Comparison of Biological, Pharmacological Characteristics, Indications, Contraindications, Efficacy, and Adverse Effects of Inactivated Whole-Virus COVID-19 Vaccines Sinopharm, CoronaVac, and Covaxin: An Observational Study
Abstract
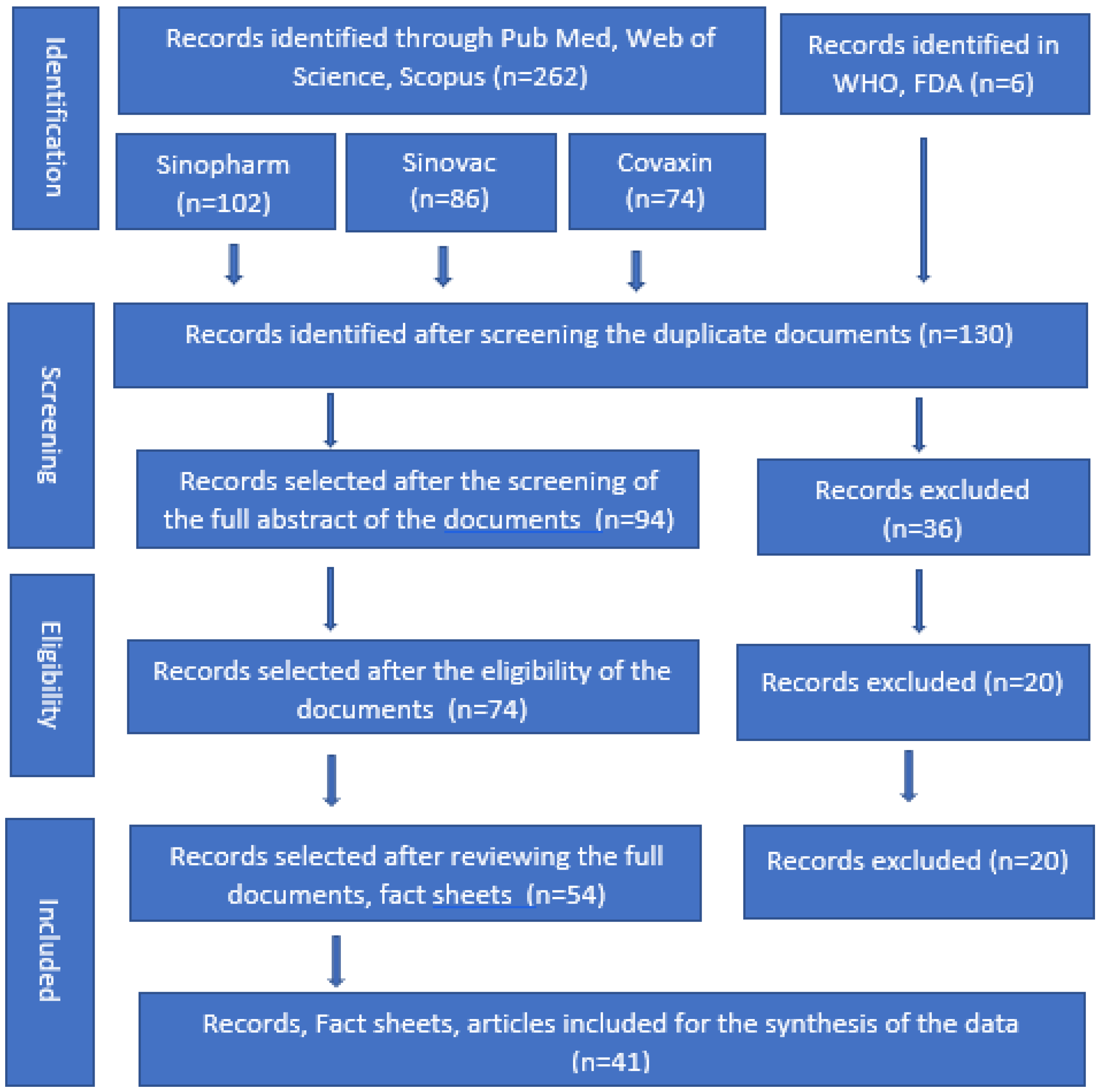
:1. Introduction
2. Materials and Methods
3. Results
| Author Name, Year | Country, Type of Study | Age Range | Efficacy |
|---|---|---|---|
| Sinopharm Vaccine | |||
| WHO, 2022 [10] Facts sheet [11] | China, RCT phase 3 | 18–59 years | Efficacy after 2 doses with 14–21-day interval was 79% (CI: 66–87%) |
| AlHosani et al., 2022 [29] | UAE, cohort study | 15 years and above | Prevent hospitalization = 79.8% (78~81.4%); critical care = 92.2% (89.7~94.1%; deaths = 97.1% (83~99.9%) |
| Mousa et al., 2022 [30] | UAE, evidence-based | 3782, above 18 years | Full vaccination prevents hospital admission against Delta variant = 95% (94–97%) |
| Al-Momani et al., 2022 [31] | Jordan, cross-sectional | 536, over 18 years | Sinopharm vaccine efficacy was 67% (95% CI 52–78%) |
| Al Kaabi et al., 2021 [32] | Asia, phase 3 trial | 40,382 participants | 72.8% (95% CI, 58.1–82.4%) for WIV04 and 78.1% (95% CI, 64.8–86.3%) for HB02 symptomatic COVID-19 cases |
| Al Kaabi et al., 2021 [33] | UAE, randomized phase 3 trial | 3,147,869 adults >18 years | Effectiveness was 79.6% (CI: 77.7–81.3) against hospitalization, 86% (CI: 82.2–89.0) against critical care admission, and 84.1% (CI: 70.8–91.3) against death due to COVID-19 |
| Silva-Valencia et al., 2021 [34] | Peru, retrospective | Infection 40.3% (38.9–41.6%); COVID-19 mortality 88.7% (85.1–91.4%) | |
| Silva-Valencia et al., 2021 [35] | Peru, cohort study | Effectiveness was 50% (CI: 49–52%) against infection and 94% (95% CI: 91–96%) against COVID-19-allied mortality | |
| Nadeem et al., 2022 [36] | Case-control, Pakistan | 3426, aged >60 years | Efficacy against symptomatic COVID-19 infection was 94.3% |
| Rearte et al., 2022 [37] | Retrospective, Argentina | 237,330, >60 years old | Efficacy against symptomatic COVID-19 infection was 85.0% (84.0–86.0) |
| CoronaVac/Sinovac Vaccine | |||
| Wei et al., 2022 [38] | Case-control, Hong Kong | 32,823 cases ≥65 years | Hospitalization 74.0% (95% CI, 71.8–75.8%). Deaths 86.4% (95% CI, 85.8–87.0%) |
| Wong et al., 2023 [39] | National data, Malaysia | 1,158,235 >18 years | CoronaVac is 88.8% (CI 95%: 84.9, 91.7) |
