Abstract
We are developing cytotoxic immunoconjugates (CICs) targeting the envelope protein (Env) of the Human Immunodeficiency Virus, type 1 (HIV) to purge the persistent reservoirs of viral infection. We have previously studied the ability of multiple monoclonal antibodies (mAbs) to deliver CICs to an HIV-infected cell. We have found that CICs targeted to the membrane-spanning gp41 domain of Env are most efficacious, in part because their killing is enhanced in the presence of soluble CD4. The ability of a mAb to deliver a CIC does not correlate with its ability to neutralize nor mediate Ab-dependent cellular cytotoxicity. In the current study, we seek to define the most effective anti-gp41 mAbs for delivering CICs to HIV-infected cells. To do this, we have evaluated a panel of human anti-gp41 mAbs for their ability to bind and kill two different Env-expressing cell lines: persistently infected H9/NL4-3 and constitutively transfected HEK293/92UG. We measured the binding and cytotoxicity of each mAb in the presence and absence of soluble CD4. We found that mAbs to the immunodominant helix-loop-helix region (ID-loop) of gp41 are most effective, whereas neutralizing mAbs to the fusion peptide, gp120/gp41 interface, and the membrane proximal external region (MPER) are relatively ineffective at delivering CICs. There was only a weak correlation between antigen exposure and killing activity. The results show that the ability to deliver an effective IC and neutralization are distinct functions of mAbs.
1. Introduction
The reservoir of cells carrying functional HIV proviruses that persist during effective antiviral therapy (ART) is the barrier to curing HIV infection [1,2]. Patients may be maintained with undetectable viral loads for years on ART, a state termed clinical latency. But when ART is stopped, viremia rapidly returns. This is due to the retention of a functional provirus, viral sequences integrated as DNA into the genome of long-lived T lymphocytes. HIV utilizes the same activation signals as the T-cell, so immune stimulation also results in virus production. If this occurs on ART, the infection is contained by the drugs. Thus, patients require continuous antiviral therapy. The degree of HIV transcription in cells of the persistent reservoir during clinical latency is a matter of considerable study [1,3,4,5,6]. The degree of virus transcription within this reservoir is a key factor in considering approaches to the eradication of the persistent reservoir. Some approaches do not require virus transcription because the therapeutic agent can inactivate the virus and not interfere with the function of uninfected cells. These include: 1. genetic manipulations, including CRISPR-mediated gene inactivation; 2. enhancing immune responses via the administration of engineered mAbs, therapeutic immunization, and checkpoint inhibition; and 3. the use of drugs that specifically inactivate HIV [7,8,9,10]. Other approaches require some degree of virus transcription to target the therapies to cells that express the virus. If this does not occur spontaneously, then it may be necessary to pharmacologically enhance the expression of virus, the fancifully named “kick and kill” or “activate and purge” approaches to eradicate the persistent reservoir of infection [11,12,13].
In a kick and kill regimen, patients would be treated with latency-reversing agents to activate expression of virus proteins while preventing the spread of infection by continued (or even intensified) ART. Multiple different agents have been proposed as latency-disrupting agents, including histone-deacetylase inhibitors, non-canonical activators of NFκB signaling, SMAC mimetics, interleukins, and toll-like receptor agonists [14,15,16,17,18,19,20,21,22,23]. Many of these agents have been tested in humans and macaques and have been found to be effective in increasing virus transcription. However, a major concern remains whether the entire reservoir can be activated to the level necessary to result in its elimination. Once the virus can be induced to come out of hiding, proposals for purging the exposed cells encompass a variety of methods. Initially, removal of activated cells depended upon host immune response and/or viral cytopathic effect. When this proved ineffective, attention shifted to the use of mAbs and therapeutic immunization in combination with virus activation [18,21,22,23,24]. Promising results have been obtained in macaques. Presumably, the mechanisms of action of mAb-based therapies are a combination of neutralization and Fc-mediated functions, such as complement activation and antibody dependent cellular cytotoxicity (ADCC). We and others have put our efforts into the development of cytotoxic immunoconjugates (CICs) as a more effective approach to killing cells that express HIV proteins [25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41], demonstrating the ability of an immunotoxin to kill target cells that are not killed by complement [27]. CICs contain two domains: a targeting domain that binds to the cells marked for killing and the toxic domain. If the toxic moiety is a small drug, the agent is termed an antibody–drug conjugate; if a protein toxin is used, it is an immunotoxin. By combining a highly specific targeting moiety with an extremely toxic molecule, CIC’s can achieve effective killing of target cells with surprisingly little off-target toxicity. The in vivo efficacy of immunotoxins directed to the gp41 domain of the HIV envelope protein (Env) has been demonstrated in mice and macaques [37,41].
In the studies presented here, we focus on optimizing the targeting domain, anti-gp41 mAbs. Env is the sole HIV protein expressed intact on the surface of infected cells. We have previously examined large panels of anti-Env mAbs to identify those most effective at delivering CICs, and we identified the immunodominant (ID)-loop of gp41 as the most effective target [34,40,42]. The efficacy of gp41-targeted CICs is further enhanced by the addition of soluble CD4 (sCD4), which increases both gp41 exposure on the cell surface and internalization of Env [32,34,40]. Here we examine a panel of 30 anti-gp41 mAbs for binding to and killing Env-expressing cells in the presence and absence of sCD4. We used flow cytometry to quantify binding and an indirect immunotoxin assay to measure cytotoxicity. We again demonstrated the primacy of the ID-loop region of gp41 as a target for CIC killing. We found little correlation between CIC and neutralization targets. A minimum degree of binding to cell-surface Env was required for cytotoxicity; beyond that, there was little correlation between cell-surface antigen expression and cell killing.
2. Materials and Methods
2.1. Reagents and Cells
The mAbs used in these studies and their sources are listed in Table 1; the mAbs are displayed based upon epitope specificity, with those targeting the amino terminus shown at the top of the table, and descending moves towards the C-terminus, although there are anomalies due to conformational epitopes. In addition to these mAbs, we have previously tested other anti-gp41 for delivering immunotoxins to HIV-infected cells, including 12.6D, 15.5A, 16.11A, 2.2B, 41.1, 41.4, T15G1, and 8ANC195 [34,40,42].
