Engineered Phage-Based Cancer Vaccines: Current Advances and Future Directions
Abstract
1. Introduction
2. Bacteriophages: Exploring the Intricacies of Their Structure and Biology
2.1. The Structure and Evolution of Bacteriophage
2.2. Phages in the Human Body and Their Pharmacokinetics
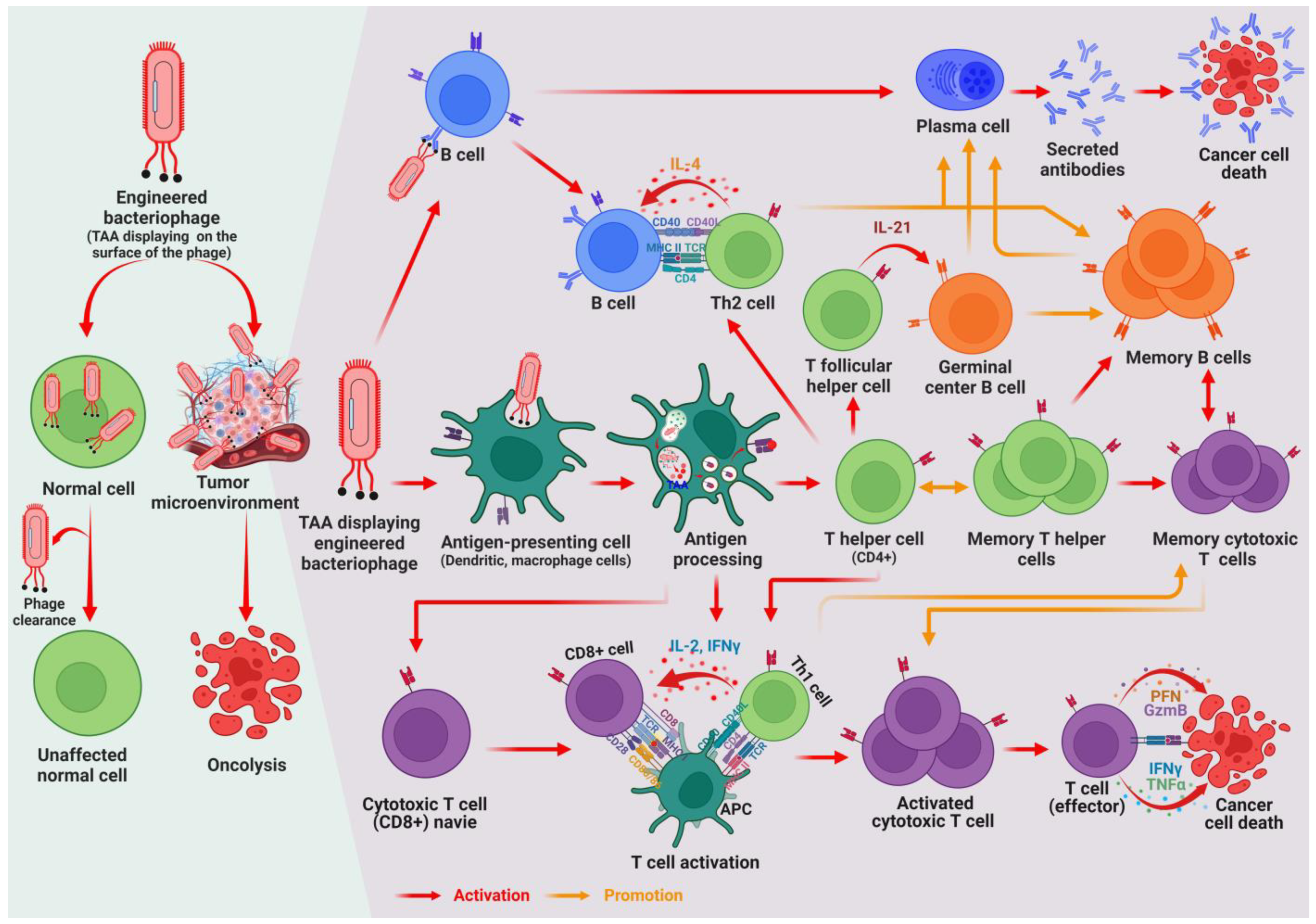
3. Exploring Phage–Immune Cell Interactions and Administration Modalities
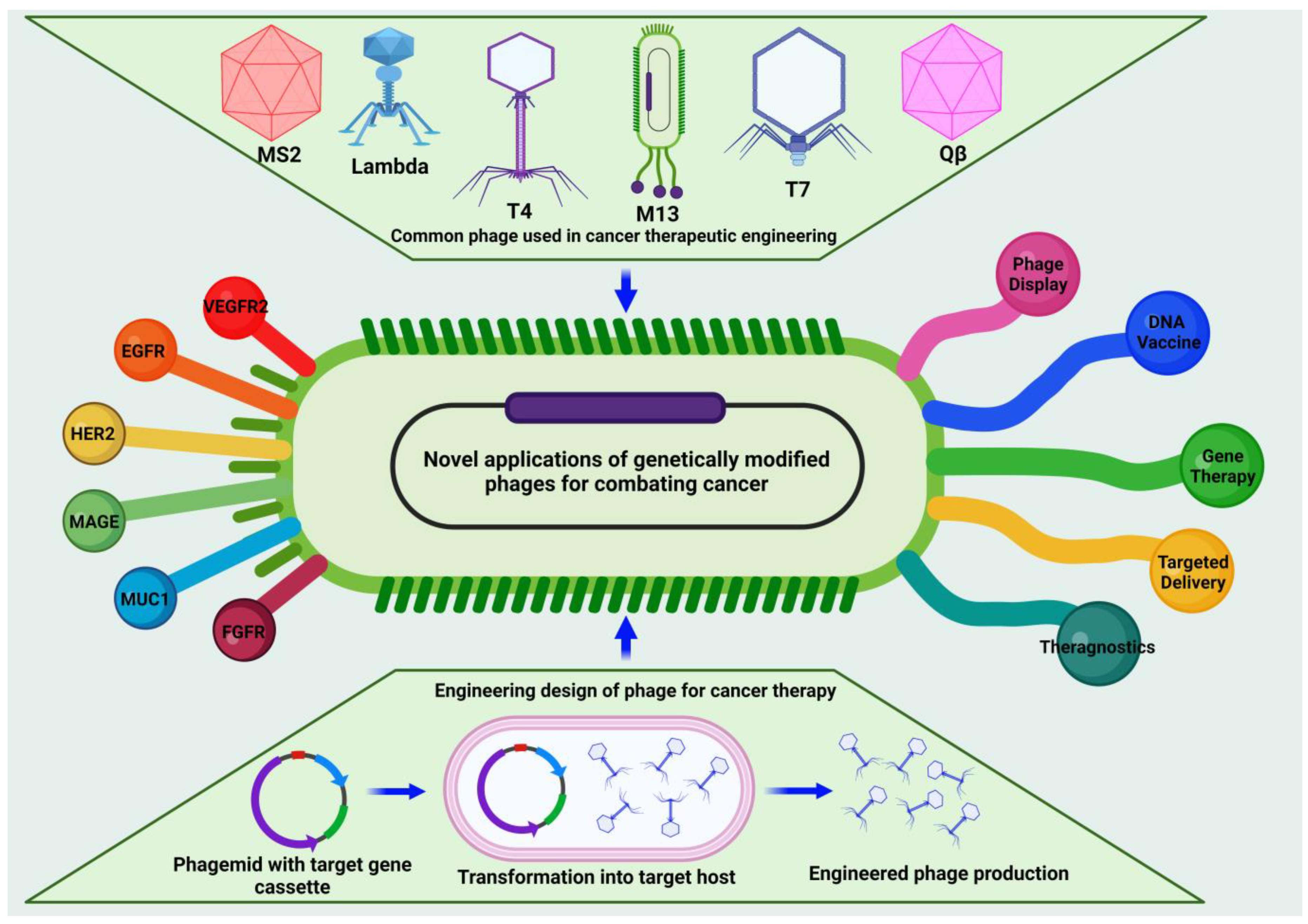
4. Phage Engineering: Innovative Approaches for Design and Development
4.1. Filamentous Phage Display Systems
4.2. T4 Phage Display Systems
4.3. T7 Phage Display Systems
4.4. Phage Lambda Display Systems
4.5. MS2 Phage Display Systems
4.6. Qβ Phage Display Systems
5. Revolutionary Strategy of Engineered Bacteriophages for Combating Cancer
5.1. Role of Phages as Antigen Display Systems for Vaccines
5.1.1. VEGFR2 Antigen Display
5.1.2. EGFR Antigen Display
5.1.3. HER2 Antigen Display
5.1.4. MAGE Antigen Display
5.1.5. MUC1 Antigen Display
5.1.6. FGFR Antigen Display
5.1.7. Flt4 Antigen Display
5.1.8. Exemplary Array of Additional TAA Mimotope Variants
5.2. Role of Phage Nanocarriers in Transforming Cancer Treatment
5.2.1. DNA Vaccines of Phages
5.2.2. Gene Therapy of Phages
5.3. Combination Therapy of Phages for Targeted Cancer Treatment
6. Advancement of Phage Use in Humans: Clinical Trials and Patents
7. Challenges and Potential of Phage-Based Nanomedicine
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Urruticoechea, A.; Alemany, R.; Balart, J.; Villanueva, A.; Vinals, F.; Capella, G. Recent advances in cancer therapy: An overview. Curr. Pharm. Des. 2010, 16, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Vardy, J.; Rourke, S.; Tannock, I.F. Evaluation of cognitive function associated with chemotherapy: A review of published studies and recommendations for future research. J. Clin. Oncol. 2007, 25, 2455–2463. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Joo, K.M.; Jin, J.; Nam, D.-H. Cancer stem cells and their mechanism of chemo-radiation resistance. Int. J. Stem. Cells 2009, 2, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Vardy, J.; Tannock, I. Cognitive function after chemotherapy in adults with solid tumours. Crit. Rev. Oncol./Hematol. 2007, 63, 183–202. [Google Scholar] [CrossRef]
- Amer, M.H. Gene therapy for cancer: Present status and future perspective. Mol. Cell. Ther. 2014, 2, 27. [Google Scholar] [CrossRef]
- Ailia, M.J.; Yoo, S.Y. In Vivo Oncolytic Virotherapy in Murine Models of Hepatocellular Carcinoma: A Systematic Review. Vaccines 2022, 10, 1541. [Google Scholar] [CrossRef]
- Muthukutty, P.; Woo, H.Y.; Ragothaman, M.; Yoo, S.Y. Recent Advances in Cancer Immunotherapy Delivery Modalities. Pharmaceutics 2023, 15, 504. [Google Scholar] [CrossRef]
- Agrawal, P.; Bhalla, S.; Usmani, S.S.; Singh, S.; Chaudhary, K.; Raghava, G.P.; Gautam, A. CPPsite 2.0: A repository of experimentally validated cell-penetrating peptides. Nucleic Acids Res. 2016, 44, D1098–D1103. [Google Scholar] [CrossRef]
- Arap, W.; Kolonin, M.G.; Trepel, M.; Lahdenranta, J.; Cardó-Vila, M.; Giordano, R.J.; Mintz, P.J.; Ardelt, P.U.; Yao, V.J.; Vidal, C.I. Steps toward mapping the human vasculature by phage display. Nat. Med. 2002, 8, 121–127. [Google Scholar] [CrossRef]
- Arap, W.; Pasqualini, R.; Ruoslahti, E. Cancer treatment by targeted drug delivery to tumor vasculature in a mouse model. Science 1998, 279, 377–380. [Google Scholar] [CrossRef]
- Bhattarai, S.R.; Yoo, S.Y.; Lee, S.-W.; Dean, D. Engineered phage-based therapeutic materials inhibit Chlamydia trachomatis intracellular infection. Biomaterials 2012, 33, 5166–5174. [Google Scholar] [CrossRef] [PubMed]
- Chae, S.Y.; Shrestha, K.R.; Jeong, S.-N.; Park, G.; Yoo, S.Y. Bioinspired RGD-engineered bacteriophage nanofiber cues against oxidative stress. Biomacromolecules 2019, 20, 3658–3671. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-Y.; Lee, H.; Kim, Y.; Yoo, S.Y.; Chung, W.-J.; Kim, G. Phage as versatile nanoink for printing 3-D cell-laden scaffolds. Acta Biomater. 2016, 29, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-S.; Kang, J.-I.; Chung, W.-J.; Lee, D.H.; Lee, B.Y.; Lee, S.-W.; Yoo, S.Y. Engineered phage matrix stiffness-modulating osteogenic differentiation. ACS Appl. Mater. Interfaces 2018, 10, 4349–4358. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, S.W.; Ji, S.T.; Kim, Y.J.; Jang, W.B.; Oh, J.-W.; Kim, J.; Yoo, S.Y.; Beak, S.H.; Kwon, S.-M. Engineered M13 nanofiber accelerates ischemic neovascularization by enhancing endothelial progenitor cells. Tissue Eng. Regen. Med. 2017, 14, 787–802. [Google Scholar] [CrossRef]
- Moon, J.-S.; Kim, W.-G.; Kim, C.; Park, G.-T.; Heo, J.; Yoo, S.Y.; Oh, J.-W. M13 bacteriophage-based self-assembly structures and their functional capabilities. Mini-Rev. Org. Chem. 2015, 12, 271–281. [Google Scholar] [CrossRef]
- Shrestha, K.R.; Lee, D.H.; Chung, W.; Lee, S.-W.; Lee, B.Y.; Yoo, S.Y. Biomimetic virus-based soft niche for ischemic diseases. Biomaterials 2022, 288, 121747. [Google Scholar] [CrossRef]
- Sugimoto, R.; Lee, J.H.; Lee, J.-H.; Jin, H.-E.; Yoo, S.Y.; Lee, S.-W. Bacteriophage nanofiber fabrication using near field electrospinning. RSC Adv. 2019, 9, 39111–39118. [Google Scholar] [CrossRef]
- Yoo, S.Y.; Chung, W.-J.; Kim, T.H.; Le, M.; Lee, S.-W. Facile patterning of genetically engineered M13 bacteriophage for directional growth of human fibroblast cells. Soft Matter 2011, 7, 363–368. [Google Scholar] [CrossRef]
- Yoo, S.Y.; Jin, H.E.; Choi, D.S.; Kobayashi, M.; Farouz, Y.; Wang, S.; Lee, S.W. M13 bacteriophage and adeno-associated virus hybrid for novel tissue engineering material with gene delivery functions. Adv. Healthc. Mater. 2016, 5, 88–93. [Google Scholar] [CrossRef]
- Yoo, S.Y.; Kobayashi, M.; Lee, P.P.; Lee, S.-W. Early osteogenic differentiation of mouse preosteoblasts induced by collagen-derived DGEA-peptide on nanofibrous phage tissue matrices. Biomacromolecules 2011, 12, 987–996. [Google Scholar] [CrossRef]
- Yoo, S.Y.; Merzlyak, A.; Lee, S.-W. Facile growth factor immobilization platform based on engineered phage matrices. Soft Matter 2011, 7, 1660–1666. [Google Scholar] [CrossRef]
- Yoo, S.Y.; Merzlyak, A.; Lee, S.-W. Synthetic phage for tissue regeneration. Mediat. Inflamm. 2014, 192790. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.Y.; Shrestha, K.R.; Jeong, S.-N.; Kang, J.-I.; Lee, S.-W. Engineered phage nanofibers induce angiogenesis. Nanoscale 2017, 9, 17109–17117. [Google Scholar] [CrossRef]
- Moon, J.-S.; Kim, W.-G.; Shin, D.-M.; Lee, S.-Y.; Kim, C.; Lee, Y.; Han, J.; Kim, K.; Yoo, S.Y.; Oh, J.-W. Bioinspired M-13 bacteriophage-based photonic nose for differential cell recognition. Chem. Sci. 2017, 8, 921–927. [Google Scholar] [CrossRef]
- Au, G.G.; Lincz, L.F.; Enno, A.; Shafren, D.R. Oncolytic Coxsackievirus A21 as a novel therapy for multiple myeloma. Br. J. Haematol. 2007, 137, 133–141. [Google Scholar] [CrossRef]
- Bachrach, G.; Leizerovici-Zigmond, M.; Zlotkin, A.; Naor, R.; Steinberg, D. Bacteriophage isolation from human saliva. Lett. Appl. Microbiol. 2003, 36, 50–53. [Google Scholar] [CrossRef] [PubMed]
- Bais, S.; Bartee, E.; Rahman, M.M.; McFadden, G.; Cogle, C.R. Oncolytic virotherapy for hematological malignancies. Adv. Virol. 2012, 2012, 186512. [Google Scholar] [CrossRef] [PubMed]