| Jara et al., 2022 [40] | Chile, Cohort ≥18 years | 10.2 million | Overall efficacy was 65.9% (65.2–66.6); for prevention of hospitalization, it was 87.5% (86.7–88.2); for ICU admission, it was 90.3% (CI: 89.1–91.4); for COVID deaths, it was 86.3% (CI: 84.5–87.9) |
| Tanriover et al., 2021, [41] | Turkey, RCT | 13,000 RCT ≥18 years | Protection against symptomatic disease 83.5% (65–92); 100% (65–92); hospitalization 100% (20–100) |
| Fadlyana et al., 2021 [42] | Indonesia, RCT | 1620 ≥18 years | Protection against symptomatic disease 65% (20–85) |
| Palacios et al., 2021 [43] | Brazil, RCT | 12,688 ≥ 18 years | Protection against symptomatic disease 51% (36–62); protection against hospitalization 100% (56–100) |
| Vokó et al., 2022 [44] | Hungry, retrospective | 895,465 | Estimated effectiveness against SARS-CoV-2 infection was 68.7% (95% CI 67.2%-70.1%) |
| Cerqueira-Silva et al., 2022 [45] | Brazil, case control | 14,362,482 | Efficacy at 14–30 days after the second dose was 55.0% (CI: 54.3–55.7) against confirmed infection and 82.1% (95% CI: 81.4–82.8) against severe outcomes (mean 68.55) |
| Covaxin Vaccine | |||
| WHO [14,15] | - | - | 68%; all variants of COVID-19 were 71% (CI: 50–84); Kappa 90% (95% CI: 30–100); and Delta 65% (95% CI: 33–83). |
| Zare et al., 2022 [46] | Iran | 214 people 19–64 years | 67% |
| Behera et al., 2022 [47] | Case-control study, India | 670 people, 29.1 years | After age and gender adjustment, vaccine effectiveness was 22% CI: 0.52–1.17; 29% (CI: 0.47–1.08) |
| Ella et al., 2021 [48] | Phase 3 clinical trial | 25,798, age ≥ 18 years | Overall vaccine efficacy was 77·8% (95% CI 65·2–86·4). |
| Malhotra et al., 2022 [49] | Retrospective cohort, India | 15,244 HCWs, age 36.6 years | The efficacy against the infection was 86% (95% CI, 77–92%) |
| Desai et al., 2022 [50] | Case-control study, India | 3732 > 18 years | Adjusted efficacy against symptomatic cases after 2 doses was 50% (95% CI 33–62) |
4. Discussion
5. Study Strengths and Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meo, S.A.; Alsomali, A.H.; Almushawah, A.A.; Halepoto, D.M. Seasonal variations impact on SARS-CoV-2 incidence and mortality in southern and northern hemispheres: Two years pandemic period-based study. J. King Saud Univ. Sci. 2022, 34, 102335. [Google Scholar] [CrossRef]
- World Health Organization (WHO). WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 25 March 2023).