Soluble two-domain CD4183 (sCD4) was obtained from the Aids Reagent Program [43]. The secondary immunotoxin consisted of affinity purified goat anti-human IgG (H + L chain specific, Invitrogen, Waltham, MA, USA) conjugated via the disulfide-cleavable heterobifunctional cross-linking reagent SPDP (Pierce Chemical Company, Rockford IL, USA) to deglycosylated ricin A chain at a 2–3 fold molar excess of ricin to antibody, as described elsewhere [40,44]. The deglycosylated ricin A chain was the kind gift of Ellen Vitetta. The same polyclonal Ab was purchased from Invitrogen conjugated to FITC. Two cell lines expressing HIV Env were used in these studies. H9/NL4-3 cells were infected with the molecularly cloned HIV isolate NL4-3, which utilizes Env from the prototype lab-adapted HIV isolate IIIB [45] and were the gift of Bruce Chesebro and Kathy Wehrly at NIAID [31]. Through serial passage, >99% of H9/NL4-3 cells maintain production of infectious virus and expression of cell surface Env [40]. 293T/92UG cells are HEK-293T cells transfected to express the Env of the clade A clinical isolate 92UG037.8 and were the kind gift of Bing Chen, Boston Children’s Hospital and Harvard Medical School [46]. Cell-surface Env on 92UG cells is found in its native trimeric configuration. Cells were cultured in RPMI 1640 (H9/NL4-3) or DMEM (92UG) supplemented with 10% fetal bovine serum (Hyclone) and grown at 37° in a 5% CO2 humidified atmosphere.
2.2. Flow Cytometry to Detect Cell-Surface Env Expression
Cells were cultured in the presence or absence of sCD4 (500 ng/mL) for 24 h before harvest for flow cytometry. Cells were washed twice in PBS with 1% bovine serum albumin and 0.01% Na azide (PBA), and 105 were placed in 100µL in 96-well round bottom plates and incubated with 3µg/mL of anti-gp41 mAb with or without sCD4 (500 ng/mL) for 1 h at room temperature. Cells were washed twice with PBA and incubated with 1 µg/mL of FITC-conjugated goat anti-human IgG. Following a 1 h incubation at room temperature in the dark, the cells were washed twice and fixed in 2% paraformaldehyde. Using an Accuri C6 flow cytometer (BD Biosciences, San Jose, CA, USA), we analyzed 104 events gated by forward and side scatter to exclude debris using FloJo software (Treestar/BD Biosciences). Data are presented as the median fluorescence intensity ± standard error of the mean.
2.3. Indirect Immunotoxin Assay
The ability of mAbs to deliver a cytotoxic payload was measured using an indirect cytotoxicity assay, as described previously [40,42,44]. The specificity of this assay has been demonstrated with target cells not expressing cell-surface Env (H9 and 293T) and with irrelevant mAbs. Target cells (1–2 104, depending on cell type) were plated in triplicate in 96-well flat bottom tissue culture plates in 100 µL tissue culture medium in the presence of the indicated concentration of mAb, plus or minus sCD4 (500 ng/mL) for 1 h at 37°. As a negative control in every experiment, wells in the absence of mAb were included. The secondary immunotoxin, goat anti-human IgG conjugated to ricin A chain, was then added at a final concentration of 300 ng/mL in 100 µL, and the cells were incubated for 3 days. Wells receiving the secondary immunotoxin but not mAb serve as the negative control and are used in the calculation of percent cytotoxicity. MTS/PMS substrate (CellTiter AQueous, Promega) was added to the wells for the final 3 h of incubation. At the completion of incubation, absorbance (A) at 490 nm was read on a microplate reader (BioTek). Percent cytotoxicity was calculated according to the formula:
{1−[(AmAb − Ano cells)/(Ano mab − Ano cells)]} × 100.
Results are shown as mean and SEM. If error bars are not visible in the figures, the error is smaller than the symbol.
2.4. Statistical Analyses
Flow cytometry was analyzed using FloJo’s built-in statistical packages. GraphPad Prism v8.3 (GraphPad Software, Boston, MA, USA) was used for Pearson correlation analysis and construction of figures.
3. Results
3.1. Binding and Cytotoxicity of Anti-gp41 on Cells Transfected to Express Cell-Surface Env
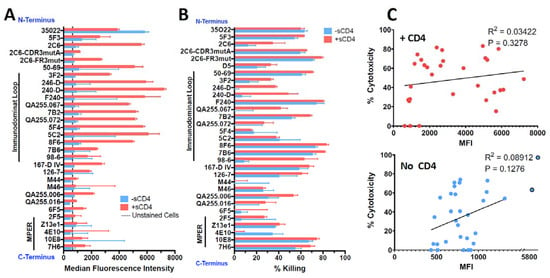
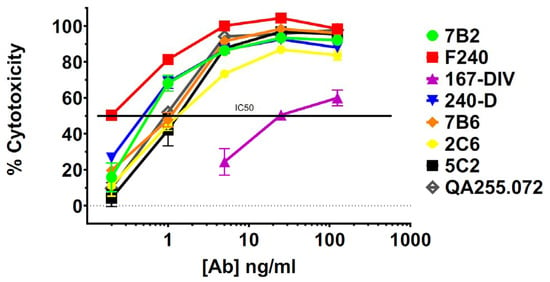
HEK293T cells transfected to express Env of the clade A isolate 92UG037.8 (henceforth 92UG) were the kind gift of Dr. Bing Chen. The mAbs were tested for binding to 92UG by flow cytometry and for cytotoxicity with an indirect immunotoxin assay (using anti-human IgG conjugated with ricin A chain). We have used this assay previously and shown it to be predictive of which mAbs will function best when directly conjugated to a toxin or cytotoxic drug [40,44]. Testing was performed in the presence or absence of sCD4, which has been shown to increase exposure and internalization of gp41 epitopes, as well as enhance the cytotoxicity of gp41-targeted CICs [32,34,40,42]. Cell-surface expression of all epitopes was markedly increased in the presence of sCD4, particularly in the ID-loop region (Figure 1A). The exception to this was mAb 35O22, which recognizes the gp120/gp41 interface. The diminished expression of this epitope reflects the ability of sCD4 to dissociate gp120 and gp41 [70]. Cytotoxicity in the indirect immunotoxin assay is shown in Figure 1B. Previously published experiments with isotype controls and untransfected HEK293 cells have demonstrated that cytotoxicity in this assay is Env-specific [40,42]. With only a single exception, M44, all mAbs were able to deliver a cytotoxic signal. At this relatively high concentration of mAb (125 ng/mL), some mAbs required sCD4 to produce or enhance cytotoxicity, while others were equally effective with or without sCD4. Surprisingly cytotoxicity of mAb 4E10 was depressed by sCD4. In Figure 1C, we show that there was no correlation between epitope expression and cytotoxicity. Because we tested the mAbs for cytotoxicity at a relatively high concentration, we chose the most effective mAbs and titrated them in an indirect immunotoxin assay in the presence of sCD4 (Figure 2). The mAb concentration that resulted in 50% killing (IC50) of 92UG ranged from 0.2 to 1 ng for the seven most effective mAbs, all of which target epitopes in the ID-loop domain.