- Bearden, C.M.; Agarwal, A.; Book, B.K.; Vieira, C.A.; Sidner, R.A.; Ochs, H.D.; Young, M.; Pescovitz, M.D. Rituximab inhibits the in vivo primary and secondary antibody response to a neoantigen, bacteriophage phiX174. Am. J. Transplant. 2005, 5, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Burrows, F.J.; Thorpe, P.E. Vascular targeting—A new approach to the therapy of solid tumors. Pharmacol. Ther. 1994, 64, 155–174. [Google Scholar] [CrossRef]
- Bazan, J.; Całkosiński, I.; Gamian, A. Phage display—A powerful technique for immunotherapy: 1. Introduction and potential of therapeutic applications. Hum. Vaccines Immunother. 2012, 8, 1817–1828. [Google Scholar] [CrossRef] [PubMed]
- Hajitou, A. Targeted systemic gene therapy and molecular imaging of cancer: Contribution of the vascular-targeted AAVP vector. Adv. Genet. 2010, 69, 65–82. [Google Scholar] [PubMed]
- Clark, J.R.; March, J.B. Bacterial viruses as human vaccines? Expert. Rev. Vaccines 2004, 3, 463–476. [Google Scholar] [CrossRef]
- Twort, F.W. Further investigations on the nature of ultra-microscopic viruses and their cultivation. Epidemiol. Infect. 1936, 36, 204–235. [Google Scholar] [CrossRef]
- Taylor, M.W. Viruses and Man: A History of Interactions; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Schless, R.A. Staphylococcus aureus meningitis: Treatment with specific bacteriophage. Am. J. Dis. Child. 1932, 44, 813–822. [Google Scholar] [CrossRef]
- Summers, W.C.S. Felix D’herelle and the Origins of Molecular Biology; Yale University Press: London, UK, 1999. [Google Scholar]
- Rice, T.B. Use of bacteriophage filtrates in treatment of suppurative conditions: Report of 300 cases. Am. J. Med. Sci. 1930, 179, 345–360. [Google Scholar] [CrossRef]
- De la Cruz, V.; Lal, A.; McCutchan, T.F. Immunogenicity and epitope mapping of foreign sequences via genetically engineered filamentous phage. J. Biol. Chem. 1988, 263, 4318–4322. [Google Scholar] [CrossRef]
- Bartolacci, C.; Andreani, C.; Curcio, C.; Occhipinti, S.; Massaccesi, L.; Giovarelli, M.; Galeazzi, R.; Iezzi, M.; Tilio, M.; Gambini, V. Phage-Based Anti-HER2 Vaccination Can Circumvent Immune Tolerance against Breast CancerPhages against HER2+ Breast Cancer. Cancer Immunol. Res. 2018, 6, 1486–1498. [Google Scholar] [CrossRef]
- Asadi-Ghalehni, M.; Ghaemmaghami, M.; Klimka, A.; Javanmardi, M.; Navari, M.; Rasaee, M.J. Cancer immunotherapy by a recombinant phage vaccine displaying EGFR mimotope: An in vivo study. Immunopharmacol. Immunotoxicol. 2015, 37, 274–279. [Google Scholar] [CrossRef]
- Adhya, S.; Merril, C.R.; Biswas, B. Therapeutic and prophylactic applications of bacteriophage components in modern medicine. Cold Spring Harb. Perspect. Med. 2014, 4, a012518. [Google Scholar] [CrossRef]
- Liu, A.; Abbineni, G.; Mao, C. Nanocomposite films assembled from genetically engineered filamentous viruses and gold nanoparticles: Nanoarchitecture-and humidity-tunable surface plasmon resonance spectra. Adv. Mater. 2009, 21, 1001–1005. [Google Scholar] [CrossRef]
- Larocca, D.; Witte, A.; Johnson, W.; Pierce, G.F.; Baird, A. Targeting bacteriophage to mammalian cell surface receptors for gene delivery. Hum. Gene Ther. 1998, 9, 2393–2399. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Han, H.; Wang, Z.; Kuang, L.; Wang, L.; Yu, L.; Wu, M.; Zhou, Z.; Qian, M. Targeted Drug Delivery to Hepatocarcinoma In vivo by Phage-Displayed Specific Binding PeptideTargeted Drug Delivery to Hepatocarcinoma by Peptide. Mol. Cancer Res. 2010, 8, 135–144. [Google Scholar] [CrossRef]
- Hatfull, G.F.; Hendrix, R.W. Bacteriophages and their genomes. Curr. Opin. Virol. 2011, 1, 298–303. [Google Scholar] [CrossRef]
- Kostyuchenko, V.A.; Leiman, P.G.; Chipman, P.R.; Kanamaru, S.; Van Raaij, M.J.; Arisaka, F.; Mesyanzhinov, V.V.; Rossmann, M.G. Three-dimensional structure of bacteriophage T4 baseplate. Nat. Struct. Mol. Biol. 2003, 10, 688–693. [Google Scholar] [CrossRef]
- Straus, S.K.; Bo, H.E. Filamentous bacteriophage proteins and assembly. Virus Protein Nucl. Complexes 2018, 88, 261–279. [Google Scholar]
- Dams, D.; Brøndsted, L.; Drulis-Kawa, Z.; Briers, Y. Engineering of receptor-binding proteins in bacteriophages and phage tail-like bacteriocins. Biochem. Soc. Trans. 2019, 47, 449–460. [Google Scholar] [CrossRef]
- Maghsoodi, A.; Chatterjee, A.; Andricioaei, I.; Perkins, N.C. How the phage T4 injection machinery works including energetics, forces, and dynamic pathway. Proc. Natl. Acad. Sci. USA 2019, 116, 25097–25105. [Google Scholar] [CrossRef]
- Hay, I.D.; Lithgow, T. Filamentous phages: Masters of a microbial sharing economy. EMBO Rep. 2019, 20, e47427. [Google Scholar] [CrossRef]
- Mäntynen, S.; Laanto, E.; Oksanen, H.M.; Poranen, M.M.; Díaz-Muñoz, S.L. Black box of phage–bacterium interactions: Exploring alternative phage infection strategies. Open Biol. 2021, 11, 210188. [Google Scholar] [CrossRef]
- Olszak, T.; Latka, A.; Roszniowski, B.; Valvano, M.A.; Drulis-Kawa, Z. Phage life cycles behind bacterial biodiversity. Curr. Med. Chem. 2017, 24, 3987–4001. [Google Scholar] [CrossRef]
- Żaczek, M.; Weber-Dąbrowska, B.; Międzybrodzki, R.; Górski, A. Phage prevalence in the human urinary tract—Current knowledge and therapeutic implications. Microorganisms 2020, 8, 1802. [Google Scholar] [CrossRef]
- Manrique, P.; Dills, M.; Young, M.J. The human gut phage community and its implications for health and disease. Viruses 2017, 9, 141. [Google Scholar] [CrossRef] [PubMed]
- Huh, H.; Wong, S.; Jean, J.S.; Slavcev, R. Bacteriophage interactions with mammalian tissue: Therapeutic applications. Adv. Drug. Deliv. Rev. 2019, 145, 4–17. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, S.; Baker, K.; Padman, B.S.; Patwa, R.; Dunstan, R.A.; Weston, T.A.; Schlosser, K.; Bailey, B.; Lithgow, T.; Lazarou, M. Bacteriophage transcytosis provides a mechanism to cross epithelial cell layers. MBio 2017, 8, e01874-01817. [Google Scholar] [CrossRef] [PubMed]
- Karimi, M.; Mirshekari, H.; Basri, S.M.M.; Bahrami, S.; Moghoofei, M.; Hamblin, M.R. Bacteriophages and phage-inspired nanocarriers for targeted delivery of therapeutic cargos. Adv. Drug Deliv. Rev. 2016, 106, 45–62. [Google Scholar] [CrossRef]
- Parent, K.N.; Schrad, J.R.; Cingolani, G. Breaking symmetry in viral icosahedral capsids as seen through the lenses of X-ray crystallography and cryo-electron microscopy. Viruses 2018, 10, 67. [Google Scholar] [CrossRef]
- Louten, J. Virus Structure and Classification. Essent. Hum. Virol. 2016, 19–29. [Google Scholar] [CrossRef]
- Han, J.-H.; Wang, M.S.; Das, J.; Sudheendra, L.; Vonasek, E.; Nitin, N.; Kennedy, I.M. Capture and detection of T7 bacteriophages on a nanostructured interface. ACS Appl. Mater. Interfaces 2014, 6, 4758–4765. [Google Scholar] [CrossRef]
- Passaretti, P.; Sun, Y.; Dafforn, T.R.; Oppenheimer, P.G. Determination and characterisation of the surface charge properties of the bacteriophage M13 to assist bio-nanoengineering. RSC Adv. 2020, 10, 25385–25392. [Google Scholar] [CrossRef]
- Golec, P.; Dąbrowski, K.; Hejnowicz, M.S.; Gozdek, A.; Łoś, J.M.; Węgrzyn, G.; Łobocka, M.B.; Łoś, M. A reliable method for storage of tailed phages. J. Microbiol. Methods 2011, 84, 486–489. [Google Scholar] [CrossRef]
- Jepson, C.D.; March, J.B. Bacteriophage lambda is a highly stable DNA vaccine delivery vehicle. Vaccine 2004, 22, 2413–2419. [Google Scholar] [CrossRef] [PubMed]
- Brigati, J.R.; Petrenko, V.A. Thermostability of landscape phage probes. Anal. Bioanal. Chem. 2005, 382, 1346–1350. [Google Scholar] [CrossRef] [PubMed]