- Centres for Disease Control and Prevention (CDC). Scientific Brief: SARS-CoV-2. Transmission. Available online: https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/sars-cov-2-transmission.html (accessed on 23 January 2023).
- Meyerowitz, E.A.; Richterman, A.; Gandhi, R.T.; Sax, P.E. Transmission of SARS-CoV-2: A Review of Viral, Host, and Environmental Factors. Ann. Intern. Med. 2021, 174, 69–79. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Getting the COVID-19 Vaccine. Available online: https://www.who.int/news-room/feature-stories/detail/getting-the-covid-19-vaccine (accessed on 23 January 2023).
- Song, G. Understanding public perceptions of benefits and risks of childhood vaccinations in the United States. Risk Anal. 2014, 34, 541–555. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). The Distinct Types of Vaccine. Available online: https://www.who.int/news-room/feature-stories/detail/the-race-for-a-covid-19-vaccine-explained (accessed on 20 January 2023).
- Our World in Data: Coronavirus (COVID-19) Vaccinations. Available online: https://ourworldindata.org/covid-vaccinations (accessed on 27 January 2022).
- The New York Times. Tracking Coronavirus Vaccinations Around the World. Available online: https://www.nytimes.com/interactive/2021/world/covid-vaccinations-tracker.html (accessed on 27 January 2023).
- World Health Organization. Interim Recommendations for Use of the Inactivated COVID-19 Vaccine BIBP Developed by China National Biotec Group (CNBG), Sinopharm: Interim Guidance, First Issued 7 May 2021, Updated 28 October 2021, Updated 15 March 2022. Available online: https://apps.who.int/iris/handle/10665/352470 (accessed on 12 January 2022).
- COVID-19 Vaccine Information Sheet Sinopharm Vaccine (Vero Cell Inactivated). Available online: https://covid19.health.gov.mv/wp-content/uploads/2021/10/SINOPHARM-INFORMATION-SHEET_Final-Revised-09th-June.pdf (accessed on 12 January 2022).
- World Health Organization. The Sinovac-CoronaVac COVID-19 Vaccine: What You Need to Know? Available online: https://www.who.int/news-room/feature-stories/detail/the-sinovac-covid-19-vaccine-what-you-need-to-know (accessed on 12 January 2023).
- World Health Organization. CoronaVac. Available online: https://extranet.who.int/pqweb/vaccines/who-recommendation-sinovac-covid-19-vaccine-vero-cell-inactivated-coronavac (accessed on 12 January 2023).
- World Health Organization. COVAXIN® (BBV152)–Inactivated, COVID-19 Vaccine. Available online: https://www.who.int/publications/m/item/covaxin-(bbv152)-inactivated-covid-19-vaccine (accessed on 12 January 2022).
- Facts Sheet. COVAXIN. Available online: https://www.bharatbiotech.com/images/covaxin/covaxin-factsheet.pdf (accessed on 12 January 2023).
- Ai, J.; Zhang, Y.; Zhang, H.; Zhang, Q.; Fu, Z.; Lin, K.; Song, J.; Zhao, Y.; Fan, M.; Wang, H.; et al. Safety, and immunogenicity of a third-dose homologous BBIBP-CorV boosting vaccination: Interim results from a prospective open-label study. Emerg. Microbes Infect. 2022, 11, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Hua, Q.; Zhang, H.; Yao, P.; Xu, N.; Sun, Y.; Lu, H.; Xu, F.; Liao, Y.; Yang, J.; Mao, H.; et al. Immunogenicity, and immune-persistence of the CoronaVac or Covilo inactivated COVID-19 Vaccine: A 6-month population-based cohort study. Front. Immunol. 2022, 13, 939311. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Phatak, S.R.; Singh, R.; Bhattacharjee, K.; Singh, N.K.; Gupta, A.; Sharma, A. Antibody response after first and second-dose of ChAdOx1-nCOV (CovishieldTM®) and BBV-152 (CovaxinTM®) among health care workers in India: The final results of cross-sectional coronavirus vaccine-induced antibody titre (COVAT) study. Vaccines 2021, 39, 6492–6509. [Google Scholar] [CrossRef]
- Vadrevu, K.M.; Reddy, S.; Jogdand, H.; Ganneru, B.; Mirza, N.; Tripathy, V.N.; Singh, C.; Khalatkar, V.; Prasanth, S.; Rai, S.; et al. Immunogenicity and reactogenicity of an inactivated SARS-CoV-2 vaccine (BBV152) in children aged 2-18 years: Interim data from an open-label, non-randomised, age de-escalation phase 2/3 study. Lancet Infect. Dis. 2022, 22, 1303–1312. [Google Scholar] [CrossRef] [PubMed]
- Meo, A.S.; Masood, A.; Shabbir, U.; Ali, H.; Nadeem, Z.; Meo, S.A.; Alshahrani, A.N.; AlAnazi, S.; Al-Masri, A.A.; Al-Khlaiwi, T. Adverse Effects of Sinopharm COVID-19 Vaccine among Vaccinated Medical Students and Health Care Workers. Vaccines 2023, 11, 105. [Google Scholar] [CrossRef]
- Riad, A.; Sağıroğlu, D.; Üstün, B.; Pokorná, A.; Klugarová, J.; Attia, S.; Klugar, M. Prevalence and Risk Factors of CoronaVac Side Effects: An Independent Cross-Sectional Study among Healthcare Workers in Turkey. J. Clin. Med. 2021, 10, 2629. [Google Scholar] [CrossRef]
- Bharat Biotech, Covaxin. Summary of Product Characteristics (SMPC). Available online: https://www.bharatbiotech.com/images/covaxin/covaxin-smpc.pdf (accessed on 22 January 2022).