Figure 1.
Comparison of binding and cytotoxicity of anti-gp41 mAbs assayed on 92UG cells. MAbs are shown ordered based on epitope location from N-terminus (top) to C-terminus (bottom). MAbs to MPER and immunodominant loop region are marked by bars. Data in red indicates presence of sCD4183 (500 ng/mL), blue the absence of sCD4183. (A) Indirect fluorescence. Mabs were tested at 3 µg/mL and detected with goat anti-human IgG-FITC as measured by flow cytometry. Binding is reported as median fluorescence with SEM. The vertical black line represents non-specific fluorescnce observed in the presence of anti-human FITC only. (B) Cytotoxicity. Anti-gp41 mAbs were tested at 125 ng/mL, and cytotoxicity was detected with ricin A chain conjugated anti-human Ig. Percent cytotoxicity was calculated as described in the text and shown as mean and SEM. (C) Correlation of cytotoxicity and binding with (top) and without (bottom) sCD4. P and R2 from Pearson correlation analysis.
Figure 2.
Titration of cytotoxicity of selected anti-gp41 mAbs assayed on 92UG cells. Cytotoxicity of anti-gp41 mAbs with concentrations from 0.2 to 125 ng/mL was evaluated with ricin A chain conjugated anti-human Ig in the presence of sCD4 (500 ng/mL). Percent cytotoxicity was calculated as described in the text.
3.2. Binding and Cytotoxicity of Anti-gp41 mAbs on Persistently Infected Cells
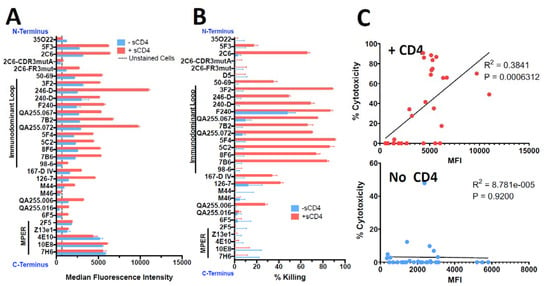
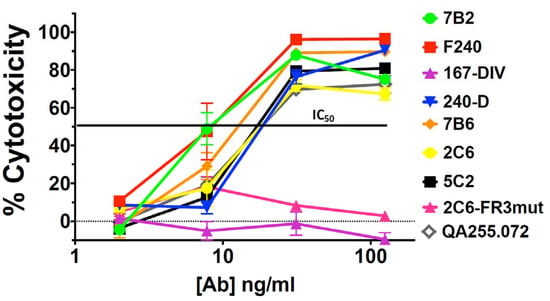
H9/NL4-3 cells are a CD4+ lymphoma cell line persistently infected with HIV, with virtually all cells expressing Env on the cell surface and secreting infectious HIV [40]. Figure 3 shows the binding and cytotoxicity of the anti-gp41 mAbs when tested on H9/NL4-3 cells. Flow cytometry demonstrated that almost all mAbs bound the cells and that sCD4 enhanced the expression of all epitopes, especially those in the ID-loop region. The mAb to the gp120/gp41 interface, 35O22, again showed decreased binding in the presence of sCD4. The set of mAbs capable of delivering a cytotoxic payload to H9/NL4-3 cells was more restricted than we observed with 92UG. Killing was primarily observed with mAbs to the ID-loop region and only in the presence of sCD4. For 2C6, mutation of the framework three region (2C6-Fr3mut) diminished binding and abrogated cytotoxicity. In published findings, this mutant diminished binding and has less ADCC in comparison to 2C6 [52]. Contrary to what we observed in 92UG, cytotoxicity was well correlated with cell-surface epitope expression in the presence of sCD4. Figure 4 shows a dose–response curve on H9/NL4-3 and demonstrates differences between the cell lines used as targets. The IC50 of the most effective mAbs was roughly 10-fold higher on H9/NL4-3 (8–15 ng/mL) than on 92UG. We have consistently observed that 92UG are more sensitive to immunotoxin killing than H9/NL4-3 [40,42], and attribute this to a diminution of the effective dose received by the cells, resulting from the secretion of immunotoxin-coated virus by the infected H9/NL4-3, but not by the transfected 92UG.
Figure 3.
Comparison of binding and cytotoxicity of anti-gp41 mAbs assayed on H9/NL4-3 cells. H9/NL4-3 cells are H9 cells persistently infected with the lab isolate NL4-3. The layout of figures and reagents are as in the caption of Figure 1. (A) Indirect fluorescence, (B) Cytotoxicity, (C) Correlation of cytotoxicity and binding.
Cytotoxicity of anti-gp41 mAbs with concentrations from 2 to 125 ng/mL was evaluated with ricin A chain conjugated anti-human Ig in the presence of sCD4183 (500 ng/mL).
4. Discussion and Conclusions
We have compared the ability of human mAbs targeted to different regions of the transmembrane domain of the HIV envelope protein, gp41, to kill two different target cells that stably express HIV Env on the cell surface. For studies such as those performed here, stable expression of Env is necessary to allow for repetitions and comparison. H9/NL4-3 is unique among persistently HIV-infected cell lines in that it maintains a productive infection with no cytopathic effect and no need to periodically replenish the cultures with uninfected cells [40]. Immunotoxin-resistant variants that have lost expression of the cell-surface envelope are found at a frequency <10−3 [30,71,72]. The ability to maintain a high level of persistent infection results from a virus mutation (8AA deletion in Vpr), perhaps combined with a cellular mutation [71,73]. It has also been surprisingly difficult to transfect cells to stably express native trimeric envelope at reasonable levels, and the HEK293T/92UG cells are a rare example of such a cell line [46]. These cell lines do not necessarily express gp120/gp41 to the same degree as the relevant cells in patients. We and others have shown that directly conjugated immunotoxins effectively treat tissue culture infections of primary human T cells [34,41,74], although even these cells may or may not be representative of what occurs in patients. In this regard, the evidence of in vivo efficacy in experimental animals [37,39,41,75] is our best argument that CICs kill cells with relevant amounts of cell-surface Env expression. The utility of H9/NL4-3 and 92UG cell lines for these experiments lies in their stable expression of native Env without cytopathic effects, not that they reflect what occurs clinically.
