SARS-CoV-2 RBD Conjugated to Polyglucin, Spermidine, and dsRNA Elicits a Strong Immune Response in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plasmid Construction, Recombinant Protein Expression, and Purification
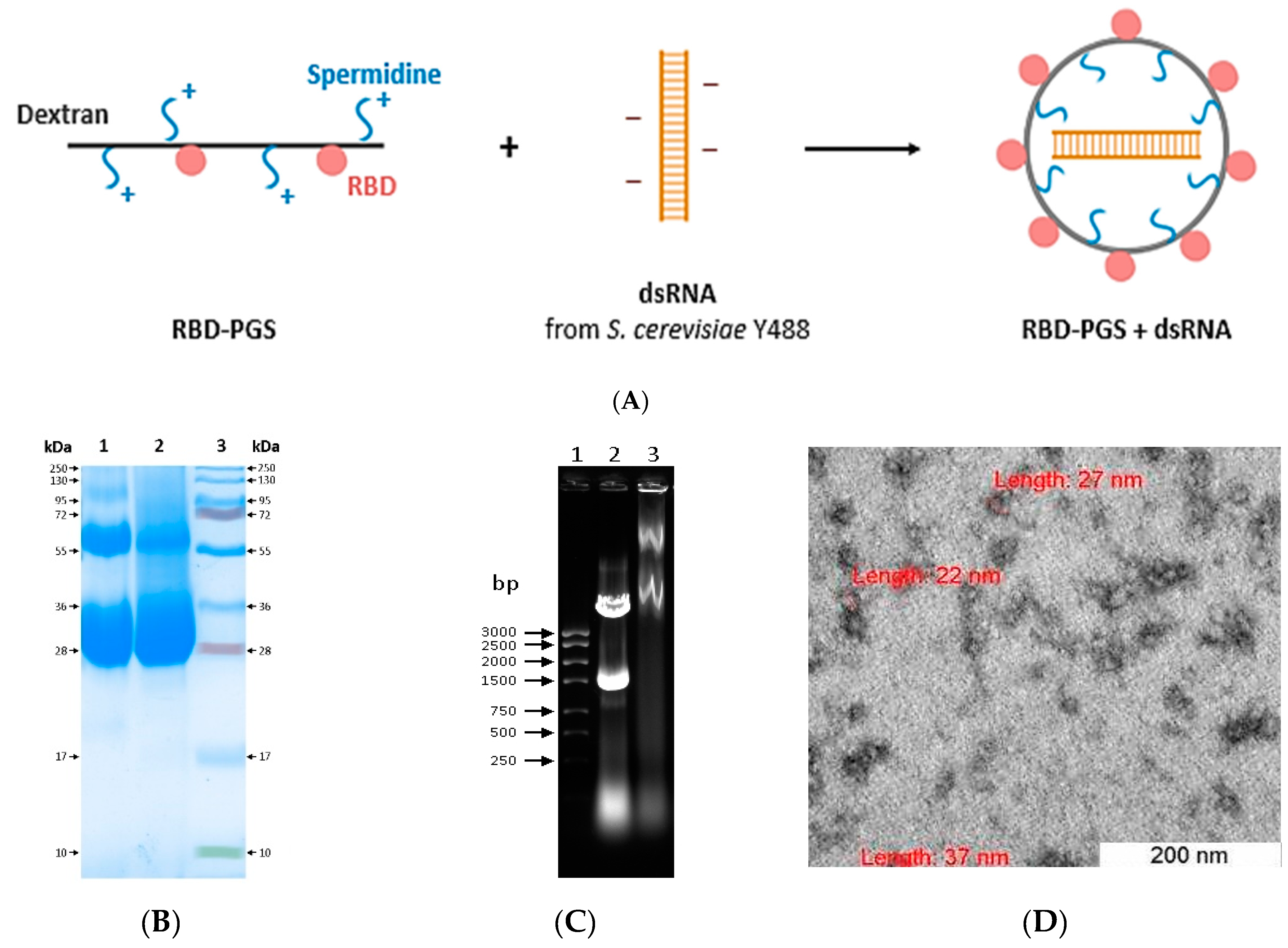
2.2. Synthesis of Polyglucin: Spermidine-RBD (PGS-RBD) Conjugates
2.3. RBD-PGS + dsRNA Particle Assembly
2.4. Dynamic and Electrophoretic Light Scattering
2.5. Animal Immunization
2.6. ELISAs
2.7. Viral Neutralization Test
2.8. IFN-γ ELISpot
2.9. Intracellular Cytokine Staining
2.10. Statistical Analysis
3. Results
3.1. Preparation and Characterization of RBD Conjugated to Polyglucin:Spermidine and dsRNA
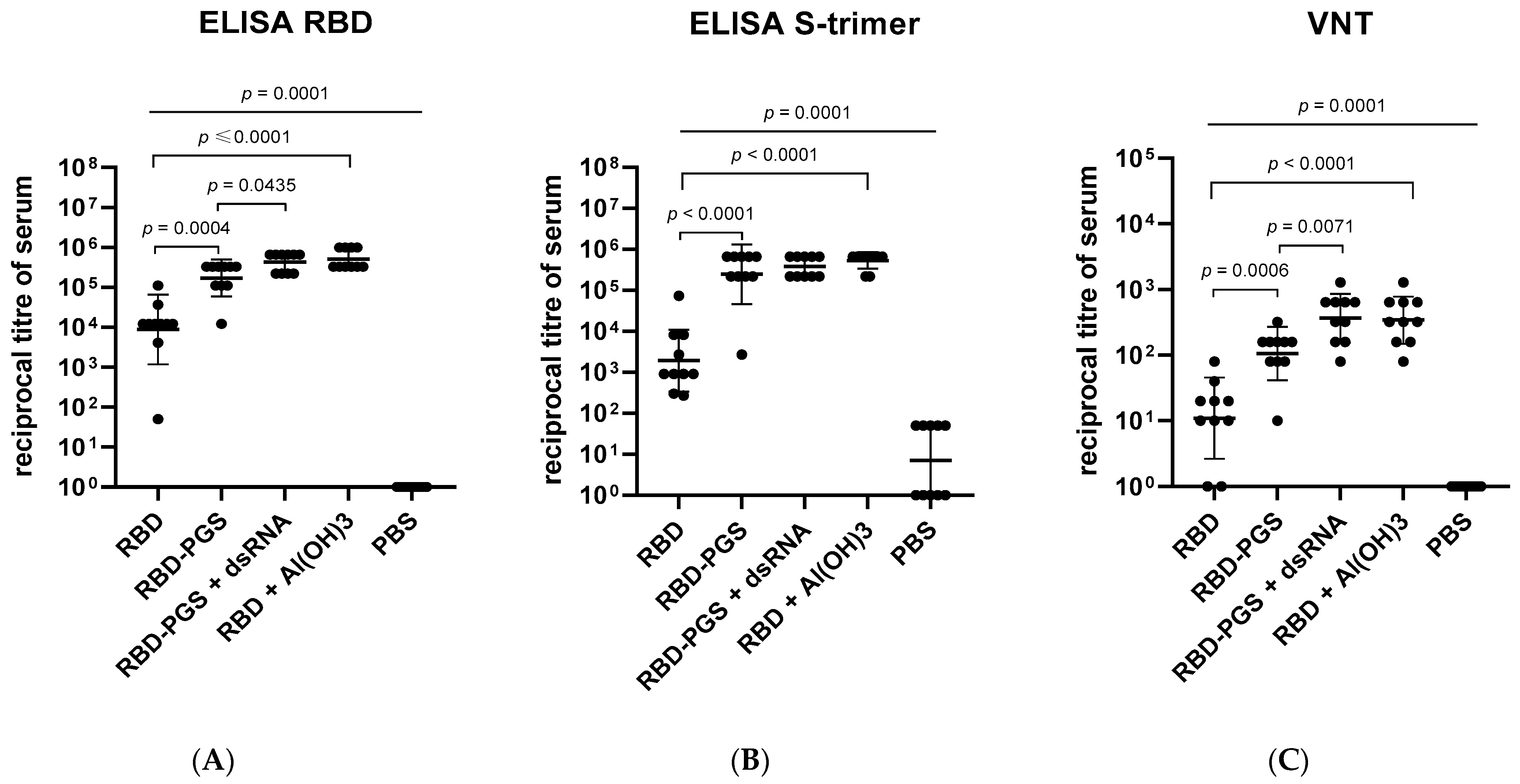
3.2. Humoral Immune Response
3.3. Cellular Immune Response
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barton, M.I.; MacGowan, S.A.; Kutuzov, M.A.; Dushek, O.; Barton, G.J.; Van Der Merwe, P.A. Effects of common mutations in the SARS-CoV-2 Spike RBD and its ligand, the human ACE2 receptor on binding affinity and kinetics. Elife 2021, 10, e70658. [Google Scholar] [CrossRef] [PubMed]
- Chavda, V.P.; Patel, A.B.; Vaghasiya, D.D. SARS-CoV-2 variants and vulnerability at the global level. J. Med. Virol. 2022, 94, 2986–3005. [Google Scholar] [CrossRef] [PubMed]
- Harvey, W.T.; Carabelli, A.M.; Jackson, B.; Gupta, R.K.; Thomson, E.C.; Harrison, E.M.; Ludden, C.; Reeve, R.; Rambaut, A.; COVID-19 Genomics UK (COG-UK) Consortium; et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat. Rev. Microbiol. 2021, 19, 409–424. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Stahel, V.P. The safety of COVID-19 mRNA vaccines: A review. Patient Saf. Surg. 2021, 15, 1–9. [Google Scholar]