- Duyvejonck, H.; Merabishvili, M.; Vaneechoutte, M.; De Soir, S.; Wright, R.; Friman, V.-P.; Verbeken, G.; De Vos, D.; Pirnay, J.-P.; Van Mechelen, E. Evaluation of the stability of bacteriophages in different solutions suitable for the production of magistral preparations in Belgium. Viruses 2021, 13, 865. [Google Scholar] [CrossRef] [PubMed]
- Górski, A.; Międzybrodzki, R.; Borysowski, J.; Dąbrowska, K.; Wierzbicki, P.; Ohams, M.; Korczak-Kowalska, G.; Olszowska-Zaremba, N.; Łusiak-Szelachowska, M.; Kłak, M. Phage as a modulator of immune responses: Practical implications for phage therapy. Adv. Virus Res. 2012, 83, 41–71. [Google Scholar]
- Oakes, R.S.; Froimchuk, E.; Jewell, C.M. Engineering biomaterials to direct innate immunity. Adv. Ther. 2019, 2, 1800157. [Google Scholar] [CrossRef]
- Chang, T.Z.; Diambou, I.; Kim, J.R.; Wang, B.; Champion, J.A. Host-and pathogen-derived adjuvant coatings on protein nanoparticle vaccines. Bioeng. Transl. Med. 2017, 2, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Iwagami, Y.; Casulli, S.; Nagaoka, K.; Kim, M.; Carlson, R.I.; Ogawa, K.; Lebowitz, M.S.; Fuller, S.; Biswas, B.; Stewart, S. Lambda phage-based vaccine induces antitumor immunity in hepatocellular carcinoma. Heliyon 2017, 3, e00407. [Google Scholar] [CrossRef]
- Dong, X.; Pan, P.; Ye, J.-J.; Zhang, Q.-L.; Zhang, X.-Z. Hybrid M13 bacteriophage-based vaccine platform for personalized cancer immunotherapy. Biomaterials 2022, 289, 121763. [Google Scholar] [CrossRef]
- Samoylov, A.; Cochran, A.; Schemera, B.; Kutzler, M.; Donovan, C.; Petrenko, V.; Bartol, F.; Samoylova, T. Humoral immune responses against gonadotropin releasing hormone elicited by immunization with phage-peptide constructs obtained via phage display. J. Biotechnol. 2015, 216, 20–28. [Google Scholar] [CrossRef]
- Palma, M. Aspects of Phage-Based Vaccines for Protein and Epitope Immunization. Vaccines 2023, 11, 436. [Google Scholar] [CrossRef] [PubMed]
- González-Mora, A.; Hernández-Pérez, J.; Iqbal, H.M.; Rito-Palomares, M.; Benavides, J. Bacteriophage-based vaccines: A potent approach for antigen delivery. Vaccines 2020, 8, 504. [Google Scholar] [CrossRef]
- Tao, P.; Zhu, J.; Mahalingam, M.; Batra, H.; Rao, V.B. Bacteriophage T4 nanoparticles for vaccine delivery against infectious diseases. Adv. Drug Deliv. Rev. 2019, 145, 57–72. [Google Scholar] [CrossRef] [PubMed]
- Hamzeh-Mivehroud, M.; Mahmoudpour, A.; Rezazadeh, H.; Dastmalchi, S. Non-specific translocation of peptide-displaying bacteriophage particles across the gastrointestinal barrier. Eur. J. Pharm. Biopharm. 2008, 70, 577–581. [Google Scholar] [CrossRef] [PubMed]
- Duerr, D.M.; White, S.J.; Schluesener, H.J. Identification of peptide sequences that induce the transport of phage across the gastrointestinal mucosal barrier. J. Virol. Methods 2004, 116, 177–180. [Google Scholar] [CrossRef]
- Aghebati-Maleki, L.; Bakhshinejad, B.; Baradaran, B.; Motallebnezhad, M.; Aghebati-Maleki, A.; Nickho, H.; Yousefi, M.; Majidi, J. Phage display as a promising approach for vaccine development. J. Biomed. Sci. 2016, 23, 66. [Google Scholar] [CrossRef]
- Febvre, H.P.; Rao, S.; Gindin, M.; Goodwin, N.D.; Finer, E.; Vivanco, J.S.; Lu, S.; Manter, D.K.; Wallace, T.C.; Weir, T.L. PHAGE study: Effects of supplemental bacteriophage intake on inflammation and gut microbiota in healthy adults. Nutrients 2019, 11, 666. [Google Scholar] [CrossRef]
- Laforêt, F.; Antoine, C.; Lebrun, S.; Gonza, I.; Goya-Jorge, E.; Douny, C.; Duprez, J.-N.; Scippo, M.-L.; Taminiau, B.; Daube, G. Impact Assessment of vB_KpnP_K1-ULIP33 Bacteriophage on the Human Gut Microbiota Using a Dynamic In Vitro Model. Viruses 2023, 15, 719. [Google Scholar] [CrossRef]
- Delmastro, P.; Meola, A.; Monaci, P.; Cortese, R.; Galfre, G. Immunogenicity of filamentous phage displaying peptide mimotopes after oral administration. Vaccine 1997, 15, 1276–1285. [Google Scholar] [CrossRef]
- Ren, Z.; Tian, C.; Zhu, Q.; Zhao, M.; Xin, A.; Nie, W.; Ling, S.; Zhu, M.; Wu, J.; Lan, H. Orally delivered foot-and-mouth disease virus capsid protomer vaccine displayed on T4 bacteriophage surface: 100% protection from potency challenge in mice. Vaccine 2008, 26, 1471–1481. [Google Scholar] [CrossRef] [PubMed]
- Hess, K.L.; Jewell, C.M. Phage display as a tool for vaccine and immunotherapy development. Bioeng. Transl. Med. 2020, 5, e10142. [Google Scholar] [CrossRef] [PubMed]
- Gray, B.P.; Brown, K.C. Combinatorial peptide libraries: Mining for cell-binding peptides. Chem. Rev. 2014, 114, 1020–1081. [Google Scholar] [CrossRef] [PubMed]
- Rakonjac, J.; Bennett, N.J.; Spagnuolo, J.; Gagic, D.; Russel, M. Filamentous bacteriophage: Biology, phage display and nanotechnology applications. Curr. Issues Mol. Biol. 2011, 13, 51–76. [Google Scholar] [PubMed]
- Yi, H.; Ghosh, D.; Ham, M.-H.; Qi, J.; Barone, P.W.; Strano, M.S.; Belcher, A.M. M13 phage-functionalized single-walled carbon nanotubes as nanoprobes for second near-infrared window fluorescence imaging of targeted tumors. Nano Lett. 2012, 12, 1176–1183. [Google Scholar] [CrossRef]
- Ahmad, G.; Dickerson, M.B.; Church, B.C.; Cai, Y.; Jones, S.E.; Naik, R.R.; King, J.S.; Summers, C.J.; Kröger, N.; Sandhage, K.H. Rapid, room-temperature formation of crystalline calcium molybdate phosphor microparticles via peptide-induced precipitation. Adv. Mater. 2006, 18, 1759–1763. [Google Scholar] [CrossRef]
- Barry, M.A.; Dower, W.J.; Johnston, S.A. Toward cell–targeting gene therapy vectors: Selection of cell–binding peptides from random peptide–presenting phage libraries. Nat. Med. 1996, 2, 299–305. [Google Scholar] [CrossRef]
- Li, J.; Feng, L.; Fan, L.; Zha, Y.; Guo, L.; Zhang, Q.; Chen, J.; Pang, Z.; Wang, Y.; Jiang, X. Targeting the brain with PEG–PLGA nanoparticles modified with phage-displayed peptides. Biomaterials 2011, 32, 4943–4950. [Google Scholar] [CrossRef]
- Abbineni, G.; Modali, S.; Safiejko-Mroczka, B.; Petrenko, V.A.; Mao, C. Evolutionary selection of new breast cancer cell-targeting peptides and phages with the cell-targeting peptides fully displayed on the major coat and their effects on actin dynamics during cell internalization. Mol. Pharm. 2010, 7, 1629–1642. [Google Scholar] [CrossRef]
- Straus, S.; Scott, W.; Symmons, M.; Marvin, D. On the structures of filamentous bacteriophage Ff (fd, f1, M13). Eur. Biophys. J. 2008, 37, 521–527. [Google Scholar] [CrossRef]
- Yang, S.H.; Chung, W.J.; McFarland, S.; Lee, S.W. Assembly of bacteriophage into functional materials. Chem. Rec. 2013, 13, 43–59. [Google Scholar] [CrossRef] [PubMed]
- Rajotte, D.; Arap, W.; Hagedorn, M.; Koivunen, E.; Pasqualini, R.; Ruoslahti, E. Molecular heterogeneity of the vascular endothelium revealed by in vivo phage display. J. Clin. Investig. 1998, 102, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, N.; Oyama, H.; Nakano, M.; Kanda, T.; Banzono, E.; Kato, Y.; Karibe, T.; Nishio, T.; Goto, J. “Cleavable” hapten–biotin conjugates: Preparation and use for the generation of anti-steroid single-domain antibody fragments. Anal. Biochem. 2009, 387, 257–266. [Google Scholar] [CrossRef]
- Even-Desrumeaux, K.; Nevoltris, D.; Lavaut, M.N.; Alim, K.; Borg, J.-P.; Audebert, S.; Kerfelec, B.; Baty, D.; Chames, P. Masked selection: A straightforward and flexible approach for the selection of binders against specific epitopes and differentially expressed proteins by phage display. Mol. Cell. Proteom. 2014, 13, 653–665. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.-M.; Jiang, Y.; Wang, J.-R.; Gong, X.-L.; Guo, B.-Y. Mimic peptides bonding specifically with the first and second extracellular loops of the CC chemokine receptor 5 derived from a phage display peptide library are potent inhibitors of experimental autoimmune encephalomyelitis. Inflamm. Res. 2011, 60, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Blanchetot, C.; Verzijl, D.; Mujić-Delić, A.; Bosch, L.; Rem, L.; Leurs, R.; Verrips, C.T.; Saunders, M.; de Haard, H.; Smit, M.J. Neutralizing nanobodies targeting diverse chemokines effectively inhibit chemokine function. J. Biol. Chem. 2013, 288, 25173–25182. [Google Scholar] [CrossRef]