The ability of a mAb to deliver a cell-killing moiety to a target cell differs from other Ab-mediated effector functions in that internalization of the CIC is necessary for it to function. ADCC, complement activation, and neutralization occur at the cell or virion surface. Previous studies, as well as these (Figure 1 and Figure 2 compared to 3 and 4), demonstrate that H9/NL4-3 are more resistant to CIC-directed cytotoxicity than 92UG [41]. We attribute this to the secretion of virus by H9/NL4-3 acting as an immunologic decoy, binding the CIC before it can reach the cell. We also observe here that once above a certain threshold binding, there is little correlation between binding of mAb to the cell, measured by fluorescence, and killing mediated by the same mAb. Epitope location is an important element in determining CIC-efficacy, and thus a weaker-binding mAb to a more effective epitope could yield greater killing. We have previously found that mAbs to the ID-loop make the most effective CICs and further confirmed this in the present studies. While multiple CICs were capable of inducing cytotoxicity in 92UG cells, the more resistant infected H9/NL4-3 cells were most effectively killed by ID-loop specific CICs, and only in the presence of sCD4. Because the ID-loop is highly mobile, it was the last domain to be visualized by cryo-EM or crystallography of Env and lies close to the cell surface [76,77]. We believe that both the mobility and proximity to the cell surface contribute to the effectiveness of CICs in this region, with CD4-mediated conformational shifts in Env initiating the first step in the internalization of the CIC [70,78,79].
There are two well-defined regions of gp41 that are targets of Ab-mediated neutralization: the N-terminal fusion peptide and MPER (Table 1). When tested for cytotoxicity on H9/NL4-3 cells, mAbs to these regions have consistently tested negative [34,40,42]. The neutralizing activity of ID-loop-specific mAbs is questionable, although recent studies have shown that mAbs to this region can prevent virus from infecting cells expressing FcγR1 (CD64) [80]. Nevertheless, among anti-gp41 mAbs, the epitope specificity of mAbs that neutralize is distinct from those that deliver CICs. Antibodies that mediate ADCC and those that can effectively deliver CICs to infected cells form partially overlapping subsets, with mAbs to the ID-loop providing both functions, whereas some anti-MPER mAbs mediate ADCC well but are ineffective in delivering CICs (Table 1, Figure 3). These observations regarding differences among anti-gp41 mAbs in neutralization, ADCC, and CIC function are consistent with earlier studies of epitopes on both gp41 and gp120 [34,40,42]. The fusion peptide is a relatively newly proposed target on gp41, and a few of the antibodies tested herein target that region. A more thorough evaluation of that region as new antibodies emerge may be warranted.
We have demonstrated the in vivo efficacy of CICs targeted with mAb 7B2 in both mice and macaques [39,41] and utilized this mAb to construct antibody–drug conjugates as well as immunotoxins [41]. This mAb identifies a highly conserved gp41 epitope, the external loop CSGKLIC. In comparative titrations 7B2 and F240 [59,60] were most effective in killing both 92UG and H9/NL4-3 cell lines (Figure 2 and Figure 4). Future development and testing of anti-HIV CICs will continue to utilize mAb 7B2 to deliver cytotoxic payloads. We are currently testing 7B2-ricin A chain in HIV-infected humanized SCID mice. We acknowledge that it is unlikely this particular immunotoxin would be used in humans due to its immunogenicity. We have worked to reduce the immunogenicity of ricin A chain by pegylation, with only minimal success [41]. We are now focusing our efforts on identifying small drugs to be used in antibody drug conjugates. Unlike in cancer, where the target is, by definition, a dividing cell, the HIV reservoir likely contains non-dividing cells, even after viral activation [1,5,6]. Toxins kill resting cells, but small cytotoxic drugs developed primarily for cancer therapy may or may not. We have now screened a growing panel of small drugs used in anti-cancer conjugates for their ability to kill resting lymphocytes. We have conjugated the identified drugs to 7B2 and are now testing them in vitro for specificity and efficacy.
Author Contributions
Conceptualization, S.H.P. and M.D.H.; methodology, G.K., F.M.C., C.W., T.P. and S.H.P.; formal analysis, G.K., F.M.C. and S.H.P.; investigation, G.K., F.M.C., C.W., T.P., S.H.P. and M.D.H.; resources, T.P., S.H.P. and M.D.H.; writing—original draft preparation, G.K., F.M.C. and S.H.P.; writing—review and editing, G.K., F.M.C., S.H.P. and M.D.H.; visualization, G.K., F.M.C. and S.H.P.; supervision, T.P. and S.H.P.; project administration, T.P. and S.H.P.; funding acquisition, S.H.P. and M.D.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by USPHS grants AI136758 (SHP) and AI 125119-01 (MDH).
Institutional Review Board Statement
The derivation of the human mAbs used here and use of human subject samples are detailed in prior publications (Table 1). No human samples were used in the current studies.All studies were conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of the relevant institutions where the mAbs were produced.
Data Availability Statement
All raw data upon which the figures in this manuscript are based are available upon request from Pincus.
Acknowledgments
We thank J. Overbaugh and B. Haynes for providing mAbs used in these studies. The AIDS Reagent Program provided additional mAbs. 92UG cells were the gift of B. Chen.
Conflicts of Interest
The authors declare no conflict of interest.
Correction Statement
This article has been republished with a minor correction to resolve spelling errors. This change does not affect the scientific content of the article.