- Dar-Odeh, N.; Abu-Hammad, O.; Qasem, F.; Jambi, S.; Alhodhodi, A.; Othman, A.; Abu-Hammad, A.; Al-Shorman, H.; Ryalat, S.; Abu-Hammad, S. Long-term adverse events of three COVID-19 vaccines as reported by vaccinated physicians and dentists, a study from Jordan and Saudi Arabia. Hum. Vaccines Immunother. 2022, 18, 2039017. [Google Scholar] [CrossRef]
- Yasmin, F.; Najeeb, H.; Siddiqui, H.F.; Asghar, M.S.; Awan, H.A.; Usama, R.M.; Salahuddin, Z.; Tahir, M.J.; Ullah, K.; Mahmmoud Fadelallah Eljack, M. Frequency of COVID-19 vaccine side effects and its associated factors among the vaccinated population of Pakistan: A cross-sectional study. Health Sci. Rep. 2023, 6, e1071. [Google Scholar] [CrossRef] [PubMed]
- Zaheri, H.; Kiani, A.; Afaghi, S.; Rahimi, F.; Banitorfi, M.; Norozi, A.K.; Hashemi, S.; Abedini, A. Lower limb arterial thrombosis followed by sub-massive pulmonary thromboembolism after Sinopharm BBIBP-CorV COVID-19 vaccination. Arch. Clin. Cases. 2022, 9, 150–153. [Google Scholar] [CrossRef]
- Wan, E.Y.F.; Wang, Y.; Chui, C.S.L.; Mok, A.H.Y.; Xu, W.; Yan, V.K.C.; Lai, F.T.T.; Li, X.; Wong, C.K.H.; Chan, E.W.Y.; et al. Safety of an inactivated, whole-virion COVID-19 vaccine (CoronaVac) in people aged 60 years or older in Hong Kong: A modified self-controlled case series. Lancet Healthy Longev. 2022, 3, e491–e500. [Google Scholar] [CrossRef] [PubMed]
- Keshavarz, P.; Yazdanpanah, F.; Emad, M.; Hajati, A.; Nejati, S.F.; Ebrahimian Sadabad, F.; Azrumelashvili, T.; Mizandari, M.; Raman, S.S. Myocarditis Following COVID-19 Vaccination: Cardiac Imaging Findings in 118 Studies. Tomography 2022, 8, 1959–1973. [Google Scholar] [CrossRef]
- Chaudhari, P.J.; Chawda, U.B.; Bhad, B.J.; Mevada, A.V.; Jha, S.G. Facial Palsy Induced by Covaxin in Adolescent Female—A Rare Case Report. Curr. Drug Saf. 2022, 18, 603. [Google Scholar] [CrossRef]
- AlHosani, F.I.; Stanciole, A.E.; Aden, B.; Timoshkin, A.; Najim, O.; Zaher, W.A.; AlDhaheri, F.A.; Al Mazrouie, S.; Rizvi, T.A.; Mustafa, F. Impact of the Sinopharm’s BBIBP-CorV vaccine in preventing hospital admissions and death in infected vaccinees: Results from a retrospective study in the emirate of Abu Dhabi, United Arab Emirates (UAE). Vaccines 2022, 40, 2003–2010. [Google Scholar] [CrossRef]
- Mousa, M.; Albreiki, M.; Alshehhi, F.; AlShamsi, S.; Marzouqi, N.A.; Alawadi, T.; Alrand, H.; Alsafar, H.; Fikri, A. Similar effectiveness of the inactivated vaccine BBIBP-CorV (Sinopharm) and the mRNA vaccine BNT162b2 (Pfizer-BioNTech) against COVID-19 related hospitalizations during the Delta outbreak in the U.A.E. J. Travel Med. 2022, 29, taac036. [Google Scholar] [CrossRef]
- Al-Momani, H.; Aldajah, K.; Alda’ajah, E.; ALjafar, Y.; Abushawer, Z. Effectiveness of Pfizer/BioNTech and Sinopharm COVID-19 vaccines in reducing hospital admissions in prince Hamza hospital, Jordan. Front. Public Health 2022, 10, 1008521. [Google Scholar] [CrossRef]
- Al Kaabi, N.; Zhang, Y.; Xia, S.; Yang, Y.; Al Qahtani, M.M.; Abdulrazzaq, N.; Al Nusair, M.; Hassany, M.; Jawad, J.S.; Abdalla, J.; et al. Effect of 2 Inactivated SARS-CoV-2 Vaccines on Symptomatic COVID-19 Infection in Adults: A Randomized Clinical Trial. JAMA 2021, 326, 35–45. [Google Scholar] [CrossRef]
- Al Kaabi, N.; Oulhaj, A.; Ganesan, S.; Al Hosani, F.I.; Najim, O.; Ibrahim, H.; Acuna, J.; Alsuwaidi, A.R.; Kamour, A.M.; Alzaabi, A.; et al. Effectiveness of BBIBP-CorV vaccine against severe outcomes of COVID-19 in Abu Dhabi, United Arab Emirates. Nat. Commun. 2022, 13, 3215. [Google Scholar] [CrossRef] [PubMed]