- Yanofsky, S.D.; Baldwin, D.N.; Butler, J.H.; Holden, F.R.; Jacobs, J.W.; Balasubramanian, P.; Chinn, J.P.; Cwirla, S.E.; Peters-Bhatt, E.; Whitehorn, E.A. High affinity type I interleukin 1 receptor antagonists discovered by screening recombinant peptide libraries. Proc. Natl. Acad. Sci. USA 1996, 93, 7381–7386. [Google Scholar] [CrossRef]
- Merzlyak, A.; Indrakanti, S.; Lee, S.-W. Genetically engineered nanofiber-like viruses for tissue regenerating materials. Nano Lett. 2009, 9, 846–852. [Google Scholar] [CrossRef]
- Merzlyak, A.; Lee, S.-W. Engineering phage materials with desired peptide display: Rational design sustained through natural selection. Bioconjugate Chem. 2009, 20, 2300–2310. [Google Scholar] [CrossRef]
- Lee, S.-W.; Mao, C.; Flynn, C.E.; Belcher, A.M. Ordering of quantum dots using genetically engineered viruses. Science 2002, 296, 892–895. [Google Scholar] [CrossRef]
- Lee, S.-W.; Wood, B.M.; Belcher, A.M. Chiral smectic C structures of virus-based films. Langmuir 2003, 19, 1592–1598. [Google Scholar] [CrossRef]
- Merzlyak, A.; Lee, S.-W. Phage as templates for hybrid materials and mediators for nanomaterial synthesis. Curr. Opin. Chem. Biol. 2006, 10, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Naik, R.R.; Stringer, S.J.; Agarwal, G.; Jones, S.E.; Stone, M.O. Biomimetic synthesis and patterning of silver nanoparticles. Nat. Mater. 2002, 1, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Bermudez, H.; Hathorne, A.P. Incorporating stimulus-responsive character into filamentous virus assemblies. Faraday Discuss. 2008, 139, 327–335. [Google Scholar] [CrossRef]
- Rohovie, M.J.; Nagasawa, M.; Swartz, J.R. Virus-like particles: Next-generation nanoparticles for targeted therapeutic delivery. Bioeng. Transl. Med. 2017, 2, 43–57. [Google Scholar] [CrossRef]
- Nagano, K.; Tsutsumi, Y. Development of novel drug delivery systems using phage display technology for clinical application of protein drugs. Proc. Jpn. Acad. Ser. B 2016, 92, 156–166. [Google Scholar] [CrossRef]
- Bakhshinejad, B.; Sadeghizadeh, M. Bacteriophages and development of nanomaterials for neural regeneration. Neural Regen. Res. 2014, 9, 1955. [Google Scholar]
- Sorokulova, I.; Olsen, E.; Vodyanoy, V. Bacteriophage biosensors for antibiotic-resistant bacteria. Expert. Rev. Med. Devices 2014, 11, 175–186. [Google Scholar] [CrossRef]
- Schmelcher, M.; Loessner, M.J. Application of bacteriophages for detection of foodborne pathogens. Bacteriophage 2014, 4, e28137. [Google Scholar] [CrossRef]
- Li, S.; Li, Y.; Chen, H.; Horikawa, S.; Shen, W.; Simonian, A.; Chin, B.A. Direct detection of Salmonella typhimurium on fresh produce using phage-based magnetoelastic biosensors. Biosens. Bioelectron. 2010, 26, 1313–1319. [Google Scholar] [CrossRef]
- Wang, H.Y.; Chang, Y.-C.; Hu, C.-W.; Kao, C.-Y.; Yu, Y.-A.; Lim, S.-K.; Mou, K.Y. Development of a Novel Cytokine Vehicle Using Filamentous Phage Display for Colorectal Cancer Treatment. ACS Synth. Biol. 2021, 10, 2087–2095. [Google Scholar] [CrossRef]
- Jin, H.-E.; Farr, R.; Lee, S.-W. Collagen mimetic peptide engineered M13 bacteriophage for collagen targeting and imaging in cancer. Biomaterials 2014, 35, 9236–9245. [Google Scholar] [CrossRef] [PubMed]
- Fokine, A.; Islam, M.Z.; Zhang, Z.; Bowman, V.D.; Rao, V.B.; Rossmann, M.G. Structure of the three N-terminal immunoglobulin domains of the highly immunogenic outer capsid protein from a T4-like bacteriophage. J. Virol. 2011, 85, 8141–8148. [Google Scholar] [CrossRef]
- Qin, L.; Fokine, A.; O’Donnell, E.; Rao, V.B.; Rossmann, M.G. Structure of the small outer capsid protein, Soc: A clamp for stabilizing capsids of T4-like phages. J. Mol. Biol. 2010, 395, 728–741. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.-J.; Black, L.W. Phage T4 SOC and HOC display of biologically active, full-length proteins on the viral capsid. Gene 1998, 215, 439–444. [Google Scholar] [CrossRef]
- Tao, P.; Mahalingam, M.; Kirtley, M.L.; van Lier, C.J.; Sha, J.; Yeager, L.A.; Chopra, A.K.; Rao, V.B. Mutated and bacteriophage T4 nanoparticle arrayed F1-V immunogens from Yersinia pestis as next generation plague vaccines. PLoS Pathog. 2013, 9, e1003495. [Google Scholar] [CrossRef]
- Li, Q.; Shivachandra, S.B.; Zhang, Z.; Rao, V.B. Assembly of the small outer capsid protein, Soc, on bacteriophage T4: A novel system for high density display of multiple large anthrax toxins and foreign proteins on phage capsid. J. Mol. Biol. 2007, 370, 1006–1019. [Google Scholar] [CrossRef] [PubMed]
- Gamkrelidze, M.; Dąbrowska, K. T4 bacteriophage as a phage display platform. Arch. Microbiol. 2014, 196, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowska, K.; Miernikiewicz, P.; Piotrowicz, A.; Hodyra, K.; Owczarek, B.; Lecion, D.; Kaźmierczak, Z.; Letarov, A.; Górski, A. Immunogenicity studies of proteins forming the T4 phage head surface. J. Virol. 2014, 88, 12551–12557. [Google Scholar] [CrossRef]
- Sanmukh, S.G.; Santos, N.J.; Barquilha, C.N.; Dos Santos, S.A.A.; Duran, B.O.S.; Delella, F.K.; Moroz, A.; Justulin, L.A.; Carvalho, H.F.; Felisbino, S.L. Exposure to Bacteriophages T4 and M13 Increases Integrin Gene Expression and Impairs Migration of Human PC-3 Prostate Cancer Cells. Antibiotics 2021, 10, 1202. [Google Scholar] [CrossRef]
- Danner, S.; Belasco, J.G. T7 phage display: A novel genetic selection system for cloning RNA-binding proteins from cDNA libraries. Proc. Natl. Acad. Sci. USA 2001, 98, 12954–12959. [Google Scholar] [CrossRef]
- Xu, H.; Bao, X.; Wang, Y.; Xu, Y.; Deng, B.; Lu, Y.; Hou, J. Engineering T7 bacteriophage as a potential DNA vaccine targeting delivery vector. Virol. J. 2018, 15, 49. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, A.; Akuta, T.; Okuyama, H.; Senda, T.; Yokoi, H.; Inokuchi, H.; Fujita, S.; Hayakawa, T.; Takeda, K.; Hasegawa, M. Protein transduction domain of HIV-1 Tat protein promotes efficient delivery of DNA into mammalian cells. J. Biol. Chem. 2001, 276, 26204–26210. [Google Scholar] [CrossRef]
- Kim, A.; Shin, T.-H.; Shin, S.-M.; Pham, C.D.; Choi, D.-K.; Kwon, M.-H.; Kim, Y.-S. Cellular internalization mechanism and intracellular trafficking of filamentous M13 phages displaying a cell-penetrating transbody and TAT peptide. PLoS ONE 2012, 7, e51813. [Google Scholar] [CrossRef]
- Dasa, S.S.K.; Jin, Q.; Chen, C.-T.; Chen, L. Target-specific copper hybrid T7 phage particles. Langmuir 2012, 28, 17372–17380. [Google Scholar] [CrossRef] [PubMed]
- Cicchini, C.; Ansuini, H.; Amicone, L.; Alonzi, T.; Nicosia, A.; Cortese, R.; Tripodi, M.; Luzzago, A. Searching for DNA–protein interactions by lambda phage display. J. Mol. Biol. 2002, 322, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Santi, E.; Capone, S.; Mennuni, C.; Lahm, A.; Tramontano, A.; Luzzago, A.; Nicosia, A. Bacteriophage lambda display of complex cDNA libraries: A new approach to functional genomics. J. Mol. Biol. 2000, 296, 497–508. [Google Scholar] [CrossRef]