- Beaudoin, C.A.; Bartas, M.; Volná, A.; Pečinka, P.; Blundell, T.L. Are There Hidden Genes in DNA/RNA Vaccines? Front Immunol. 2022, 13, 801915. [Google Scholar] [CrossRef]
- Switzer, C.; Loeb, M. Evaluating the relationship between myocarditis and mRNA vaccination. Expert Rev. Vaccines 2022, 21, 83–89. [Google Scholar] [CrossRef]
- Novak, N.; Tordesillas, L.; Cabanillas, B. Adverse rare events to vaccines for COVID-19: From hypersensitivity reactions to thrombosis and thrombocytopenia. Int. Rev. Immunol. 2022, 41, 438–447. [Google Scholar] [CrossRef]
- Das, S.K.; Paul, M.; Behera, B.C.; Thatoi, H. Current status of COVID-19 vaccination: Safety and liability concern for children, pregnant and lactating women. Expert Rev. Vaccines 2022, 21, 825–842. [Google Scholar] [CrossRef]
- Merkuleva, I.A.; Shcherbakov, D.N.; Borgoyakova, M.B.; Isaeva, A.A.; Nesmeyanova, V.S.; Volkova, N.V.; Aripov, V.S.; Shanshin, D.V.; Karpenko, L.I.; Belenkaya, S.V.; et al. Are Hamsters a Suitable Model for Evaluating the Immunogenicity of RBD-Based Anti-COVID-19 Subunit Vaccines? Viruses 2022, 14, 1060. [Google Scholar] [CrossRef]
- He, P.; Zou, Y.; Hu, Z. Advances in aluminum hydroxide-based adjuvant research and its mechanism. Hum. Vaccin. Immunother. 2015, 11, 477–488. [Google Scholar] [CrossRef]
- Bouazzaoui, A.; Abdellatif, A.A.H.; Al-Allaf, F.A.; Bogari, N.M.; Al-Dehlawi, S.; Qari, S.H. Strategies for Vaccination: Conventional Vaccine Approaches Versus New-Generation Strategies in Combination with Adjuvants. Pharmaceutics 2021, 13, 140. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Zheng, T.; Xu, K.; Han, Y.; Xu, L.; Huang, E.; An, Y.; Cheng, Y.; Li, S.; Liu, M.; et al. A Universal Design of Betacoronavirus Vaccines against COVID-19, MERS, and SARS. Cell 2020, 182, 722–733.e11. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Li, Y.; Dai, L.; Wang, J.; He, P.; Li, C.; Fang, X.; Wang, C.; Zhao, X.; Huang, E.; et al. Safety and immunogenicity of a recombinant tandem-repeat dimeric RBD-based protein subunit vaccine (ZF2001) against COVID-19 in adults: Two randomised, double-blind, placebo-controlled, phase 1 and 2 trials. Lancet Infect Dis. 2021, 21, 1107–1119. [Google Scholar] [CrossRef] [PubMed]
- Routhu, N.K.; Cheedarla, N.; Bollimpelli, V.S.; Gangadhara, S.; Edara, V.V.; Lai, L.; Sahoo, A.; Shiferaw, A.; Styles, T.M.; Floyd, K.; et al. SARS-CoV-2 RBD trimer protein adjuvanted with Alum-3M-052 protects from SARS-CoV-2 infection and immune pathology in the lung. Nat. Commun. 2021, 12, 3587. [Google Scholar] [CrossRef] [PubMed]
- Fougeroux, C.; Goksøyr, L.; Idorn, M.; Soroka, V.; Myeni, S.K.; Dagil, R.; Janitzek, C.M.; Søgaard, M.; Aves, K.L.; Horsted, E.W.; et al. Capsid-like particles decorated with the SARS-CoV-2 receptor-binding domain elicit strong virus neutralization activity. Nat. Commun. 2021, 12, 324. [Google Scholar] [CrossRef]
- Toledo-Romani, M.E.; García-Carmenate, M.; Verdecia-Sánchez, L.; Pérez-Rodríguez, S.; Rodriguez-González, M.; Valenzuela-Silva, C.; Paredes-Moreno, B.; Sanchez-Ramirez, B.; González-Mugica, R.; Hernández-Garcia, T.; et al. Safety and immunogenicity of anti-SARS-CoV-2 conjugate vaccine SOBERANA 02 in a two-dose or three-dose heterologous scheme in adults: Phase IIb Clinical Trial. Med 2022, 3, 760–773.e5. [Google Scholar] [CrossRef]