- Silva-Valencia, J.; Soto-Becerra, P.; Escobar-Agreda, S.; Fernandez-Navarro, M.; Elorreaga, O.A.; Mayta-Tristán, P.; Mezones-Holguin, E.; Solari, L. Relative vaccine effectiveness of the booster dose of COVID-19 vaccine for preventing death in individuals with a primary regimen based on the BBIBP-CorV.; ChAdOx1-S.; or BNT162b2 vaccines during the Omicron wave in Peru: A nested case-control study using national population data. Vaccines 2022, 40, 6512–6519. [Google Scholar] [CrossRef]
- Silva-Valencia, J.; Soto-Becerra, P.; Escobar-Agreda, S.; Fernández-Navarro, M.; Moscoso-Porras, M.; Solari, L.; Mayta-Tristán, P. Effectiveness of the BBIPB-CorV Vaccine in Preventing Infection and Death in Health CareWorkers in Peru 2021. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3922632 (accessed on 2 January 2023).
- Nadeem, I.; Ul Munamm, S.A.; Ur Rasool, M.; Fatimah, M.; Abu Bakar, M.; Rana, Z.K.; Khatana, U.F.; Jordon, L.; Saqlain, M.; Mahdi, N.; et al. Safety and efficacy of Sinopharm vaccine (BBIBP-CorV) in the elderly population of Faisalabad district of Pakistan. Postgrad. Med. J. 2022. [Google Scholar] [CrossRef]
- Rearte, A.; Castelli, J.M.; Rearte, R.; Fuentes, N.; Pennini, V.; Pesce, M.; Barbeira, P.B.; Iummato, L.E.; Laurora, M.; Bartolomeu, M.L.; et al. Effectiveness of rAd26-rAd5, ChAdOx1 nCoV-19, and BBIBP-CorV vaccines for risk of infection with SARS-CoV-2 and death due to COVID-19 in people older than 60 years in Argentina: A test-negative, case-control, and retrospective longitudinal study. Lancet 2022, 399, 1254–1264. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Jia, K.M.; Zhao, S.; Hung, C.T.; Mok, C.K.P.; Poon, P.K.M.; Man Leung, E.Y.; Wang, M.H.; Yam, C.H.K.; Chow, T.Y.; et al. Estimation of Vaccine Effectiveness of CoronaVac and BNT162b2 Against Severe Outcomes Over Time Among Patients With SARS-CoV-2 Omicron. JAMA Netw. Open. 2023, 6, e2254777. [Google Scholar] [CrossRef]
- Wong, M.T.J.; Dhaliwal, S.S.; Balakrishnan, V.; Nordin, F.; Norazmi, M.N.; Tye, G.J. Effectiveness of Booster Vaccinations on the Control of COVID-19 during the Spread of Omicron Variant in Malaysia. Int. J. Environ. Res. Public Health 2023, 20, 1647. [Google Scholar] [CrossRef] [PubMed]
- Jara, A.; Undurraga, E.A.; González, C.; Paredes, F.; Fontecilla, T.; Jara, G.; Pizarro, A.; Acevedo, J.; Leo, K.; Leon, F.; et al. Effectiveness of an Inactivated SARS-CoV-2 Vaccine in Chile. N. Engl. J. Med. 2021, 385, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Tanriover, M.D.; Doğanay, H.L.; Akova, M.; Güner, H.R.; Azap, A.; Akhan, S.; Köse, Ş.; Erdinç, F.Ş.; Akalın, E.H.; Tabak, Ö.F.; et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): Interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet 2021, 398, 213–222. [Google Scholar] [CrossRef]
- Fadlyana, E.; Rusmil, K.; Tarigan, R.; Rahmadi, A.R.; Prodjosoewojo, S.; Sofiatin, Y.; Khrisna, C.V.; Sari, R.M.; Setyaningsih, L.; Surachman, F.; et al. A phase I.I.I.; observer-blind, randomized, placebo-controlled study of the efficacy, safety, and immunogenicity of SARS-CoV-2 inactivated vaccine in healthy adults aged 18–59 years: An interim analysis in Indonesia. Vaccines 2021, 39, 6520–6528. [Google Scholar] [CrossRef] [PubMed]
- Palacios, R.; Batista, A.P.; Albuquerque, C.S.N.; Patiño, E.G.; Santos, J.P.; Conde, M.T.R.P.; Piorelli, R.O.; Júnior, L.C.P.; Raboni, S.M.; Ramos, F.; et al. Efficacy and Safety of a COVID-19 Inactivated Vaccine in Healthcare Professionals in Brazil: The PROFISCOV Study. 11 April 2021. Available online: https://ssrn.com/abstract=3822780 (accessed on 2 January 2023).