- Santini, C.; Brennan, D.; Mennuni, C.; Hoess, R.H.; Nicosia, A.; Cortese, R.; Luzzago, A. Eficient display of an HCV cDNA expression library as C-terminal fusion to the capsid protein D of bacteriophage lambda. J. Mol. Biol. 1998, 282, 125–135. [Google Scholar] [CrossRef]
- Mikawa, Y.G.; Maruyama, I.N.; Brenner, S. Surface display of proteins on bacteriophage λ heads. J. Mol. Biol. 1996, 262, 21–30. [Google Scholar] [CrossRef]
- Sternberg, N.; HoEss, R.H. Display of peptides and proteins on the surface of bacteriophage lambda. Proc. Natl. Acad. Sci. USA 1995, 92, 1609–1613. [Google Scholar] [CrossRef]
- Kuwabara, I.; Maruyama, H.; Mikawa, Y.G.; Zuberi, R.I.; Liu, F.-T.; Maruyama, I.N. Efficient epitope mapping by bacteriophage λ surface display. Nat. Biotechnol. 1997, 15, 74–78. [Google Scholar] [CrossRef]
- Maruyama, I.N.; Maruyama, H.I.; Brenner, S. Lambda foo: A lambda phage vector for the expression of foreign proteins. Proc. Natl. Acad. Sci. USA 1994, 91, 8273–8277. [Google Scholar] [CrossRef] [PubMed]
- Lankes, H.; Zanghi, C.; Santos, K.; Capella, C.; Duke, C.; Dewhurst, S. In vivo gene delivery and expression by bacteriophage lambda vectors. J. Appl. Microbiol. 2007, 102, 1337–1349. [Google Scholar] [CrossRef] [PubMed]
- Razazan, A.; Nicastro, J.; Slavcev, R.; Barati, N.; Arab, A.; Mosaffa, F.; Jaafari, M.R.; Behravan, J. Lambda bacteriophage nanoparticles displaying GP2, a HER2/neu derived peptide, induce prophylactic and therapeutic activities against TUBO tumor model in mice. Sci. Rep. 2019, 9, 2221. [Google Scholar] [CrossRef]
- Catala, A.; Dzieciatkowska, M.; Wang, G.; Gutierrez-Hartmann, A.; Simberg, D.; Hansen, K.C.; D’Alessandro, A.; Catalano, C.E. Targeted intracellular delivery of trastuzumab using designer phage lambda nanoparticles alters cellular programs in human breast cancer cells. ACS Nano 2021, 15, 11789–11805. [Google Scholar] [CrossRef] [PubMed]
- Bookstaver, M.L.; Tsai, S.J.; Bromberg, J.S.; Jewell, C.M. Improving vaccine and immunotherapy design using biomaterials. Trends Immunol. 2018, 39, 135–150. [Google Scholar] [CrossRef] [PubMed]
- Sanmukh, S.G.; Dos Santos, N.J.; Barquilha, C.N.; De Carvalho, M.; Dos Reis, P.P.; Delella, F.K.; Carvalho, H.F.; Latek, D.; Fehér, T.; Felisbino, S.L. Bacterial RNA virus MS2 exposure increases the expression of cancer progression genes in the LNCaP prostate cancer cell line. Oncol. Lett. 2023, 25, 86. [Google Scholar] [CrossRef]
- Reed, C.A.; Langlais, C.; Wang, I.-N.; Young, R. A2 expression and assembly regulates lysis in Qβ infections. Microbiology 2013, 159, 507–514. [Google Scholar] [CrossRef]
- Nchinda, G.W.; Al-Atoom, N.; Coats, M.T.; Cameron, J.M.; Waffo, A.B. Uniqueness of RNA coliphage Qβ Display system in directed evolutionary biotechnology. Viruses 2021, 13, 568. [Google Scholar] [CrossRef]
- Rumnieks, J.; Tars, K. Crystal structure of the read-through domain from bacteriophage Qβ A1 protein. Protein Sci. 2011, 20, 1707–1712. [Google Scholar] [CrossRef]
- Yin, Z.; Comellas-Aragones, M.; Chowdhury, S.; Bentley, P.; Kaczanowska, K.; BenMohamed, L.; Gildersleeve, J.C.; Finn, M.; Huang, X. Boosting immunity to small tumor-associated carbohydrates with bacteriophage Qβ capsids. ACS Chem. Biol. 2013, 8, 1253–1262. [Google Scholar] [CrossRef]
- Ren, S.; Zuo, S.; Zhao, M.; Wang, X.; Wang, X.; Chen, Y.; Wu, Z.; Ren, Z. Inhibition of tumor angiogenesis in lung cancer by T4 phage surface displaying mVEGFR2 vaccine. Vaccine 2011, 29, 5802–5811. [Google Scholar] [CrossRef] [PubMed]
- Barati, N.; Razazan, A.; Nicastro, J.; Slavcev, R.; Arab, A.; Mosaffa, F.; Nikpoor, A.R.; Badiee, A.; Jaafari, M.R.; Behravan, J. Immunogenicity and antitumor activity of the superlytic λF7 phage nanoparticles displaying a HER2/neu-derived peptide AE37 in a tumor model of BALB/c mice. Cancer Lett. 2018, 424, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Tang, L.; Zhou, C.; Tan, L. Immunotherapy of EGFR-positive tumor based on recombinant EGFR phage vaccine. Chin.-Ger. J. Clin. Oncol. 2006, 5, 189–193. [Google Scholar] [CrossRef]
- Fang, J.; Wang, G.; Yang, Q.; Song, J.; Wang, Y.; Wang, L. The potential of phage display virions expressing malignant tumor specific antigen MAGE-A1 epitope in murine model. Vaccine 2005, 23, 4860–4866. [Google Scholar] [CrossRef]
- Yin, Z.; Wu, X.; Kaczanowska, K.; Sungsuwan, S.; Comellas Aragones, M.; Pett, C.; Yu, J.; Baniel, C.; Westerlind, U.; Finn, M. Antitumor humoral and T cell responses by mucin-1 conjugates of bacteriophage Qβ in wild-type mice. ACS Chem. Biol. 2018, 13, 1668–1676. [Google Scholar] [CrossRef]
- Ren, S.-x.; Ren, Z.-j.; Zhao, M.-y.; Wang, X.-b.; Zuo, S.-g.; Yu, F. Antitumor activity of endogenous mFlt4 displayed on a T4 phage nanoparticle surface. Acta Pharmacol. Sin. 2009, 30, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Binetruy-Tournaire, R.; Demangel, C.; Malavaud, B.; Vassy, R.; Rouyre, S.; Kraemer, M.; Plouet, J.; Derbin, C.; Perret, G.; Mazie, J.C. Identification of a peptide blocking vascular endothelial growth factor (VEGF)-mediated angiogenesis. EMBO J. 2000, 19, 1525–1533. [Google Scholar] [CrossRef]
- Wang, A.; Cui, M.; Qu, H.; Di, J.; Wang, Z.; Xing, J.; Wu, F.; Wu, W.; Wang, X.; Shen, L. Induction of anti-EGFR immune response with mimotopes identified from a phage display peptide library by panitumumab. Oncotarget 2016, 7, 75293. [Google Scholar] [CrossRef]
- Hetian, L.; Ping, A.; Shumei, S.; Xiaoying, L.; Luowen, H.; Jian, W.; Lin, M.; Meisheng, L.; Junshan, Y.; Chengchao, S. A novel peptide isolated from a phage display library inhibits tumor growth and metastasis by blocking the binding of vascular endothelial growth factor to its kinase domain receptor. J. Biol. Chem. 2002, 277, 43137–43142. [Google Scholar] [CrossRef]
- Arab, A.; Nicastro, J.; Slavcev, R.; Razazan, A.; Barati, N.; Nikpoor, A.R.; Brojeni, A.A.M.; Mosaffa, F.; Badiee, A.; Jaafari, M.R. Lambda phage nanoparticles displaying HER2-derived E75 peptide induce effective E75-CD8+ T response. Immunol. Res. 2018, 66, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Clifton, G.T.; Peoples, G.E.; Mittendorf, E.A. The development and use of the E75 (HER2 369–377) peptide vaccine. Future Oncol. 2016, 12, 1321–1329. [Google Scholar] [CrossRef]
- Sartorius, R.; Pisu, P.; D’Apice, L.; Pizzella, L.; Romano, C.; Cortese, G.; Giorgini, A.; Santoni, A.; Velotti, F.; De Berardinis, P. The use of filamentous bacteriophage fd to deliver MAGE-A10 or MAGE-A3 HLA-A2-restricted peptides and to induce strong antitumor CTL responses. J. Immunol. 2008, 180, 3719–3728. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Huang, H.; Wang, C.; Lin, S.; Huang, Y.; Wang, Y.; Liang, G.; Yan, Q.; Xiao, J.; Wu, J. Identification of a novel peptide that blocks basic fibroblast growth factor-mediated cell proliferation. Oncotarget 2013, 4, 1819. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Duan, Y.; Zhou, H.; Li, W.; Li, F.; Guo, L.; Roeske, R.W. Selection of peptide ligands binding to fibroblast growth factor receptor 1. IUBMB Life 2002, 54, 67–72. [Google Scholar] [CrossRef]
- Lipok, M.; Szlachcic, A.; Kindela, K.; Czyrek, A.; Otlewski, J. Identification of a peptide antagonist of the FGF 1–FGFR 1 signaling axis by phage display selection. FEBS Open Bio. 2019, 9, 914–924. [Google Scholar] [CrossRef]
- Wang, W.; Chen, X.; Li, T.; Li, Y.; Wang, R.; He, D.; Luo, W.; Li, X.; Wu, X. Screening a phage display library for a novel FGF8b-binding peptide with anti-tumor effect on prostate cancer. Exp. Cell. Res. 2013, 319, 1156–1164. [Google Scholar] [CrossRef]
