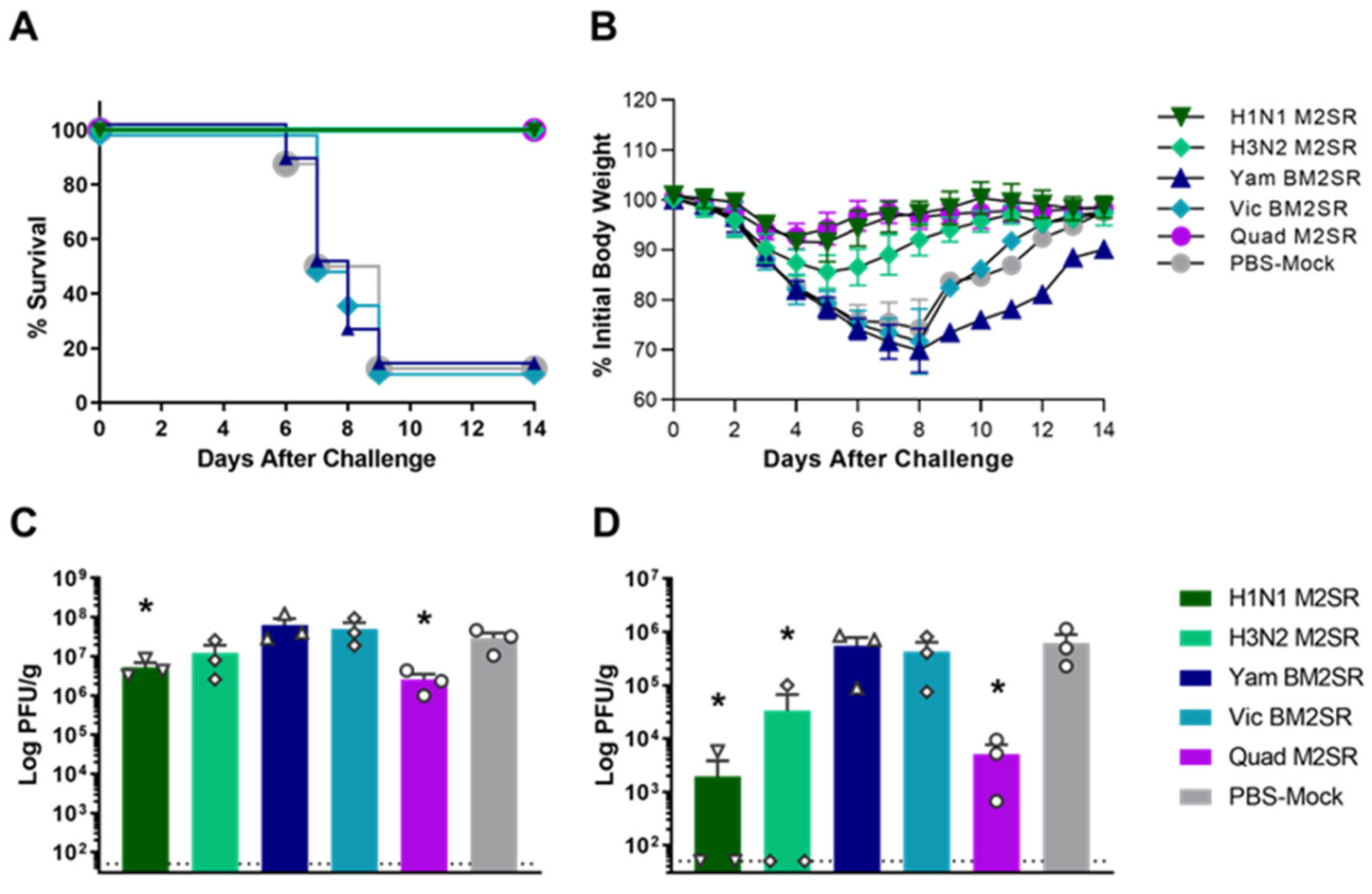
3.2. Quadrivalent M2SR Protects Mice against Lethal Influenza A and B Challenge
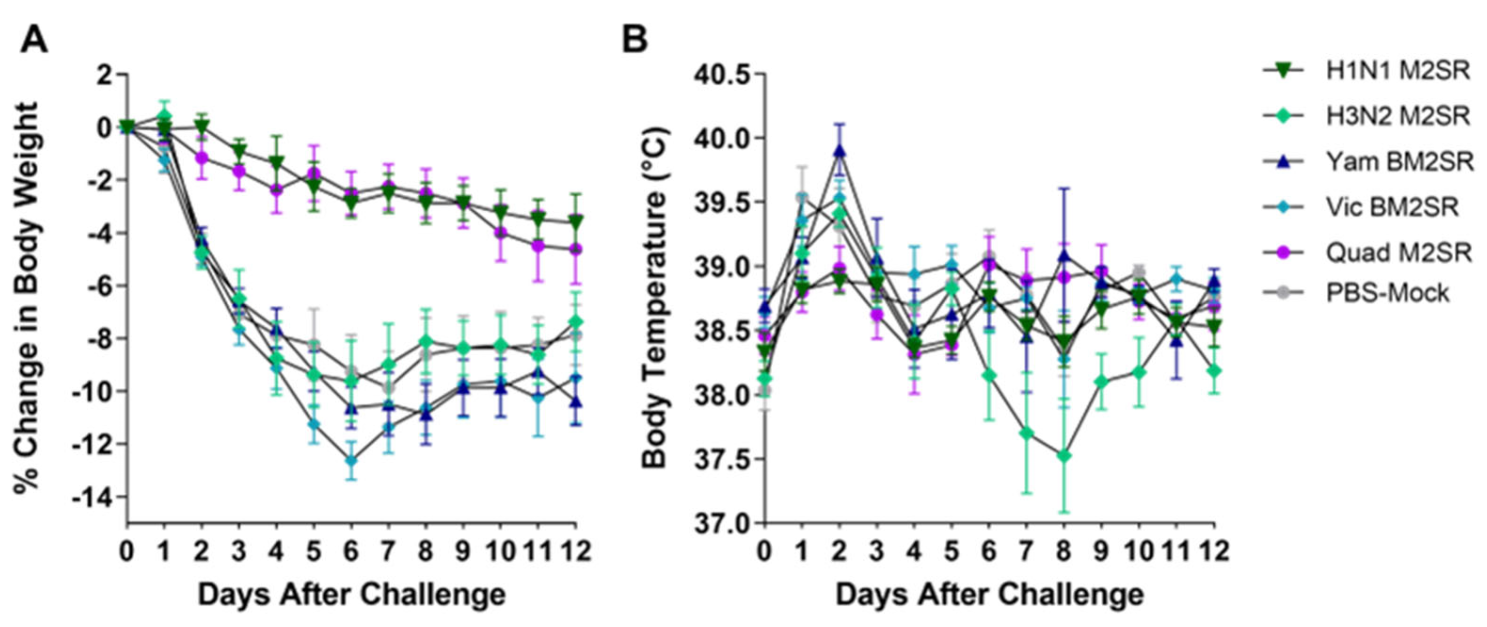
3.3. Quadrivalent M2SR Is Well Tolerated in the Ferret Model
3.4. Quadrivalent M2SR Elicits Antibody Responses toward all 4 Influenza Strains in the Ferret Model
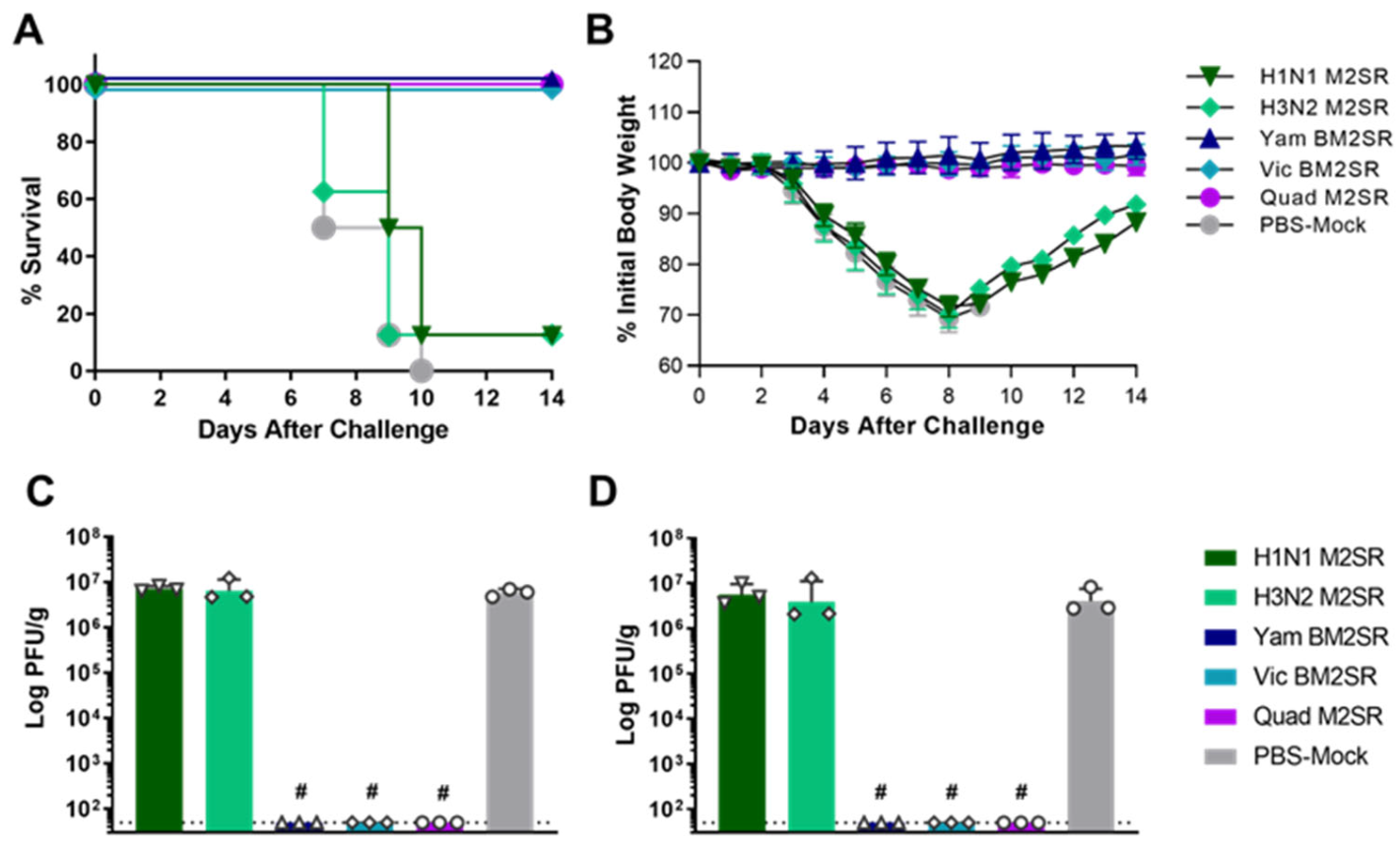
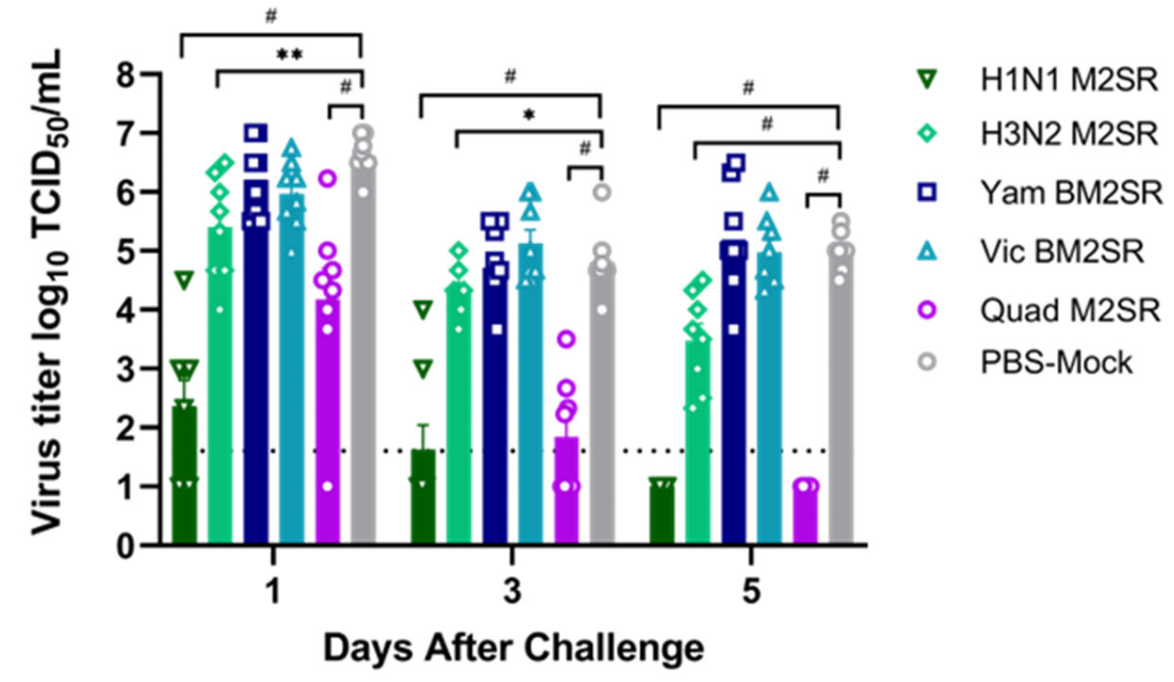
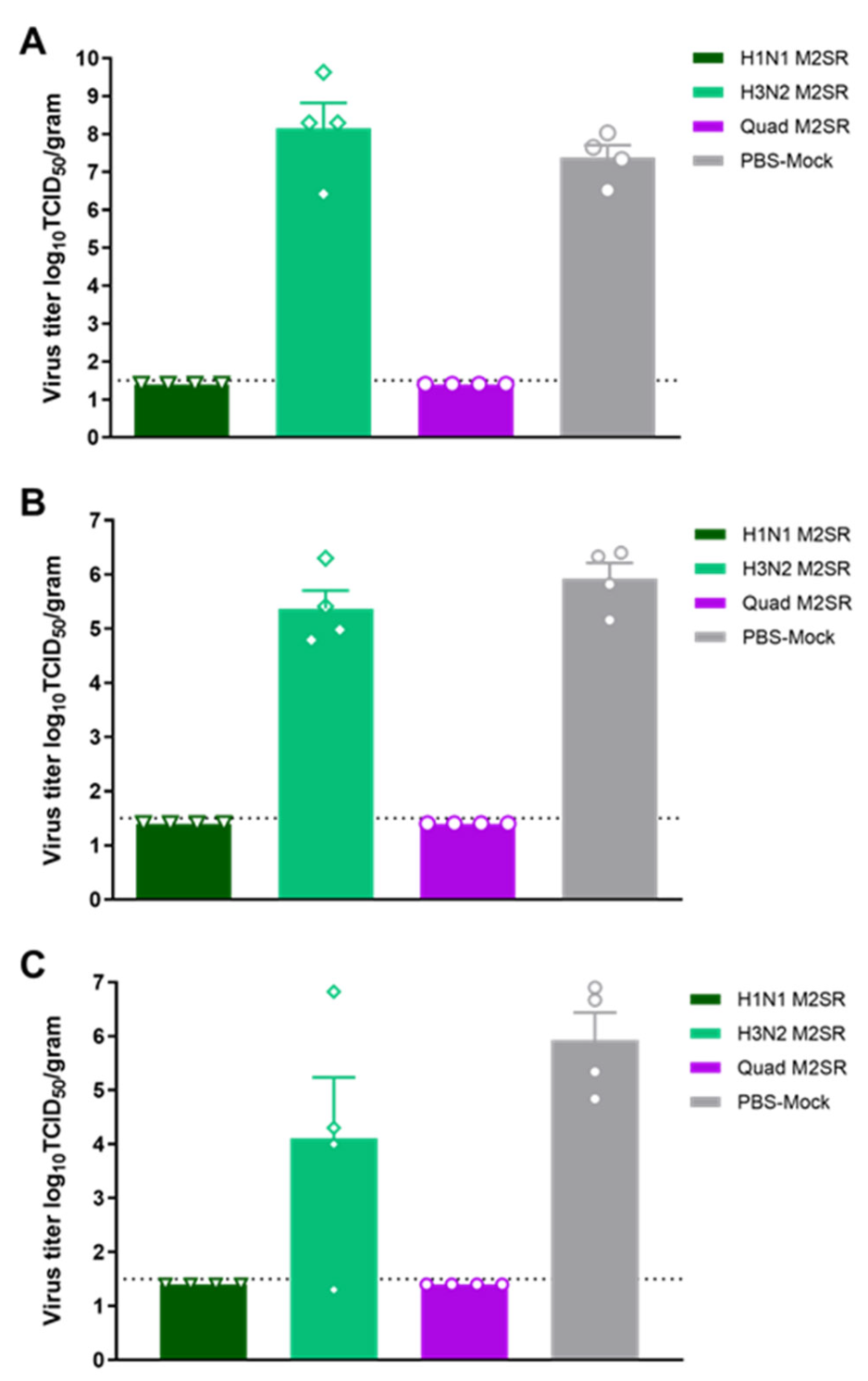
3.5. Quadrivalent M2SR Protects Ferrets against H1N1pdm Influenza a Virus Challenge