- Vokó, Z.; Kiss, Z.; Surján, G.; Surján, O.; Barcza, Z.; Pályi, B.; Formanek-Balku, E.; Molnár, G.A.; Herczeg, R.; Gyenesei, A.; et al. Nationwide effectiveness of five SARS-CoV-2 vaccines in Hungary-the HUN-VE study. Clin. Microbiol. Infect. 2022, 28, 398–404. [Google Scholar] [CrossRef]
- Cerqueira-Silva, T.; Katikireddi, S.V.; Oliveira, V.D.A.; Flores-Ortiz, R.; Júnior, J.B.; Paixão, E.S.; Robertson, C.; Penna, G.O.; Werneck, G.L.; Barreto, M.L.; et al. Vaccine effectiveness of heterologous CoronaVac plus BNT162b2 in Brazil. Nat. Med. 2022, 28, 838–843. [Google Scholar] [CrossRef]
- Zare, H.; Rezapour, H.; Fereidouni, A.; Nikpour, S.; Mahmoudzadeh, S.; Royce, S.G.; Fereidouni, M. Analysis, and comparison of anti-RBD neutralizing antibodies from AZD-1222, Sputnik, V, Sinopharm and Covaxin vaccines and its relationship with gender among health care workers. Immun. Ageing 2022, 19, 47. [Google Scholar] [CrossRef]
- Behera, P.; Singh, A.K.; Subba, S.H.; Mc, A.; Sahu, D.P.; Chandanshive, P.D.; Pradhan, S.K.; Parida, S.P.; Mishra, A.; Patro, B.K.; et al. Effectiveness of COVID-19 vaccine (Covaxin) against breakthrough SARS-CoV-2 infection in India. Hum. Vaccines Immunother. 2022, 18, 2034456. [Google Scholar] [CrossRef] [PubMed]
- Ella, R.; Reddy, S.; Jogdand, H.; Sarangi, V.; Ganneru, B.; Prasad, S.; Das, D.; Raju, D.; Praturi, U.; Sapkal, G.; et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: Interim results from a double-blind, randomised, multicentre, phase 2 trial, and 3-month follow-up of a double-blind, randomised phase 1 trial. Lancet Infect Dis. 2021, 21, 950–961. [Google Scholar] [CrossRef]
- Malhotra, S.; Mani, K.; Lodha, R.; Bakhshi, S.; Mathur, V.P.; Gupta, P.; Kedia, S.; Sankar, J.; Kumar, P.; Kumar, A.; et al. SARS-CoV-2 Reinfection Rate and Estimated Effectiveness of the Inactivated Whole Virion Vaccine BBV152 Against Reinfection Among Health Care Workers in New Delhi, India. JAMA Netw. Open 2022, 5, e2142210. [Google Scholar] [CrossRef]
- Desai, D.; Khan, A.R.; Soneja, M.; Mittal, A.; Naik, S.; Kodan, P.; Mandal, A.; Maher, G.T.; Kumar, R.; Agarwal, A.; et al. Effectiveness of an inactivated virus-based SARS-CoV-2 vaccine, BBV152, in India: A test-negative, case-control study. Lancet Infect. Dis. 2022, 22, 349–356. [Google Scholar] [CrossRef]
- Xia, S.; Zhang, Y.; Wang, Y.; Wang, H.; Yang, Y.; Gao, G.F.; Tan, W.; Wu, G.; Xu, M.; Lou, Z.; et al. Safety and immunogenicity of an inactivated COVID-19 vaccine, BBIBP-CorV.; in people younger than 18 years: A randomized, double-blind, controlled, phase 1/2 trial. Lancet Infect. Dis. 2022, 22, 196–208. [Google Scholar] [CrossRef]
- World Health Organization. COVID-19 Vaccine BIBP/Sinopharm. Available online: https://extranet.who.int/pqweb/vaccines/who-recommendation-covid-19-vaccine-bibp (accessed on 1 February 2023).