References
- Siliciano, J.D.; Siliciano, R.F. In Vivo Dynamics of the Latent Reservoir for HIV-1: New Insights and Implications for Cure. Annu. Rev. Pathol. 2022, 17, 271–294. [Google Scholar] [CrossRef]
- Mouquet, H. Hunting Down the HIV-1 Reservoir: A Starring Role for Antibodies? Immunity 2017, 46, 527–529. [Google Scholar] [CrossRef]
- Collora, J.A.; Ho, Y.-C. The loud minority: Transcriptionally active HIV-1-infected cells survive, proliferate, and persist. Cell 2022, 185, 227–229. [Google Scholar] [CrossRef] [PubMed]
- Ta, T.M.; Malik, S.; Anderson, E.M.; Jones, A.D.; Perchik, J.; Freylikh, M.; Sardo, L.; Klase, Z.A.; Izumi, T. Insights Into Persistent HIV-1 Infection and Functional Cure: Novel Capabilities and Strategies. Front. Microbiol. 2022, 13, 862270. [Google Scholar] [CrossRef]
- Clark, I.C.; Mudvari, P.; Thaploo, S.; Smith, S.; Abu-Laban, M.; Hamouda, M.; Theberge, M.; Shah, S.; Ko, S.H.; Perez, L.; et al. HIV silencing and cell survival signatures in infected T cell reservoirs. Nature 2023, 614, 318–325. [Google Scholar] [CrossRef]
- Margolis, D.M.; Archin, N.M.; Cohen, M.S.; Eron, J.J.; Ferrari, G.; Garcia, J.V.; Gay, C.L.; Goonetilleke, N.; Joseph, S.B.; Swanstrom, R.; et al. Curing HIV: Seeking to Target and Clear Persistent Infection. Cell 2020, 181, 189–206. [Google Scholar] [CrossRef]
- Maslennikova, A.; Mazurov, D. Application of CRISPR/Cas Genomic Editing Tools for HIV Therapy: Toward Precise Modifications and Multilevel Protection. Front. Cell Infect. Microbiol. 2022, 12, 880030. [Google Scholar] [CrossRef]
- Gubser, C.; Chiu, C.; Lewin, S.R.; Rasmussen, T.A. Immune checkpoint blockade in HIV. EBioMedicine 2022, 76, 103840. [Google Scholar] [CrossRef]
- Magro, G.; Calistri, A.; Parolin, C. Targeting and Understanding HIV Latency: The CRISPR System against the Provirus. Pathogens 2021, 10, 1257. [Google Scholar] [CrossRef]
- Balibar, C.J.; Klein, D.J.; Zamlynny, B.; Diamond, T.L.; Fang, Z.; Cheney, C.A.; Kristoff, J.; Lu, M.; Bukhtiyarova, M.; Ou, Y.; et al. Potent targeted activator of cell kill molecules eliminate cells expressing HIV-1. Sci. Transl. Med. 2023, 15, eabn2038. [Google Scholar] [CrossRef]
- Deeks, S.G. Shock and Kill. Nature 2012, 487, 439–440. [Google Scholar]
- Rasmussen, T.A.; Lewin, S.R. Shocking HIV out of hiding: Where are we with clinical trials of latency reversing agents? Curr. Opin. HIV AIDS 2016, 11, 394–401. [Google Scholar] [CrossRef]
- Nuhn, M.M.; Gumbs, S.B.H.; Buchholtz, N.; Jannink, L.M.; Gharu, L.; de Witte, L.D.; Wensing, A.M.J.; Lewin, S.R.; Nijhuis, M.; Symons, J. Shock and kill within the CNS: A promising HIV eradication approach? J. Leukoc. Biol. 2022, 112, 1297–1315. [Google Scholar] [CrossRef]
- Wei, D.G.; Chiang, V.; Fyne, E.; Balakrishnan, M.; Barnes, T.; Graupe, M.; Hesselgesser, J.; Irrinki, A.; Murry, J.P.; Stepan, G.; et al. Histone Deacetylase Inhibitor Romidepsin Induces HIV Expression in CD4 T Cells from Patients on Suppressive Antiretroviral Therapy at Concentrations Achieved by Clinical Dosing. PLoS Pathog. 2014, 10, e1004071. [Google Scholar] [CrossRef]
- Elliott, J.H.; Wightman, F.; Solomon, A.; Ghneim, K.; Ahlers, J.; Cameron, M.J.; Smith, M.Z.; Spelman, T.; McMahon, J.; Velayudham, P.; et al. Activation of HIV transcription with short-course vorinostat in HIV-infected patients on suppressive antiretroviral therapy. PLoS Pathog. 2014, 10, e1004473. [Google Scholar] [CrossRef]
- Borducchi, E.N.; Liu, J.; Nkolola, J.P.; Cadena, A.M.; Yu, W.H.; Fischinger, S.; Broge, T.; Abbink, P.; Mercado, N.B.; Chandrashekar, A.; et al. Antibody and TLR7 agonist delay viral rebound in SHIV-infected monkeys. Nature 2018, 563, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Martinsen, J.T.; Gunst, J.D.; Hojen, J.F.; Tolstrup, M.; Sogaard, O.S. The Use of Toll-Like Receptor Agonists in HIV-1 Cure Strategies. Front. Immunol. 2020, 11, 1112. [Google Scholar] [CrossRef]
- Dashti, A.; Waller, C.; Mavigner, M.; Schoof, N.; Bar, K.J.; Shaw, G.M.; Vanderford, T.H.; Liang, S.; Lifson, J.D.; Dunham, R.M.; et al. SMAC Mimetic Plus Triple-Combination Bispecific HIVxCD3 Retargeting Molecules in SHIV.C.CH505-Infected, Antiretroviral Therapy-Suppressed Rhesus Macaques. J. Virol. 2020, 94, e00793-20. [Google Scholar] [CrossRef]
- Molyer, B.; Kumar, A.; Angel, J.B. SMAC Mimetics as Therapeutic Agents in HIV Infection. Front. Immunol. 2021, 12, 780400. [Google Scholar] [CrossRef]
- Bricker, K.M.; Chahroudi, A.; Mavigner, M. New Latency Reversing Agents for HIV-1 Cure: Insights from Nonhuman Primate Models. Viruses 2021, 13, 1560. [Google Scholar] [CrossRef]
- Gunst, J.D.; Hojen, J.F.; Sogaard, O.S. Broadly neutralizing antibodies combined with latency-reversing agents or immune modulators as strategy for HIV-1 remission. Curr. Opin. HIV AIDS 2020, 15, 309–315. [Google Scholar] [CrossRef]
- Hsu, D.C.; Schuetz, A.; Imerbsin, R.; Silsorn, D.; Pegu, A.; Inthawong, D.; Sopanaporn, J.; Visudhiphan, P.; Chuenarom, W.; Keawboon, B.; et al. TLR7 agonist, N6-LS and PGT121 delayed viral rebound in SHIV-infected macaques after antiretroviral therapy interruption. PloS Pathog. 2021, 17, e1009339. [Google Scholar] [CrossRef]