3.6. Quadrivalent M2SR Elicits Robust Neutralizing Antibody Responses against Past- and Future-Drifted Influenza B Strains
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rolfes, M.A.; Flannery, B.; Chung, J.R.; O’Halloran, A.; Garg, S.; Belongia, E.A.; Gaglani, M.; Zimmerman, R.K.; Jackson, M.L.; Monto, A.S.; et al. Effects of Influenza Vaccination in the United States During the 2017–2018 Influenza Season. Clin. Infect. Dis. 2019, 69, 1845–1853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grohskopf, L.A.; Alyanak, E.; Ferdinands, J.M.; Broder, K.R.; Blanton, L.H.; Talbot, H.K.; Fry, A.M. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices, United States, 2021–2022 Influenza Season. MMWR Recomm. Rep. 2021, 70, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). CDC Seasonal Flu Vaccine Effectiveness Studies. Available online: https://www.cdc.gov/flu/vaccines-work/effectiveness-studies.htm (accessed on 23 December 2022).
- World Health Organization (WHO). Global Influenza Surveillance and Response System (GISRS). Available online: https://www.who.int/initiatives/global-influenza-surveillance-and-response-system (accessed on 29 March 2023).
- Flannery, B.; Clippard, J.; Zimmerman, R.K.; Nowalk, M.P.; Jackson, M.L.; Jackson, L.A.; Monto, A.S.; Petrie, J.G.; McLean, H.Q.; Belongia, E.A.; et al. Early estimates of seasonal influenza vaccine effectiveness—United States, January 2015. MMWR Morb. Mortal. Wkly. Rep. 2015, 64, 10–15. [Google Scholar] [PubMed]
- Skowronski, D.M.; Chambers, C.; Sabaiduc, S.; De Serres, G.; Winter, A.L.; Dickinson, J.A.; Krajden, M.; Gubbay, J.B.; Drews, S.J.; Martineau, C.; et al. A Perfect Storm: Impact of Genomic Variation and Serial Vaccination on Low Influenza Vaccine Effectiveness During the 2014–2015 Season. Clin. Infect. Dis. 2016, 63, 21–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Centers for Disease Control and Prevention (CDC). 2014–2015 Influenza Season Week 53 Ending January 3. 2015. Available online: http://www.cdc.gov/flu/weekly (accessed on 23 December 2022).