- Almufty, H.B.; Mohammed, S.A.; Abdullah, A.M.; Merza, M.A. Potential adverse effects of COVID19 vaccines among Iraqi population; a comparison between the three available vaccines in Iraq; a retrospective cross-sectional study. Diabetes Metab. Syndr. 2021, 15, 102207. [Google Scholar] [CrossRef] [PubMed]
- Saeed, B.Q.; Al-Shahrabi, R.; Alhaj, S.S.; Alkokhardi, Z.M.; Adrees, A.O. Side effects and perceptions following Sinopharm COVID-19 vaccination. Int. J. Infect. Dis. 2021, 111, 219–226. [Google Scholar] [CrossRef]
- Babaee, E.; Amirkafi, A.; Tehrani-Banihashemi, A.; SoleimanvandiAzar, N.; Eshrati, B.; Rampisheh, Z.; Asadi-Aliabadi, M.; Nojomi, M. Adverse effects following COVID-19 vaccination in Iran. BMC Infect. Dis. 2022, 22, 476. [Google Scholar] [CrossRef] [PubMed]
- Strategic Advisory Group of Experts on Immunization-SAGE (WHO). Working Group on COVID-19 Vaccines. Evidence Assessment: Sinovac/CoronaVac COVID-19 Vaccine. 29 April 2021 Apr. Available online: https://cdn.who.int/media/docs/default-source/immunization/sage/2021/april/5_sage29apr2021_critical-evidence_sinovac.pdf (accessed on 2 January 2023).
- Wilder-Smith, A.; Mulholland, K. Effectiveness of an Inactivated SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 385, 946. [Google Scholar] [CrossRef]
- British Society of Immunology. Types of Vaccines for COVID-19. Available online: https://www.immunology.org/public-information/vaccine-resources/covid-19/covid-19-vaccine-infographics/types-covid19-vaccines (accessed on 1 February 2023).
- Kumar, N.P.; Padmapriyadarsini, C.; Devi, K.U.; Banurekha, V.; Nancy, A.; Kumar, C.G.; Murhekar, M.V.; Gupta, N.; Panda, S.; Babu, S. Antibody responses to the BBV152 vaccine in individuals previously infected with SARS-CoV-2: A pilot study. Indian J. Med. Res. 2021, 153, 671. [Google Scholar] [PubMed]
- Sapkal, G.N.; Yadav, P.D.; Ella, R.; Deshpande, G.R.; Sahay, R.R.; Gupta, N.; Vadrevu, K.M.; Abraham, P.; Panda, S.; Bhargava, B. Inactivated COVID-19 vaccine BBV152/COVAXIN effectively neutralizes recently emerged B.1.1.7 variant of SARS-CoV-2. J. Travel Med. 2021, 28, taab051. [Google Scholar] [CrossRef] [PubMed]


| Characteristics | Sinopharm | CoronaVac/Sinovac | Covaxin |
|---|---|---|---|
| Generic name | Sinopharm [10,11] | CoronaVac [12,13] | Covaxin vaccine [14,15] |
| Brand name | BIBP-CorV [10,11] | COVID-19 Vero Cell [12,13] | BBV152, COVAXIN [14,15] |
| Type of Vaccine | Whole-virion inactivated vaccine [10,11] | Whole-virion inactivated vaccine [12,13] | Whole-virion inactivated vaccine [14,15] |
| Manufacturer, country | CNPGC, Beijing, China [10,11] | Sinovac Biotech, China [12,13] | Bharat Biotech, India [14,15] |
| FDA/WHO approval | 7 May 2021, updated 14 October 2021; 15 March 2022 [10,11] | 24 May 2021 [12,13] | 3 November 2021 [14,15] |
| Dose(s) | Two doses, 0.5 mL each, with a 3–4 week interval [10,11] | Two doses, 0.5 mL each, with a 28-day interval [12,13] | Two doses, 0.5 mL each, with a 28-day interval [14,15] |
| Booster shots | 4–6 months following primary series vaccination [10,11] | 4–6 months following primary series vaccination [12,13] | 4–6 months following primary series vaccination [14,15] |
| Route of administration | Intramuscular injection [10,11] | Intramuscular injection [12,13] | Intramuscular injection [14,15] |
| Storage | Store the box in a refrigerator at +2 to +8 °C. [10,11] | Store the box in a refrigerator at +2 to +8 °C [12,13] | Store the box in a refrigerator at +2 to +8 °C [14,15] |
| Vaccination cost | About USD 30 (GBP 22–26) per dose [10,11] | About USD 5–14 per dose [12,13] | About USD 2 per dose [14,15] |
| Effectiveness | Two doses at an interval of 21 days, efficacy 79% (CI: 66–87%); 84% (CI: 80–88%); 86% (CI: 80–91%); and 94% (CI: 62–100%) [10,11] | In two doses, with 2–4 week intervals, efficacy was 51% (CI: 36–62%) in symptomatic patients; 100% (CI: 17–100%) in severe cases; and 100% (CI: 56–100%) against hospitalization [12,13] | 68% for all variants of COVID-19 and 71% (CI: 50–84%); Kappa 90% (95% CI: 30–100%); and Delta 65% (95% CI: 33–83%) [14,15] |
| Effective age | 18 years and above [10,11] | 18 years and above [12,13] | 18 years and above [14,15] |