- Moldt, B.; Chandrashekar, A.; Borducchi, E.N.; Nkolola, J.P.; Stephenson, H.; Nagel, M.; Hung, M.; Goldsmith, J.; Pace, C.S.; Carr, B.; et al. HIV envelope antibodies and TLR7 agonist partially prevent viral rebound in chronically SHIV-infected monkeys. PLoS Pathog. 2022, 18, e1010467. [Google Scholar] [CrossRef]
- Bricker, K.M.; Obregon-Perko, V.; Uddin, F.; Williams, B.; Uffman, E.A.; Garrido, C.; Fouda, G.G.; Geleziunas, R.; Robb, M.; Michael, N.; et al. Therapeutic vaccination of SIV-infected, ART-treated infant rhesus macaques using Ad48/MVA in combination with TLR-7 stimulation. PLoS Pathog. 2020, 16, e1008954. [Google Scholar] [CrossRef]
- Chaudhary, V.K.; Queen, C.; Junghans, R.P.; Waldmann, T.A.; FitzGerald, D.J.; Pastan, I. A recombinant immunotoxin consisting of two antibody variable domains fused to Pseudomonas exotoxin. Nature 1989, 339, 394–398. [Google Scholar]
- Till, M.A.; Zolla-Pazner, S.; Gorny, M.K.; Patton, J.S.; Uhr, J.W.; Vitetta, E.S. Human immunodeficiency virus-infected T cells and monocytes are killed by monoclonal human anti-gp41 antibodies coupled to ricin A chain. Proc. Natl. Acad. Sci. USA 1989, 86, 1987–1991. [Google Scholar]
- Pincus, S.H.; Wehrly, K.; Chesebro, B. Treatment of HIV tissue culture infection with monoclonal antibody-ricin A chain conjugates. J. Immunol. 1989, 142, 3070–3075. [Google Scholar]
- Till, M.A.; Ghetie, V.; May, R.D.; Auerbach, P.C.; Zolla-Pazner, S.; Gorny, M.K.; Gregory, T.; Uhr, J.W.; Vitetta, E.S. Immunoconjugates containing ricin A chain and either human anti-gp41 or CD4 kill H9 cells infected with different isolates of HIV, but do not inhibit normal T or B cell function. J. Acquir. Immune Defic. Syndr. 1990, 3, 609–614. [Google Scholar]
- Ashorn, P.; Moss, B.; Weinstein, J.N.; Chaudhary, V.K.; FitzGerald, D.J.; Pastan, I.; Berger, E.A. Elimination of infectious human immunodeficiency virus from human T-cell cultures by synergistic action of CD4-Pseudomonas exotoxin and reverse transcriptase inhibitors. Proc. Natl. Acad. Sci. USA 1990, 87, 8889–8893. [Google Scholar]
- Pincus, S.H.; Wehrly, K.; Tschachler, E.; Hayes, S.F.; Buller, R.S.; Reitz, M. Variants selected by treatment of human immunodeficiency virus-infected cells with an immunotoxin. J. Exp. Med. 1990, 172, 745–757. [Google Scholar]
- Pincus, S.H.; Cole, R.L.; Hersh, E.M.; Lake, D.; Masuho, Y.; Durda, P.J.; McClure, J. In vitro efficacy of anti-HIV immunotoxins targeted by various antibodies to the envelope protein. J. Immunol. 1991, 146, 4315–4324. [Google Scholar]
- Pincus, S.H.; McClure, J. Soluble CD4 enhances the efficacy of immunotoxins directed against gp41 of the human immunodeficiency virus. Proc. Natl. Acad. Sci. USA 1993, 90, 332–336. [Google Scholar]
- Pincus, S.H.; Tolstikov, V.V. Anti-human immunodeficiency virus immunoconjugates. Adv. Pharmacol. 1995, 32, 205–242. [Google Scholar]
- Pincus, S.H.; Wehrly, K.; Cole, R.; Fang, H.; Lewis, G.K.; McClure, J.; Conley, A.J.; Wahren, B.; Posner, M.R.; Notkins, A.L.; et al. In vitro effects of anti-HIV immunotoxins directed against multiple epitopes on the HIV-1 envelope glycoprotein gp160. AIDS Res. Hum. Retrovir. 1996, 12, 1041–1051. [Google Scholar]
- McHugh, L.; Hu, S.; Lee, B.K.; Santora, K.; Kennedy, P.E.; Berger, E.A.; Pastan, I.; Hamer, D.H. Increased affinity and stability of an anti-HIV-1 envelope immunotoxin by structure-based mutagenesis. J. Biol. Chem. 2002, 277, 34383–34390. [Google Scholar]
- Brooks, D.G.; Hamer, D.H.; Arlen, P.A.; Gao, L.; Bristol, G.; Kitchen, C.M.R.; Berger, E.A.; Zack, J.A. Molecular characterization, reactivation, and depletion of latent HIV. Immunity 2003, 19, 413–423. [Google Scholar]
- Pincus, S.H.; Fang, H.; Wilkinson, R.A.; Marcotte, T.K.; Robinson, J.E.; Olson, W.C. In vivo efficacy of anti-gp41, but not anti-gp120, immunotoxins in a mouse model of HIV infection. J. Immunol. 2003, 170, 2236–2241. [Google Scholar]
- Berger, E.A.; Pastan, I. Immunotoxin complementation of HAART to deplete persisting HIV-infected cell reservoirs. PLoS Pathog. 2010, 6, e1000803. [Google Scholar] [CrossRef]
- Denton, P.W.; Long, J.M.; Wietgrefe, S.W.; Sykes, C.; Spagnuolo, R.A.; Snyder, O.D.; Perkey, K.; Archin, N.M.; Choudhary, S.K.; Yang, K.; et al. Targeted Cytotoxic Therapy Kills Persisting HIV Infected Cells During ART. PLoS Pathog. 2014, 10, e1003872. [Google Scholar] [CrossRef]
- Pincus, S.H.; Song, K.; Maresh, G.A.; Hamer, D.H.; Dimitrov, D.S.; Chen, W.; Zhang, M.-Y.; Ghetie, V.F.; Chan-Hui, P.-Y.; Robinson, J.E.; et al. Identification of Human Anti-HIV gp160 Monoclonal Antibodies That Make Effective Immunotoxins. J. Virol 2017, 91, e01955-16. [Google Scholar] [CrossRef]
- Pincus, S.H.; Song, K.; Maresh, G.A.; Frank, A.; Worthylake, D.; Chung, H.K.; Polacino, P.; Hamer, D.H.; Coyne, C.P.; Rosenblum, M.G.; et al. Design and In Vivo Characterization of Immunoconjugates Targeting HIV gp160. J. Virol. 2017, 91, e01360-16. [Google Scholar] [CrossRef]
- Pincus, S.H.; Craig, R.B.; Weachter, L.; LaBranche, C.C.; Nabi, R.; Watt, C.; Raymond, M.; Peters, T.; Song, K.; Maresh, G.A.; et al. Bispecific Anti-HIV Immunoadhesins That Bind Gp120 and Gp41 Have Broad and Potent HIV-Neutralizing Activity. Vaccines 2021, 9, 744. [Google Scholar] [CrossRef]