- Sarawar, S.; Hatta, Y.; Watanabe, S.; Dias, P.; Neumann, G.; Kawaoka, Y.; Bilsel, P. M2SR, a novel live single replication influenza virus vaccine, provides effective heterosubtypic protection in mice. Vaccine 2016, 34, 5090–5098. [Google Scholar] [CrossRef] [Green Version]
- Hatta, Y.; Boltz, D.; Sarawar, S.; Kawaoka, Y.; Neumann, G.; Bilsel, P. M2SR, a novel live influenza vaccine, protects mice and ferrets against highly pathogenic avian influenza. Vaccine 2017, 35, 4177–4183. [Google Scholar] [CrossRef]
- Moser, M.J.; Hatta, Y.; Gabaglia, C.; Sanchez, A.; Dias, P.; Sarawar, S.; Kawaoka, Y.; Hatta, M.; Neumann, G.; Bilsel, P. Single-replication BM2SR vaccine provides sterilizing immunity and cross-lineage influenza B virus protection in mice. Vaccine 2019, 37, 4533–4542. [Google Scholar] [CrossRef]
- Hatta, Y.; Boltz, D.; Sarawar, S.; Kawaoka, Y.; Neumann, G.; Bilsel, P. Novel influenza vaccine M2SR protects against drifted H1N1 and H3N2 influenza virus challenge in ferrets with pre-existing immunity. Vaccine 2018, 36, 5097–5103. [Google Scholar] [CrossRef]
- Eiden, J.; Fierro, C.; Schwartz, H.; Adams, M.; Ellis, K.J.; Aitchison, R.; Herber, R.; Hatta, Y.; Marshall, D.; Moser, M.J.; et al. Intranasal M2SR (M2-deficient Single Replication) H3N2 Influenza Vaccine Provides Enhanced Mucosal and Serum Antibodies in Adults. J. Infect. Dis. 2022, 227, 103–112. [Google Scholar] [CrossRef]
- Eiden, J.; Gordon, G.; Fierro, C.; Herber, R.; Aitchison, R.; Belshe, R.; Greenberg, H.; Hoft, D.; Hatta, Y.; Moser, M.J.; et al. Safety and Immunogenicity of M2-Deficient, Single Replication, Live Influenza Vaccine (M2SR) in Adults. Vaccines 2021, 9, 1388. [Google Scholar] [CrossRef]
- Eiden, J.; Volckaert, B.; Rudenko, O.; Aitchison, R.; Herber, R.; Belshe, R.; Greenberg, H.; Coelingh, K.; Marshall, D.; Kawaoka, Y.; et al. M2-Deficient Single-Replication Influenza Vaccine-Induced Immune Responses Associated with Protection against Human Challenge with Highly Drifted H3N2 Influenza Strain. J. Infect. Dis. 2022, 226, 83–90. [Google Scholar] [CrossRef]
- Iwatsuki-Horimoto, K.; Horimoto, T.; Noda, T.; Kiso, M.; Maeda, J.; Watanabe, S.; Muramoto, Y.; Fujii, K.; Kawaoka, Y. The cytoplasmic tail of the influenza A virus M2 protein plays a role in viral assembly. J. Virol. 2006, 80, 5233–5240. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, T.; Watanabe, S.; Kim, J.H.; Hatta, M.; Kawaoka, Y. Novel approach to the development of effective H5N1 influenza A virus vaccines: Use of M2 cytoplasmic tail mutants. J. Virol. 2008, 82, 2486–2492. [Google Scholar] [CrossRef] [Green Version]
- Hadfield, J.; Megill, C.; Bell, S.M.; Huddleston, J.; Potter, B.; Callender, C.; Sagulenko, P.; Bedford, T.; Neher, R.A. Nextstrain: Real-time tracking of pathogen evolution. Bioinformatics 2018, 34, 4121–4123. [Google Scholar] [CrossRef] [Green Version]
- Ambrose, C.S.; Levin, M.J. The rationale for quadrivalent influenza vaccines. Hum. Vaccines Immunother. 2012, 8, 81–88. [Google Scholar] [CrossRef] [Green Version]
- van de Sandt, C.E.; Dou, Y.; Vogelzang-van Trierum, S.E.; Westgeest, K.B.; Pronk, M.R.; Osterhaus, A.; Fouchier, R.A.M.; Rimmelzwaan, G.F.; Hillaire, M.L.B. Influenza B virus-specific CD8+ T-lymphocytes strongly cross-react with viruses of the opposing influenza B lineage. J. Gen. Virol. 2015, 96, 2061–2073. [Google Scholar] [CrossRef]