- Asadi-Ghalehni, M.; Rasaee, M.J.; Asl, N.N.; Khosravani, M.; Rajabibazl, M.; Modjtahedi, H.; Sadroddiny, E. Construction of a recombinant phage-vaccine capable of reducing the growth rate of an established LL2 tumor model. Iran. J. Allergy Asthma Immunol. 2018, 17, 240–249. [Google Scholar]
- Eriksson, F.; Culp, W.D.; Massey, R.; Egevad, L.; Garland, D.; Persson, M.A.; Pisa, P. Tumor specific phage particles promote tumor regression in a mouse melanoma model. Cancer Immunol. Immunother. 2007, 56, 677–687. [Google Scholar] [CrossRef]
- Yayon, A.; Aviezer, D.; Safran, M.; Gross, J.L.; Heldman, Y.; Cabilly, S.; Givol, D.; Katchalski-Katzir, E. Isolation of peptides that inhibit binding of basic fibroblast growth factor to its receptor from a random phage-epitope library. Proc. Natl. Acad. Sci. USA 1993, 90, 10643–10647. [Google Scholar] [CrossRef]
- Wu, C.-H.; Liu, I.-J.; Lu, R.-M.; Wu, H.-C. Advancement and applications of peptide phage display technology in biomedical science. J. Biomed. Sci. 2016, 23, 8. [Google Scholar] [CrossRef] [PubMed]
- An, P.; Lei, H.; Zhang, J.; Song, S.; He, L.; Jin, G.; Liu, X.; Wu, J.; Meng, L.; Liu, M. Suppression of tumor growth and metastasis by a VEGFR-1 antagonizing peptide identified from a phage display library. Int. J. Cancer 2004, 111, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Pardoll, D. Cancer and the immune system: Basic concepts and targets for intervention. Semin. Oncol. 2015, 42, 523–538. [Google Scholar] [CrossRef] [PubMed]
- Zuo, S.; Dai, G.; Wang, L.; Wen, Y.; Huang, Z.; Yang, W.; Ma, W.; Ren, X. Suppression of angiogenesis and tumor growth by recombinant T4 phages displaying extracellular domain of vascular endothelial growth factor receptor 2. Arch. Virol. 2019, 164, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Hurwitz, H. Integrating the anti–VEGF-A humanized monoclonal antibody bevacizumab with chemotherapy in advanced colorectal cancer. Clin. Color. Cancer 2004, 4, S62–S68. [Google Scholar] [CrossRef]
- Zhang, J.; Li, H.; Wang, X.; Qi, H.; Miao, X.; Zhang, T.; Chen, G.; Wang, M. Phage-derived fully human antibody scFv fragment directed against human vascular endothelial growth factor receptor 2 blocked its interaction with VEGF. Biotechnol. Prog. 2012, 28, 981–989. [Google Scholar] [CrossRef]
- Lamdan, H.; Gavilondo, J.V.; Munoz, Y.; Pupo, A.; Huerta, V.; Musacchio, A.; Pérez, L.; Ayala, M.; Rojas, G.; Balint, R.F. Affinity maturation and fine functional mapping of an antibody fragment against a novel neutralizing epitope on human vascular endothelial growth factor. Mol. BioSyst. 2013, 9, 2097–2106. [Google Scholar] [CrossRef]
- Kordi, S.; Rahmati-Yamchi, M.; Vostakolaei, M.A.; Etemadie, A.; Barzegari, A.; Abdolalizadeh, J. Isolation of a novel anti-kdr3 single-chain variable fragment antibody from a phage display library. Iran. J. Allergy Asthma Immunol. 2019, 18, 289–299. [Google Scholar] [CrossRef]
- Giordano, R.J.; Cardó-Vila, M.; Salameh, A.; Anobom, C.D.; Zeitlin, B.D.; Hawke, D.H.; Valente, A.P.; Almeida, F.C.; Nör, J.E.; Sidman, R.L. From combinatorial peptide selection to drug prototype (I): Targeting the vascular endothelial growth factor receptor pathway. Proc. Natl. Acad. Sci. USA 2010, 107, 5112–5117. [Google Scholar] [CrossRef]
- Lu, L.; Chen, H.; Hao, D.; Zhang, X.; Wang, F. The functions and applications of A7R in anti-angiogenic therapy, imaging and drug delivery systems. Asian J. Pharm. Sci. 2019, 14, 595–608. [Google Scholar] [CrossRef]
- Giordano, R.J.; Cardó-Vila, M.; Lahdenranta, J.; Pasqualini, R.; Arap, W. Biopanning and Rapid Analysis of Selective Interactive Ligands; Nature Publishing Group US New York: New York, NY, USA, 2001. [Google Scholar]
- Roskoski, R., Jr. The ErbB/HER family of protein-tyrosine kinases and cancer. Pharmacol. Res. 2014, 79, 34–74. [Google Scholar] [CrossRef]
- Engelman, J.A.; Cantley, L.C. A sweet new role for EGFR in cancer. Cancer Cell 2008, 13, 375–376. [Google Scholar] [CrossRef]
- Rajaram, P.; Chandra, P.; Ticku, S.; Pallavi, B.; Rudresh, K.; Mansabdar, P. Epidermal growth factor receptor: Role in human cancer. Indian J. Dent. Res. 2017, 28, 687. [Google Scholar] [PubMed]
- Yavari, B.; Mahjub, R.; Saidijam, M.; Raigani, M.; Soleimani, M. The potential use of peptides in cancer treatment. Curr. Protein Pept. Sci. 2018, 19, 759–770. [Google Scholar] [CrossRef] [PubMed]
- Roovers, R.C.; Laeremans, T.; Huang, L.; De Taeye, S.; Verkleij, A.J.; Revets, H.; de Haard, H.J.; van Bergen en Henegouwen, P.M.P. Efficient inhibition of EGFR signalling and of tumour growth by antagonistic anti-EGFR Nanobodies. Cancer Immunol. Immunother. 2007, 56, 303–317. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Martínez, D.; Infante, Y.C.; Ramírez, B.S.; Rojas, G. Domain-level epitope mapping of polyclonal antibodies against HER-1 and HER-2 receptors using phage display technology. Sci. Rep. 2022, 12, 12268. [Google Scholar] [CrossRef]
- Lamtha, T.; Tabtimmai, L.; Bangphoomi, K.; Kiriwan, D.; Malik, A.A.; Chaicumpa, W.; van Bergen En Henegouwen, P.M.; Choowongkomon, K. Generation of a nanobody against HER2 tyrosine kinase using phage display library screening for HER2-positive breast cancer therapy development. Protein Eng. Des. Sel. 2021, 34, gzab030. [Google Scholar] [CrossRef]
- Wang, J.; Lamolinara, A.; Conti, L.; Giangrossi, M.; Cui, L.; Morelli, M.B.; Amantini, C.; Falconi, M.; Bartolacci, C.; Andreani, C. HER2-Displaying M13 Bacteriophages induce Therapeutic Immunity against Breast Cancer. Cancers 2022, 14, 4054. [Google Scholar] [CrossRef]
- Shadidi, M.; Sørensen, D.; Dybwad, A.; Furset, G.; Sioud, M. Mucosal vaccination with phage-displayed tumour antigens identified through proteomics-based strategy inhibits the growth and metastasis of 4T1 breast adenocarcinoma. Int. J. Oncol. 2008, 32, 241–247. [Google Scholar] [CrossRef]
- Wu, Y.; Wan, Y.; Bian, J.; Zhao, J.; Jia, Z.; Zhou, L.; Zhou, W.; Tan, Y. Phage display particles expressing tumor-specific antigens induce preventive and therapeutic anti-tumor immunity in murine p815 model. Int. J. Cancer 2002, 98, 748–753. [Google Scholar] [CrossRef]
- Rähni, A.; Jaago, M.; Sadam, H.; Pupina, N.; Pihlak, A.; Tuvikene, J.; Annuk, M.; Mägi, A.; Timmusk, T.; Ghaemmaghami, A.M. Melanoma-specific antigen-associated antitumor antibody reactivity as an immune-related biomarker for targeted immunotherapies. Commun. Med. 2022, 2, 48. [Google Scholar] [CrossRef] [PubMed]
- Odales, J.; Servín-Blanco, R.; Martínez-Cortés, F.; Valle, J.G.; Domínguez-Romero, A.N.; Gevorkian, G.; Manoutcharian, K. Antitumor efficacy of MUC1-derived variable epitope library treatments in a mouse model of breast cancer. Vaccine 2022, 40, 4796–4805. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.; Olsen, S.K.; Ibrahimi, O.A. Structural basis for fibroblast growth factor receptor activation. Cytokine Growth Factor. Rev. 2005, 16, 107–137. [Google Scholar] [CrossRef]
- Dodé, C.; Levilliers, J.; Dupont, J.-M.; De Paepe, A.; Le Dû, N.; Soussi-Yanicostas, N.; Coimbra, R.S.; Delmaghani, S.; Compain-Nouaille, S.; Baverel, F. Loss-of-function mutations in FGFR1 cause autosomal dominant Kallmann syndrome. Nat. Genet. 2003, 33, 463–465. [Google Scholar] [CrossRef]
- Kan, S.-h.; Elanko, N.; Johnson, D.; Cornejo-Roldan, L.; Cook, J.; Reich, E.W.; Tomkins, S.; Verloes, A.; Twigg, S.R.; Rannan-Eliya, S. Genomic screening of fibroblast growth-factor receptor 2 reveals a wide spectrum of mutations in patients with syndromic craniosynostosis. Am. J. Hum. Genet. 2002, 70, 472–486. [Google Scholar] [CrossRef] [PubMed]