- Garlick, R.L.; Kirschner, R.J.; Eckenrode, F.M.; Tarpley, W.G.; Tomich, C.S. Escherichia coli expression, purification, and biological activity of a truncated soluble CD4. AIDS Res. Hum. Retrovir. 1990, 6, 465–479. [Google Scholar]
- Craig, R.B.; Summa, C.M.; Corti, M.; Pincus, S.H. Anti-HIV Double Variable Domain Immunoglobulins Binding Both gp41 and gp120 for Targeted Delivery of Immunoconjugates. PLoS ONE 2012, 7, e46778. [Google Scholar] [CrossRef]
- Adachi, A.; Gendelman, H.E.; Koenig, S.; Folks, T.; Willey, R.; Rabson, A.; Martin, M.A. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J. Virol. 1986, 59, 284–291. [Google Scholar]
- Chen, J.; Kovacs, J.M.; Peng, H.; Rits-Volloch, S.; Lu, J.; Park, D.; Zablowsky, E.; Seaman, M.S.; Chen, B. Effect of the cytoplasmic domain on antigenic characteristics of HIV-1 envelope glycoprotein. Science 2015, 349, 191–195. [Google Scholar] [CrossRef]
- Huang, J.; Kang, B.H.; Pancera, M.; Lee, J.H.; Tong, T.; Feng, Y.; Georgiev, I.S.; Chuang, G.-Y.; Druz, A.; Doria-Rose, N.A.; et al. Broad and potent HIV-1 neutralization by a human antibody that binds the gp41-gp120 interface. Nature 2014, 515, 138–142. [Google Scholar] [CrossRef]
- Pham, T.N.; Lukhele, S.; Dallaire, F.; Perron, G.; Cohen, E.A. Enhancing Virion Tethering by BST2 Sensitizes Productively and Latently HIV-infected T cells to ADCC Mediated by Broadly Neutralizing Antibodies. Sci. Rep. 2016, 6, 37225. [Google Scholar] [CrossRef]
- Williams, K.L.; Stumpf, M.; Naiman, N.E.; Ding, S.; Garrett, M.; Gobillot, T.; Vézina, D.; Dusenbury, K.; Ramadoss, N.S.; Basom, R.; et al. Identification of HIV gp41-specific antibodies that mediate killing of infected cells. PLoS Pathog. 2019, 15, e1007572. [Google Scholar] [CrossRef]
- Lai, R.P.; Hock, M.; Radzimanowski, J.; Tonks, P.; Hulsik, D.L.; Effantin, G.; Seilly, D.J.; Dreja, H.; Kliche, A.; Wagner, R.; et al. A fusion intermediate gp41 immunogen elicits neutralizing antibodies to HIV-1. J. Biol. Chem. 2014, 289, 29912–29926. [Google Scholar] [CrossRef]
- Buchacher, A.; Predl, R.; Strutzenberger, K.; Steinfellner, W.; Trkola, A.; Purtscher, M.; Gruber, G.; Tauer, C.; Steindl, F.; Jungbauer, A. Generation of human monoclonal antibodies against HIV-1 proteins; electrofusion and Epstein-Barr virus transformation for peripheral blood lymphocyte immortalization. AIDS Res. Hum. Retrovir. 1994, 10, 359–369. [Google Scholar]
- Sojar, H.; Baron, S.; Sullivan, J.T.; Garrett, M.; van Haaren, M.M.; Hoffman, J.; Overbaugh, J.; Doranz, B.J.; Hicar, M.D. Monoclonal Antibody 2C6 Targets a Cross-Clade Conformational Epitope in gp41 with Highly Active Antibody-Dependent Cell Cytotoxicity. J. Virol. 2019, 93, e00772-19. [Google Scholar] [CrossRef]
- Hicar, M.D.; Chen, X.; Kalams, S.A.; Sojar, H.; Landucci, G.; Forthal, D.N.; Spearman, P.; Crowe, J.E., Jr. Low frequency of broadly neutralizing HIV antibodies during chronic infection even in quaternary epitope targeting antibodies containing large numbers of somatic mutations. Mol. Immunol. 2016, 70, 94–103. [Google Scholar] [CrossRef]
- DeCotes, D.; Baron, S.; Hoffman, J.; Garrett, M.; Sojar, H.; Hicar, M.D. Highly mutated monoclonal antibody 3F2 targets a conformational and strain-restricted epitope in human immunodeficiency virus gp41 with significant antibody-dependent cell cytotoxicity. Arch. Virol. 2022, 167, 2193–2201. [Google Scholar] [CrossRef]
- Pollara, J.; Bonsignori, M.; Moody, M.A.; Pazgier, M.; Haynes, B.F.; Ferrari, G. Epitope specificity of human immunodeficiency virus-1 antibody dependent cellular cytotoxicity [ADCC] responses. Curr. HIV Res. 2013, 11, 378–387. [Google Scholar] [CrossRef]
- Xu, J.-Y.; Gorny, M.K.; Palker, T.; Karwowska, S.; Zolla-Pazner, S. Epitope mapping of two immunodominant domains of gp41, the transmembrane protein of human immunodeficiency virus type 1, using ten human monoclonal antibodies. J. Virol. 1991, 65, 4832–4838. [Google Scholar]
- Gorny, M.K.; Gianakakos, V.; Sharpe, S.; Zolla-Pazner, S. Generation of human monoclonal antibodies to human immunodeficiency virus. Proc. Natl. Acad. Sci. USA 1989, 86, 1624–1628. [Google Scholar]
- Robinson, W.E.; Gorney, M.K.; Xu, J.Y.; Mitchell, W.M.; Zolla-Pazner, S. Two Immunodominant Domains of gp4l Bind Antibodies Which Enhance Human Immunodeficiency Virus Type 1 Infection In Vitro. J. Virol. 1991, 65, 4169–4176. [Google Scholar]
- von Bredow, B.; Arias, J.F.; Heyer, L.N.; Moldt, B.; Le, K.; Robinson, J.E.; Zolla-Pazner, S.; Burton, D.R.; Evans, D.T. Comparison of Antibody-Dependent Cell-Mediated Cytotoxicity and Virus Neutralization by HIV-1 Env-specific Monoclonal Antibodies. J. Virol. 2016, 90, 6127–6139. [Google Scholar] [CrossRef]
- Cavacini, L.A.; Emes, C.L.; Wisnewski, A.V.; Power, J.; Lewis, G.; Montefiori, D.; Posner, M.R. Functional and molecular characterization of human monoclonal antibody reactive with the immunodominant region of HIV type 1 glycoprotein 41. AIDS Res. Hum. Retrovir. 1998, 14, 1271–1280. [Google Scholar] [CrossRef]
- Hicar, M.D.; Chen, X.; Sulli, C.; Barnes, T.; Goodman, J.; Sojar, H.; Briney, B.; Willis, J.; Chukwuma, V.U.; Kalams, S.A.; et al. Human Antibodies that Recognize Novel Immunodominant Quaternary Epitopes on the HIV-1 Env Protein. PLoS ONE 2016, 11, e0158861. [Google Scholar] [CrossRef]