- Kiseleva, I.; Krutikova, E.; Stepanova, E.; Donina, S.; Pisareva, M.; Krivitskaya, V.; Rekstin, A.; Sparrow, E.G.; Torelli, G.; Rudenko, L. Cross-Protective Efficacy of Monovalent Live Influenza B Vaccines against Genetically Different Lineages of B/Victoria and B/Yamagata in Ferrets. Biomed. Res. Int. 2018, 2018, 9695628. [Google Scholar] [CrossRef] [Green Version]
- Bandell, A.; Woo, J.; Coelingh, K. Protective efficacy of live-attenuated influenza vaccine (multivalent, Ann Arbor strain): A literature review addressing interference. Expert Rev. Vaccines 2011, 10, 1131–1141. [Google Scholar] [CrossRef] [Green Version]
- Wright, P.F.; Hoen, A.G.; Ilyushina, N.A.; Brown, E.P.; Ackerman, M.E.; Wieland-Alter, W.; Connor, R.I.; Jegaskanda, S.; Rosenberg-Hasson, Y.; Haynes, B.C.; et al. Correlates of Immunity to Influenza as Determined by Challenge of Children with Live, Attenuated Influenza Vaccine. Open Forum Infect. Dis. 2016, 3, ofw108. [Google Scholar] [CrossRef] [Green Version]
- Jegaskanda, S.; Luke, C.; Hickman, H.D.; Sangster, M.Y.; Wieland-Alter, W.F.; McBride, J.M.; Yewdell, J.W.; Wright, P.F.; Treanor, J.; Rosenberger, C.M.; et al. Generation and Protective Ability of Influenza Virus-Specific Antibody-Dependent Cellular Cytotoxicity in Humans Elicited by Vaccination, Natural Infection, and Experimental Challenge. J. Infect. Dis. 2016, 214, 945–952. [Google Scholar] [CrossRef] [Green Version]
- Sarawar, S.; Gabaglia, C.R.; Sanchez, A.; Hatta, Y.; Dias, P.; Neumann, G.; Kawaoka, Y.; Bilsel, P. Longevity and Mechanism of Heterosubtypic Protection Induced by M2SR (M2-Deficient Single-Replication) Live Influenza Virus Vaccine in Mice. Vaccines 2022, 10, 2131. [Google Scholar] [CrossRef] [PubMed]
- Belshe, R.B. The need for quadrivalent vaccine against seasonal influenza. Vaccine 2010, 28 (Suppl. S4), D45–D53. [Google Scholar] [CrossRef] [PubMed]

| Vaccine | HA and NA Components | Dose |
|---|---|---|
| H1N1 M2SR | A/California/07/2009 | 1 × 107 TCID50 |
| H3N2 M2SR | A/Brisbane/10/2007 | 1 × 107 TCID50 |
| Vic M2SR | B/Brisbane/60/2008 | 1 × 107 TCID50 |
| Yam M2SR | B/Wisconsin/01/2010 | 1 × 107 TCID50 |
| M2SR Quad | A/California/07/2009 | 4 × 107 TCID50 |
| A/Brisbane/10/2007 | ||
| B/Brisbane/60/2008 | ||
| B/Wisconsin/01/2010 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hill-Batorski, L.; Hatta, Y.; Moser, M.J.; Sarawar, S.; Neumann, G.; Kawaoka, Y.; Bilsel, P. Quadrivalent Formulation of Intranasal Influenza Vaccine M2SR (M2-Deficient Single Replication) Protects against Drifted Influenza A and B Virus Challenge. Vaccines 2023, 11, 798. https://doi.org/10.3390/vaccines11040798
Hill-Batorski L, Hatta Y, Moser MJ, Sarawar S, Neumann G, Kawaoka Y, Bilsel P. Quadrivalent Formulation of Intranasal Influenza Vaccine M2SR (M2-Deficient Single Replication) Protects against Drifted Influenza A and B Virus Challenge. Vaccines. 2023; 11(4):798. https://doi.org/10.3390/vaccines11040798
Chicago/Turabian StyleHill-Batorski, Lindsay, Yasuko Hatta, Michael J. Moser, Sally Sarawar, Gabriele Neumann, Yoshihiro Kawaoka, and Pamuk Bilsel. 2023. "Quadrivalent Formulation of Intranasal Influenza Vaccine M2SR (M2-Deficient Single Replication) Protects against Drifted Influenza A and B Virus Challenge" Vaccines 11, no. 4: 798. https://doi.org/10.3390/vaccines11040798