- Knudsen, N.P.; Olsen, A.; Buonsanti, C.; Follmann, F.; Zhang, Y.; Coler, R.N.; Fox, C.B.; Meinke, A.; D’Oro, U.; Casini, D.; et al. Different human vaccine adjuvants promote distinct antigen-independent immunological signatures tailored to different pathogens. Sci. Rep. 2016, 6, 19570. [Google Scholar] [CrossRef] [Green Version]
- Cordeiro, A.S.; Alonso, M.J.; de la Fuente, M. Nanoengineering of vaccines using natural polysaccharides. Biopolymers 2015, 33, 1279–1293. [Google Scholar] [CrossRef]
- Tkachuk, A.P.; Gushchin, V.A.; Potapov, V.D.; Demidenko, A.V.; Lunin, V.G.; Gintsburg, A.L. Multi-subunit BCG booster vaccine GamTBvac: Assessment of immunogenicity and protective efficacy in murine and guinea pig TB models. PLoS ONE 2017, 12, e0176784. [Google Scholar] [CrossRef] [Green Version]
- Baldwin, A.D.; Kiick, K.L. Polysaccharide-modified synthetic polymeric biomaterials. Biopolymers 2010, 94, 128–140. [Google Scholar] [CrossRef] [Green Version]
- D’Agostino, L.; di Pietro, M.; Di Luccia, A. Nuclear aggregates of polyamines are supramolecular structures that play a crucial role in genomic DNA protection and conformation. FEBS J. 2005, 272, 3777–3787. [Google Scholar] [CrossRef] [PubMed]
- Bateneva, A.V.; Gamaley, S.G.; Lebedev, R.L.; Danilenko, E.D. Stimulating effect of double-stranded yeast RNA on the activity of interferon system genes. Med. Immunol. 2021, 22, 1155–1162. [Google Scholar] [CrossRef]
- Volosnikova, E.A. The Study of the Process of Forming Conjugates to Create Vaccine Constructs. Siberian Sci. Med. J. 2011, 31, 141–145. Available online: https://elibrary.ru/item.asp?id=17303490 (accessed on 11 November 2022).