- Forthal, D.N.; Landucci, G.; Gorny, M.K.; Zolla-Pazner, S.; Robinson, W.E., Jr. Functional activities of 20 human immunodeficiency virus type 1 (HIV-1)-specific human monoclonal antibodies. AIDS Res. Hum. Retrovir. 1995, 11, 1095–1099. [Google Scholar] [CrossRef]
- Choudhry, V.; Zhang, M.Y.; Sidorov, I.A.; Louis, J.M.; Harris, I.; Dimitrov, A.S.; Bouma, P.; Cham, F.; Choudhary, A.; Rybak, S.M.; et al. Cross-reactive HIV-1 neutralizing monoclonal antibodies selected by screening of an immune human phage library against an envelope glycoprotein (gp140) isolated from a patient (R2) with broadly HIV-1 neutralizing antibodies. Virology 2007, 363, 79–90. [Google Scholar] [CrossRef]
- Wrotniak, B.H.; Garrett, M.; Baron, S.; Sojar, H.; Shon, A.; Asiago-Reddy, E.; Yager, J.; Kalams, S.; Croix, M.; Hicar, M.D. Antibody dependent cell cytotoxicity is maintained by the unmutated common ancestor of 6F5, a Gp41 conformational epitope targeting antibody that utilizes heavy chain VH1-2. Vaccine 2022, 40, 4174–4181. [Google Scholar] [CrossRef]
- Muster, T.; Steindl, F.; Purtscher, M.; Trkola, A.; Klima, A.; Himmler, G.; Ruker, F.; Katinger, H. A conserved neutralizing epitope on gp41 of human immunodeficiency virus type 1. J. Virol. 1993, 67, 6642–6647. [Google Scholar]
- Muster, T.; Guinea, R.; Trkola, A.; Purtscher, M.; Klima, A.; Steindl, F.; Palese, P.; Katinger, H. Cross-neutralizing activity against divergent HIV-1 isolates induced by the gp41 sequence ELDKWAS. J. Virol. 1994, 68, 4031–4034. [Google Scholar]
- Zwick, M.B.; Labrijn, A.F.; Wang, M.; Spenlehauer, C.; Saphire, E.O.; Binley, J.M.; Moore, J.P.; Stiegler, G.; Katinger, H.; Burton, D.R.; et al. Broadly neutralizing antibodies targeted to the membrane-proximal external region of human immunodeficiency virus type 1 glycoprotein gp41. J. Virol. 2001, 75, 10892–10905. [Google Scholar] [CrossRef]
- Nelson, J.; Brunel, F.; Jensen, R.; Crooks, E.; Cardoso, R.; Wang, M.; Hessell, A.; Wilson, I.; Binley, J.; Dawson, P.; et al. An Affinity-Enhanced Neutralizing Antibody against the Membrane-Proximal External Region of Human Immunodeficiency Virus Type 1 gp41 Recognizes an Epitope between Those of 2F5 and 4E10. J. Virol. 2007, 81, 4033–4043. [Google Scholar] [CrossRef]
- Bruel, T.; Guivel-Benhassine, F.; Amraoui, S.; Malbec, M.; Richard, L.; Bourdic, K.; Donahue, D.A.; Lorin, V.; Casartelli, N.; Noël, N.; et al. Elimination of HIV-1-infected cells by broadly neutralizing antibodies. Nat. Commun. 2016, 7, 10844. [Google Scholar] [CrossRef]
- Moore, J.P.; McKeating, J.A.; Weiss, R.A.; Sattentau, Q.J. Dissociation of gp120 from HIV-1 virions induced by soluble CD4. Science 1990, 250, 1139–1142. [Google Scholar]
- Fang, H.; Pincus, S.H. Unique insertion sequence and pattern of CD4 expression in variants selected with immunotoxins from HIV-infected T cells. J. Virol. 1995, 69, 75. [Google Scholar]
- Duensing, T.D.; Fang, H.; Dorward, D.W.; Pincus, S.H. Processing of the envelope glycoprotein gp160 in immunotoxin-resistant cell lines chronically infected with HIV-1. J. Virol. 1995, 69, 7122–7131. [Google Scholar]
- Fang, H.; Pincus, S.H. Spontaneous activation of HIV-1 in an immunotoxin resistant variant cell line. AIDS Res. Hum. Retrovir. 1999, 15, 1345–1349. [Google Scholar]
- Kennedy, P.E.; Bera, T.K.; Wang, Q.C.; Gallo, M.; Wagner, W.; Lewis, M.G.; Berger, E.A.; Pastan, I. Anti-HIV-1 immunotoxin 3B3(Fv)-PE38: Enhanced potency against clinical isolates in human PBMCs and macrophages, and negligible hepatotoxicity in macaques. J. Leukoc. Biol. 2006, 80, 1175–1182. [Google Scholar]
- Goldstein, H.; Pettoello-Mantovani, M.; Bera, T.K.; Pastan, I.; Berger, E.A. Chimeric toxins targeted to the human immunodeficiency virus type 1 envelope glycoprotein augment the in vivo activity of combination antiretroviral therapy in thy/liv-SCID-Hu mice. J. Inf. Dis. 2000, 181, 921–926. [Google Scholar]
- Lyumkis, D.; Julien, J.-P.; de Val, N.; Cupo, A.; Potter, C.S.; Klasse, P.J.; Burton, D.R.; Sanders, R.W.; Moore, J.P.; Carragher, B.; et al. Cryo-EM Structure of a Fully Glycosylated Soluble Cleaved HIV-1 Envelope Trimer. Science 2013, 342, 1484–1490. [Google Scholar] [CrossRef]
- Julien, J.-P.; Cupo, A.; Sok, D.; Stanfield, R.L.; Lyumkis, D.; Deller, M.C.; Klasse, P.J.; Burton, D.R.; Sanders, R.W.; Moore, J.P.; et al. Crystal Structure of a Soluble Cleaved HIV-1 Envelope Trimer. Science 2013, 342, 1477–1483. [Google Scholar] [CrossRef]
- Sattentau, Q.J.; Moore, J.P. Conformational changes induced in the human immunodeficiency virus envelope glycoprotein by soluble CD4 binding. J. Exp. Med. 1991, 174, 407–415. [Google Scholar]
- Pancera, M.; Majeed, S.; Ban, Y.-E.A.; Chen, L.; Huang, C.-C.; Kong, L.; Kwon, Y.D.; Stuckey, J.; Zhou, T.; Robinson, J.E.; et al. Structure of HIV-1 gp120 with gp41-interactive region reveals layered envelope architecture and basis of conformational mobility. Proc. Natl. Acad. Sci. USA 2010, 107, 1166–1171. [Google Scholar] [CrossRef]
- Montefiori, D.C.; Filsinger Interrante, M.V.; Bell, B.N.; Rubio, A.A.; Joyce, J.G.; Shiver, J.W.; LaBranche, C.C.; Kim, P.S. The high-affinity immunoglobulin receptor FcγRI potentiates HIV-1 neutralization via antibodies against the gp41 N-heptad repeat. Proc. Natl. Acad. Sci. USA 2021, 118, e2018027118. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).