- Webster, M.K.; Donoghue, D.J. FGFR activation in skeletal disorders: Too much of a good thing. Trends Genet. 1997, 13, 178–182. [Google Scholar] [CrossRef]
- Wang, J.; Stockton, D.W.; Ittmann, M. The fibroblast growth factor receptor-4 Arg388 allele is associated with prostate cancer initiation and progression. Clin. Cancer Res. 2004, 10, 6169–6178. [Google Scholar] [CrossRef]
- Massari, F.; Ciccarese, C.; Santoni, M.; Lopez-Beltran, A.; Scarpelli, M.; Montironi, R.; Cheng, L. Targeting fibroblast growth factor receptor (FGFR) pathway in renal cell carcinoma. Expert Rev. Anticancer Ther. 2015, 15, 1367–1369. [Google Scholar] [CrossRef]
- Shi, S.; Li, X.; You, B.; Shan, Y.; Cao, X.; You, Y. High expression of FGFR4 enhances tumor growth and metastasis in nasopharyngeal carcinoma. J. Cancer 2015, 6, 1245. [Google Scholar] [CrossRef]
- Rodriguez-Vida, A.; Saggese, M.; Hughes, S.; Rudman, S.; Chowdhury, S.; Smith, N.R.; Lawrence, P.; Rooney, C.; Dougherty, B.; Landers, D. Complexity of FGFR signalling in metastatic urothelial cancer. J. Hematol. Oncol. 2015, 8, 119. [Google Scholar] [CrossRef]
- Criscitiello, C.; Esposito, A.; De Placido, S.; Curigliano, G. Targeting fibroblast growth factor receptor pathway in breast cancer. Curr. Opin. Oncol. 2015, 27, 452–456. [Google Scholar] [CrossRef]
- Wu, X.; Yan, Q.; Huang, Y.; Huang, H.; Su, Z.; Xiao, J.; Zeng, Y.; Wang, Y.; Nie, C.; Yang, Y. Isolation of a novel basic FGF-binding peptide with potent antiangiogenetic activity. J. Cell. Mol. Med. 2010, 14, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Lin, S.; Li, X.; Wu, X. Mechanism of inhibitory effect of P7 on 3T3 cell proliferation induced by basic fibroblast growth factor. Acta Pharmacol. Sin. 2010, 45, 314–317. [Google Scholar]
- Li, Q.; Gao, S.; Yu, Y.; Wang, W.; Chen, X.; Wang, R.; Li, T.; Wang, C.; Li, X.; Wu, X. A novel bFGF antagonist peptide inhibits breast cancer cell growth. Mol. Med. Rep. 2012, 6, 210–214. [Google Scholar] [PubMed]
- Chen, Q.; Yang, Z.; Chen, X.; Shu, L.; Qu, W. Peptide P7 inhibits the bFGF-stimulated proliferation and invasion of SKOV3 cells. Exp. Ther. Med. 2019, 17, 3003–3008. [Google Scholar] [CrossRef] [PubMed]
- Asadi-Ghalehni, M.; Rasaee, M.J.; RajabiBazl, M.; Khosravani, M.; Motaghinejad, M.; Javanmardi, M.; Khalili, S.; Modjtahedi, H.; Sadroddiny, E. A novel recombinant anti-epidermal growth factor receptor peptide vaccine capable of active immunization and reduction of tumor volume in a mouse model. Microbiol. Immunol. 2017, 61, 531–538. [Google Scholar] [CrossRef]
- Clark, J.R.; Bartley, K.; Jepson, C.D.; Craik, V.; March, J.B. Comparison of a bacteriophage-delivered DNA vaccine and a commercially available recombinant protein vaccine against hepatitis B. FEMS Immunol. Med. Microbiol. 2011, 61, 197–204. [Google Scholar] [CrossRef]
- Khan, K.H. DNA vaccines: Roles against diseases. Germs 2013, 3, 26. [Google Scholar] [CrossRef]
- March, J.B.; Clark, J.R.; Jepson, C.D. Genetic immunisation against hepatitis B using whole bacteriophage λ particles. Vaccine 2004, 22, 1666–1671. [Google Scholar] [CrossRef]
- Coban, C.; Koyama, S.; Takeshita, F.; Akira, S.; Ishii, K.J. Molecular and cellular mechanisms of DNA vaccines. Hum. Vaccines 2008, 4, 453–457. [Google Scholar] [CrossRef]
- Li, L.; Saade, F.; Petrovsky, N. The future of human DNA vaccines. J. Biotechnol. 2012, 162, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Hobernik, D.; Bros, M. DNA vaccines—How far from clinical use? Int. J. Mol. Sci. 2018, 19, 3605. [Google Scholar] [CrossRef] [PubMed]
- Folegatti, P.M.; Ewer, K.J.; Aley, P.K.; Angus, B.; Becker, S.; Belij-Rammerstorfer, S.; Bellamy, D.; Bibi, S.; Bittaye, M.; Clutterbuck, E.A. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: A preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 2020, 396, 467–478. [Google Scholar] [CrossRef]
- Nicastro, J.; Sheldon, K.; Slavcev, R.A. Bacteriophage lambda display systems: Developments and applications. Appl. Microbiol. Biotechnol. 2014, 98, 2853–2866. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.R.; March, J.B. Bacteriophage-mediated nucleic acid immunisation. FEMS Immunol. Med. Microbiol. 2004, 40, 21–26. [Google Scholar] [CrossRef]
- Ghaemi, A.; Soleimanjahi, H.; Gill, P.; Hassan, Z.; Jahromi, S.R.M.; Roohvand, F. Recombinant λ-phage nanobioparticles for tumor therapy in mice models. Genet. Vaccines Ther. 2010, 8, 3. [Google Scholar] [CrossRef]
- Ghaemi, A.; Soleimanjahi, H.; Gill, P.; Hassan, Z.M.; Razeghi, S.; Fazeli, M.; Razavinikoo, S.M.H. Protection of mice by a λ-based therapeutic vaccine against cancer associated with human papillomavirus type 16. Intervirology 2011, 54, 105–112. [Google Scholar] [CrossRef]
- Thomas, B.S.; Nishikawa, S.; Ito, K.; Chopra, P.; Sharma, N.; Evans, D.H.; Tyrrell, D.L.J.; Bathe, O.F.; Rancourt, D.E. Peptide vaccination is superior to genetic vaccination using a recombineered bacteriophage λ subunit vaccine. Vaccine 2012, 30, 998–1008. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, H.; Bamdad, T.; Jamali, A.; Pouyanfard, S.; Mohammadi, M.G. Evaluation of humoral and cellular immune responses against HSV-1 using genetic immunization by filamentous phage particles: A comparative approach to conventional DNA vaccine. J. Virol. Methods 2010, 163, 440–444. [Google Scholar] [CrossRef]
- Ye, X.; Liu, Z.; Hemida, M.G.; Yang, D. Targeted delivery of mutant tolerant anti-coxsackievirus artificial microRNAs using folate conjugated bacteriophage Phi29 pRNA. PLoS ONE 2011, 6, e21215. [Google Scholar] [CrossRef]
- Bao, Q.; Li, X.; Han, G.; Zhu, Y.; Mao, C.; Yang, M. Phage-based vaccines. Adv. Drug. Deliv. Rev. 2019, 145, 40–56. [Google Scholar] [CrossRef]
- Kaufmann, K.B.; Büning, H.; Galy, A.; Schambach, A.; Grez, M. Gene therapy on the move. EMBO Mol. Med. 2013, 5, 1642–1661. [Google Scholar] [CrossRef] [PubMed]
- Ramamoorth, M.; Narvekar, A. Non viral vectors in gene therapy-an overview. J. Clin. Diagn. Res. JCDR 2015, 9, GE01. [Google Scholar] [CrossRef] [PubMed]
- Bouard, D.; Alazard-Dany, N.; Cosset, F.L. Viral vectors: From virology to transgene expression. Br. J. Pharmacol. 2009, 157, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Waehler, R.; Russell, S.J.; Curiel, D.T. Engineering targeted viral vectors for gene therapy. Nat. Rev. Genet. 2007, 8, 573–587. [Google Scholar] [CrossRef] [PubMed]
- Hosseinidoust, Z. Phage-mediated gene therapy. Curr. Gene Ther. 2017, 17, 120–126. [Google Scholar] [CrossRef]
- Hood, J.D.; Bednarski, M.; Frausto, R.; Guccione, S.; Reisfeld, R.A.; Xiang, R.; Cheresh, D.A. Tumor regression by targeted gene delivery to the neovasculature. Science 2002, 296, 2404–2407. [Google Scholar] [CrossRef]
- Hajitou, A.; Trepel, M.; Lilley, C.E.; Soghomonyan, S.; Alauddin, M.M.; Marini, F.C.; Restel, B.H.; Ozawa, M.G.; Moya, C.A.; Rangel, R. A hybrid vector for ligand-directed tumor targeting and molecular imaging. Cell 2006, 125, 385–398. [Google Scholar] [CrossRef]
- Hajitou, A.; Lev, D.C.; Hannay, J.A.; Korchin, B.; Staquicini, F.I.; Soghomonyan, S.; Alauddin, M.M.; Benjamin, R.S.; Pollock, R.E.; Gelovani, J.G. A preclinical model for predicting drug response in soft-tissue sarcoma with targeted AAVP molecular imaging. Proc. Natl. Acad. Sci. USA 2008, 105, 4471–4476. [Google Scholar] [CrossRef]
- Przystal, J.M.; Umukoro, E.; Stoneham, C.A.; Yata, T.; O’Neill, K.; Syed, N.; Hajitou, A. Proteasome inhibition in cancer is associated with enhanced tumor targeting by the adeno-associated virus/phage. Mol. Oncol. 2013, 7, 55–66. [Google Scholar] [CrossRef]