- Merkuleva, I.A.; Shcherbakov, D.N.; Borgoyakova, M.B.; Shanshin, D.V.; Rudometov, A.P.; Karpenko, L.I.; Belenkaya, S.V.; Isaeva, A.A.; Nesmeyanova, V.S.; Kazachinskaia, E.I.; et al. Comparative Immunogenicity of the Recombinant Receptor-Binding Domain of Protein S SARS-CoV-2 Obtained in Prokaryotic and Mammalian Expression Systems. Vaccines 2022, 10, 96. [Google Scholar] [CrossRef] [PubMed]
- Kleanthous, H.; Silverman, J.M.; Makar, K.W.; Yoon, I.K.; Jackson, N.; Vaughn, D.W. Scientific rationale for developing potent RBD-based vaccines targeting COVID-19. NPJ Vaccines 2021, 6, 128. [Google Scholar] [CrossRef]
- Zang, J.; Gu, C.; Zhou, B.; Zhang, C.; Yang, Y.; Xu, S.; Bai, L.; Zhang, R.; Deng, Q.; Yuan, Z.; et al. Immunization with the receptor-binding domain of SARS-CoV-2 elicits antibodies cross-neutralizing SARS-CoV-2 and SARS-CoV without antibody-dependent enhancement. Cell Discov. 2020, 6, 61. [Google Scholar] [CrossRef]
- Yang, J.; Wang, W.; Chen, Z.; Lu, S.; Yang, F.; Bi, Z.; Bao, L.; Mo, F.; Li, X.; Huang, Y.; et al. A vaccine targeting the RBD of the S protein of SARS-CoV-2 induces protective immunity. Nature 2020, 586, 572–577. [Google Scholar] [CrossRef]
- Malladi, S.K.; Singh, R.; Pandey, S.; Gayathri, S.; Kanjo, K.; Ahmed, S.; Khan, M.S.; Kalita, P.; Girish, N.; Upadhyaya, A.; et al. Design of a highly thermotolerant, immunogenic SARS-CoV-2 spike fragment. J. Biol. Chem. 2021, 296, 100025. [Google Scholar] [CrossRef]
- Jaume, M.; Yip, M.S.; Kam, Y.W.; Cheung, C.Y.; Kien, F.; Roberts, A.; Li, P.H.; Dutry, I.; Escriou, N.; Daeron, M.; et al. SARS-CoV subunit vaccine: Antibody-mediated neutralisation and enhancement. Hong Kong Med. J. 2012, 18 (Suppl. 2), 31–36. [Google Scholar]
- Quinlan, B.D.; Mou, H.; Zhang, L.; Guo, Y.; He, W.; Ojha, A.; Parcells, M.S.; Luo, G.; Li, W.; Zhong, G.; et al. The SARS-CoV-2 receptor-binding domain elicits a potent neutralizing response without antibody-dependent enhancement. Biorxiv 2020. [Google Scholar] [CrossRef]
- Thuluva, S.; Paradkar, V.; Gunneri, S.R.; Yerroju, V.; Mogulla, R.; Turaga, K.; Kyasani, M.; Manoharan, S.K.; Medigeshi, G.; Singh, J.; et al. Evaluation of safety and immunogenicity of receptor-binding domain-based COVID-19 vaccine (Corbevax) to select the optimum formulation in open-label, multicentre, and randomised phase-1/2 and phase-2 clinical trials. EBioMedicine 2022, 83, 104217. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Zhao, G.; Chan, C.C.; Sun, S.; Chen, M.; Liu, Z.; Guo, H.; He, Y.; Zhou, Y.; Zheng, B.J.; et al. Recombinant receptor-binding domain of SARS-CoV spike protein expressed in mammalian, insect and E. coli cells elicits potent neutralizing antibody and protective immunity. Virology 2009, 393, 144–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, N.; Ji, Q.; Liu, Z.; Tang, K.; Xie, Y.; Li, K.; Zhou, J.; Li, S.; Shang, H.; Shi, Z.; et al. Effect of Different Adjuvants on Immune Responses Elicited by Protein-Based Subunit Vaccines against SARS-CoV-2 and Its Delta Variant. Viruses 2022, 14, 501. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, K.A.; Rodriguez-Aponte, S.A.; Dalvie, N.C.; Lee, J.H.; Abraham, W.; Carnathan, D.G.; Jimenez, L.E.; Ngo, J.T.; Chang, J.; Zhang, Z.; et al. Phosphate-mediated coanchoring of RBD immunogens and molecular adjuvants to alum potentiates humoral immunity against SARS-CoV-2. Sci. Adv. 2021, 7, eabj6538. [Google Scholar] [CrossRef] [PubMed]
- Coria, L.M.; Saposnik, L.M.; Pueblas Castro, C.; Castro, E.F.; Bruno, L.A.; Stone, W.B.; Pérez, P.S.; Darriba, M.L.; Chemes, L.B.; Alcain, J.; et al. A Novel Bacterial Protease Inhibitor Adjuvant in RBD-Based COVID-19 Vaccine Formulations Containing Alum Increases Neutralizing Antibodies, Specific Germinal Center B Cells and Confers Protection Against SARS-CoV-2 Infection in Mice. Front. Immunol. 2022, 13, 844837. [Google Scholar] [CrossRef]
- Valdes-Balbin, Y.; Santana-Mederos, D.; Paquet, F.; Fernandez, S.; Climent, Y.; Chiodo, F.; Rodríguez, L.; Sanchez Ramirez, B.; Leon, K.; Hernandez, T.; et al. Molecular Aspects Concerning the Use of the SARS-CoV-2 Receptor Binding Domain as a Target for Preventive Vaccines. ACS Cent. Sci. 2021, 7, 757–767. [Google Scholar] [CrossRef]
- Sahin, U.; Muik, A.; Derhovanessian, E.; Vogler, I.; Kranz, L.M.; Vormehr, M.; Baum, A.; Pascal, K.; Quandt, J.; Maurus, D.; et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature 2020, 586, 594–599. [Google Scholar] [CrossRef]
- Cohen, A.A.; Gnanapragasam, P.; Lee, Y.E.; Hoffman, P.R.; Ou, S.; Kakutani, L.M.; Keeffe, J.R.; Wu, H.J.; Howarth, M.; West, A.P.; et al. Mosaic nanoparticles elicit cross-reactive immune responses to zoonotic coronaviruses in mice. Science 2021, 371, 735–741. [Google Scholar] [CrossRef]
- Karpenko, L.I.; Bazhan, S.I.; Bogryantseva, M.P.; Ryndyuk, N.N.; Ginko, Z.I.; Kuzubov, V.I.; Lebedev, L.R.; Kaplina, O.N.; Reguzova, A.Y.; Ryzhikov, A.B.; et al. Results of phase I clinical trials of a combined vaccine against HIV-1 based on synthetic polyepitope immunogens. Russ. J. Bioorg. Chem. 2016, 42, 170–182. [Google Scholar] [CrossRef]
- Gilbert, P.B.; Montefiori, D.C.; McDermott, A.B.; Fong, Y.; Benkeser, D.; Deng, W.; Zhou, H.; Houchens, C.R.; Martins, K.; Jayashankar, L.; et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science 2022, 375, 43–50. [Google Scholar] [CrossRef]