| Pregnant females | WHO suggests the use of the vaccine in pregnant women when the benefits outweigh the potential risks [10,11] | Pregnant women data are lacking. WHO suggests use in pregnancy, when the benefits outweigh the risks [12,13] | Data on pregnant women are insufficient. Minor adverse events were found [14,15] |
| Breastfeeding | WHO suggests use in breastfeeding women [10,11] | WHO recommends the use in lactating women as in other adults [12,13] | WHO recommends use in lactating women as in other adults [14,15] |
| People with comorbidities | Data are insufficient [10,11] | Recommended for persons with comorbidities [12,13] | Data are insufficient [14,15] |
| Mechanism of action | The inactivated vaccine contains the killed “SARS-CoV-2 virus, recognized by the immune system, triggers a response, and builds immune memory” to fight SARS-CoV-2 [10,11] | The inactivated vaccine contains the killed “SARS-CoV-2 virus, recognized by the immune system, triggers a response and builds immune memory" to fight SARS-CoV-2 [12,13] | The inactivated vaccine contains the killed “SARS-CoV-2 virus, recognized by the immune system, triggers a response, and builds immune memory” to fight SARS-CoV-2 [14,15] |
| Indications | For active immunization against SARS-CoV-2 | For active immunization against SARS-CoV-2 | For active immunization against SARS-CoV-2 |
| Contraindications | Known history of anaphylaxis, if developed after the first dose should not receive a second dose and acute symptoms [10,11] | Known history of anaphylaxis, if developed anaphylaxis after the first dose and should not receive a second dose [12,13] | Known history of anaphylaxis, if developed, should not receive a second dose, and acute infection or fever [14,15]. |
| Characteristics | Sinopharm | CoronaVac/Sinovac | Covaxin |
|---|---|---|---|
| Immunogenicity/neutralizing antibodies/duration of immunity | The median level of antibody and IgG level increased from 11.12 to 2607.50 and 4.07 to 619.20 BAU/mL on day 14 [16] | Neutralizing antibodies to live SARS-CoV-2; 77.9% seroconverted was by 28 days after the second dose [17] | Two doses of vaccines with an interval of 28 days showed 95.0% seropositivity to anti-spike antibodies. [18,19] |
| Local adverse effects | Pain at the injection site, redness, [20] | Injection site pain (41.5%) [21] | Injection site pain, swelling, redness, and itching [15]. Adverse effects were mild or moderately common after the first dose [22] |
| Systemic adverse effects | Fatigue, headache, myalgia, general lethargy, body ache, arthralgia, nausea, chills, fever, dizziness [20,23,24], and thromboembolism [25] | Fatigue, headache, muscle pain, and joint pain were common systemic effects [21]. In total, 57.49 per 100,000 people with thromboembolism [26] | Headache, fever, malaise, body aches, nausea, vomiting [15], myocarditis [27], and facial paralysis [28] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meo, S.A.; ElToukhy, R.A.; Meo, A.S.; Klonoff, D.C. Comparison of Biological, Pharmacological Characteristics, Indications, Contraindications, Efficacy, and Adverse Effects of Inactivated Whole-Virus COVID-19 Vaccines Sinopharm, CoronaVac, and Covaxin: An Observational Study. Vaccines 2023, 11, 826. https://doi.org/10.3390/vaccines11040826
Meo SA, ElToukhy RA, Meo AS, Klonoff DC. Comparison of Biological, Pharmacological Characteristics, Indications, Contraindications, Efficacy, and Adverse Effects of Inactivated Whole-Virus COVID-19 Vaccines Sinopharm, CoronaVac, and Covaxin: An Observational Study. Vaccines. 2023; 11(4):826. https://doi.org/10.3390/vaccines11040826
Chicago/Turabian StyleMeo, Sultan Ayoub, Riham A. ElToukhy, Anusha Sultan Meo, and David C. Klonoff. 2023. "Comparison of Biological, Pharmacological Characteristics, Indications, Contraindications, Efficacy, and Adverse Effects of Inactivated Whole-Virus COVID-19 Vaccines Sinopharm, CoronaVac, and Covaxin: An Observational Study" Vaccines 11, no. 4: 826. https://doi.org/10.3390/vaccines11040826
APA StyleMeo, S. A., ElToukhy, R. A., Meo, A. S., & Klonoff, D. C. (2023). Comparison of Biological, Pharmacological Characteristics, Indications, Contraindications, Efficacy, and Adverse Effects of Inactivated Whole-Virus COVID-19 Vaccines Sinopharm, CoronaVac, and Covaxin: An Observational Study. Vaccines, 11(4), 826. https://doi.org/10.3390/vaccines11040826