- Alter, G.; Yu, J.; Liu, J.; Chandrashekar, A.; Borducchi, E.N.; Tostanoski, L.H.; McMahan, K.; Jacob-Dolan, C.; Martinez, D.R.; Chang, A.; et al. Immunogenicity of Ad26.COV2.S vaccine against SARS-CoV-2 variants in humans. Nature 2021, 596, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Sadoff, J.; Gray, G.; Vandebosch, A.; Cárdenas, V.; Shukarev, G.; Grinsztejn, B.; Goepfert, P.A.; Truyers, C.; Fennema, H.; Spiessens, B.; et al. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against COVID-19. N. Engl. J. Med. 2021, 384, 2187–2201. [Google Scholar] [CrossRef] [PubMed]
- Heath, P.T.; Galiza, E.P.; Baxter, D.N.; Boffito, M.; Browne, D.; Burns, F.; Chadwick, D.R.; Clark, R.; Cosgrove, C.; Galloway, J.; et al. Safety and Efficacy of NVX-CoV2373 COVID-19 Vaccine. N. Engl. J. Med. 2021, 385, 1172–1183. [Google Scholar] [CrossRef] [PubMed]
- Shinde, V.; Bhikha, S.; Hoosain, Z.; Archary, M.; Bhorat, Q.; Fairlie, L.; Lalloo, U.; Masilela, M.S.L.; Moodley, D.; Hanley, S.; et al. Efficacy of nvx-cov2373 COVID-19 vaccine against the b.1.351 variant. N. Engl. J. Med. 2021, 384, 1899–1909. [Google Scholar] [CrossRef]
- Wu, Z.; Hu, Y.; Xu, M.; Chen, Z.; Yang, W.; Jiang, Z.; Li, M.; Jin, H.; Cui, G.; Chen, P.; et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy adults aged 60 years and older: A randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect. Dis. 2021, 21, 803–812. [Google Scholar] [CrossRef]
- Ella, R.; Reddy, S.; Jogdand, H.; Sarangi, V.; Ganneru, B.; Prasad, S.; Das, D.; Raju, D.; Praturi, U.; Sapkal, G.; et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: Interim results from a double-blind, randomised, multicentre, phase 2 trial, and 3-month follow-up of a double-blind, randomised phase 1 trial. Lancet Infect. Dis. 2021, 21, 950–961. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Volosnikova, E.A.; Merkuleva, I.A.; Esina, T.I.; Shcherbakov, D.N.; Borgoyakova, M.B.; Isaeva, A.A.; Nesmeyanova, V.S.; Volkova, N.V.; Belenkaya, S.V.; Zaykovskaya, A.V.; et al. SARS-CoV-2 RBD Conjugated to Polyglucin, Spermidine, and dsRNA Elicits a Strong Immune Response in Mice. Vaccines 2023, 11, 808. https://doi.org/10.3390/vaccines11040808
Volosnikova EA, Merkuleva IA, Esina TI, Shcherbakov DN, Borgoyakova MB, Isaeva AA, Nesmeyanova VS, Volkova NV, Belenkaya SV, Zaykovskaya AV, et al. SARS-CoV-2 RBD Conjugated to Polyglucin, Spermidine, and dsRNA Elicits a Strong Immune Response in Mice. Vaccines. 2023; 11(4):808. https://doi.org/10.3390/vaccines11040808
Chicago/Turabian StyleVolosnikova, Ekaterina A., Iuliia A. Merkuleva, Tatiana I. Esina, Dmitry N. Shcherbakov, Mariya B. Borgoyakova, Anastasiya A. Isaeva, Valentina S. Nesmeyanova, Natalia V. Volkova, Svetlana V. Belenkaya, Anna V. Zaykovskaya, and et al. 2023. "SARS-CoV-2 RBD Conjugated to Polyglucin, Spermidine, and dsRNA Elicits a Strong Immune Response in Mice" Vaccines 11, no. 4: 808. https://doi.org/10.3390/vaccines11040808