- Kia, A.; Przystal, J.M.; Nianiaris, N.; Mazarakis, N.D.; Mintz, P.J.; Hajitou, A. Dual Systemic Tumor Targeting with Ligand-Directed Phage and Grp78 Promoter Induces Tumor RegressionLigand Systemic Targeting of the Grp78 Promoter. Mol. Cancer Ther. 2012, 11, 2566–2577. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.L.; Yuan, Z.; Cardó-Vila, M.; Sanchez Claros, C.; Adem, A.; Cui, M.-H.; Branch, C.A.; Gelovani, J.G.; Libutti, S.K.; Sidman, R.L. AAVP displaying octreotide for ligand-directed therapeutic transgene delivery in neuroendocrine tumors of the pancreas. Proc. Natl. Acad. Sci. USA 2016, 113, 2466–2471. [Google Scholar] [CrossRef]
- Chongchai, A.; Waramit, S.; Suwan, K.; Al-Bahrani, M.; Udomruk, S.; Phitak, T.; Kongtawelert, P.; Pothacharoen, P.; Hajitou, A. Bacteriophage-mediated therapy of chondrosarcoma by selective delivery of the tumor necrosis factor alpha (TNFα) gene. FASEB J. 2021, 35, e21487. [Google Scholar] [CrossRef]
- Asavarut, P.; Waramit, S.; Suwan, K.; Marais, G.J.; Chongchai, A.; Benjathummarak, S.; Al-Bahrani, M.; Vila-Gomez, P.; Williams, M.; Kongtawelert, P. Systemically targeted cancer immunotherapy and gene delivery using transmorphic particles. EMBO Mol. Med. 2022, 14, e15418. [Google Scholar] [CrossRef] [PubMed]
- Yang Zhou, J.; Suwan, K.; Hajitou, A. Initial steps for the development of a phage-mediated gene replacement therapy using CRISPR-Cas9 technology. J. Clin. Med. 2020, 9, 1498. [Google Scholar] [CrossRef]
- Ghosh, D.; Peng, X.; Leal, J.; Mohanty, R.P. Peptides as drug delivery vehicles across biological barriers. J. Pharm. Investig. 2018, 48, 89–111. [Google Scholar] [CrossRef]
- Shadidi, M.; Sioud, M. Identification of novel carrier peptides for the specific delivery of therapeutics into cancer cells. FASEB J. 2003, 17, 256–258. [Google Scholar] [CrossRef] [PubMed]
- Fukuta, T.; Asai, T.; Kiyokawa, Y.; Nakada, T.; Bessyo-Hirashima, K.; Fukaya, N.; Hyodo, K.; Takase, K.; Kikuchi, H.; Oku, N. Targeted delivery of anticancer drugs to tumor vessels by use of liposomes modified with a peptide identified by phage biopanning with human endothelial progenitor cells. Int. J. Pharm. 2017, 524, 364–372. [Google Scholar] [CrossRef]
- Cai, X.-M.; Xie, H.-L.; Liu, M.-Z.; Zha, X.-L. Inhibition of cell growth and invasion by epidermal growth factor-targeted phagemid particles carrying siRNA against focal adhesion kinase in the presence of hydroxycamptothecin. BMC Biotechnol. 2008, 8, 74. [Google Scholar] [CrossRef]
- Cohen, B.A.; Bergkvist, M. Targeted in vitro photodynamic therapy via aptamer-labeled, porphyrin-loaded virus capsids. J. Photochem. Photobiol. B Biol. 2013, 121, 67–74. [Google Scholar] [CrossRef]
- Gandra, N.; Abbineni, G.; Qu, X.; Huai, Y.; Wang, L.; Mao, C. Bacteriophage bionanowire as a carrier for both cancer-targeting peptides and photosensitizers and its use in selective cancer cell killing by photodynamic therapy. Small 2013, 9, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Pan, P.; Zheng, D.-W.; Bao, P.; Zeng, X.; Zhang, X.-Z. Bioinorganic hybrid bacteriophage for modulation of intestinal microbiota to remodel tumor-immune microenvironment against colorectal cancer. Sci. Adv. 2020, 6, eaba1590. [Google Scholar] [CrossRef] [PubMed]
- Maji, M.; Mazumder, S.; Bhattacharya, S.; Choudhury, S.T.; Sabur, A.; Shadab, M.; Bhattacharya, P.; Ali, N. A lipid based antigen delivery system efficiently facilitates MHC class-I antigen presentation in dendritic cells to stimulate CD8+ T cells. Sci. Rep. 2016, 6, 27206. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Wu, Y.; Bian, J.; Wang, X.; Zhou, W.; Jia, Z.; Tan, Y.; Zhou, L. Induction of hepatitis B virus-specific cytotoxic T lymphocytes response in vivo by filamentous phage display vaccine. Vaccine 2001, 19, 2918–2923. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, F.; Tsagozis, P.; Lundberg, K.; Parsa, R.; Mangsbo, S.M.; Persson, M.A.; Harris, R.A.; Pisa, P. Tumor-specific bacteriophages induce tumor destruction through activation of tumor-associated macrophages. J. Immunol. 2009, 182, 3105–3111. [Google Scholar] [CrossRef]
- Matsuzaki, S.; Uchiyama, J.; Takemura-Uchiyama, I.; Daibata, M. Perspective: The age of the phage. Nature 2014, 509, S9. [Google Scholar] [CrossRef]
- Young, R.; Gill, J.J. Phage therapy redux—What is to be done? Science 2015, 350, 1163–1164. [Google Scholar] [CrossRef]
- Ledford, H. Cancer-fighting viruses near market. Nature 2015, 526, 622–623. [Google Scholar] [CrossRef]
- Roehnisch, T.; Then, C.; Nagel, W.; Blumenthal, C.; Braciak, T.; Donzeau, M.; Böhm, T.; Flaig, M.; Bourquin, C.; Oduncu, F.S. Phage idiotype vaccination: First phase I/II clinical trial in patients with multiple myeloma. J. Transl. Med. 2014, 12, 119. [Google Scholar] [CrossRef]
- Dąbrowska, K. Phage therapy: What factors shape phage pharmacokinetics and bioavailability? Systematic and critical review. Med. Res. Rev. 2019, 39, 2000–2025. [Google Scholar] [CrossRef]
- Veeranarayanan, S.; Azam, A.H.; Kiga, K.; Watanabe, S.; Cui, L. Bacteriophages as solid tumor theragnostic agents. Int. J. Mol. Sci. 2022, 23, 402. [Google Scholar] [CrossRef] [PubMed]
- Yao, V.J.; Ozawa, M.G.; Trepel, M.; Arap, W.; McDonald, D.M.; Pasqualini, R. Targeting pancreatic islets with phage display assisted by laser pressure catapult microdissection. Am. J. Pathol. 2005, 166, 625–636. [Google Scholar] [CrossRef] [PubMed]
- Morrison, D.; Ulevitch, R. The effects of bacterial endotoxins on host mediation systems. A review. Am. J. Pathol. 1978, 93, 526. [Google Scholar]
- Liu, D.; Van Belleghem, J.D.; de Vries, C.R.; Burgener, E.; Chen, Q.; Manasherob, R.; Aronson, J.R.; Amanatullah, D.F.; Tamma, P.D.; Suh, G.A. The safety and toxicity of phage therapy: A review of animal and clinical studies. Viruses 2021, 13, 1268. [Google Scholar] [CrossRef] [PubMed]
- Bordier, C. Phase separation of integral membrane proteins in Triton X-114 solution. J. Biol. Chem. 1981, 256, 1604–1607. [Google Scholar] [CrossRef]
- Szermer-Olearnik, B.; Boratyński, J. Removal of endotoxins from bacteriophage preparations by extraction with organic solvents. PLoS ONE 2015, 10, e0122672. [Google Scholar] [CrossRef]
| Phage Type | Structure | Family | Dimension | Size (kb) | Genome | Coat Protein | Copy Number | Life Cycle |
|---|---|---|---|---|---|---|---|---|
| M13 | Filamentous | Inoviridae | 900 × 7 nm | 6.4 | ssDNA | pIII, pVIII | 5, 2700 | Lysogenic |
| fd | Filamentous | Inoviridae | 900 × 7 nm | 6.4 | ssDNA | pIII, pVIII | 5, 2700 | Lysogenic |
| T4 | Icosahedral with tail | Myoviridae | 120 × 86 nm | 168 | dsDNA | HOC, SOC | 155, 870 | Lytic |
| T7 | Icosahedral with tail | Podoviridae | 56 × 29 nm | 40 | dsDNA | gp10B | 415 | Lytic |
| λ | Icosahedral with tail | Siphoviridae | 60 × 150 nm | 48.5 | dsDNA | gpD, gpE, gpV | 405, 405, 192 | Lysogenic/Lytic |
| MS2 | Icosahedral | Leviviridae | 26 nm | 3.57 | ssRNA | Coat protein | 178 | Lytic |
| Qβ | Icosahedral | Leviviridae | 28 nm | 4.2 | ssRNA | Coat protein | 180 | Lytic |
| Peptide Sequence | Target | Biological Applications | Phage/Phage Library | References |
|---|---|---|---|---|
| DKSEKFARDA | GM-CSF | Colorectal cancer | M13 | [113] |
| CDCRGDCFC | TAA | Breast cancer | T7 | [127] |
| EADPTGHSY | MAGE A1 | Antitumor | Fd | [147] |
| ATWLPPR | VEGF | Anti-angiogenesis | M13 | [150] |
| DTDWVRMRDSAR, VPGWSQAFMALA | EGFR | Lung cancer | Ph.D-12 | [151] |
| HTMYYHHYQHHL | VEGF | Breast carcinoma | Ph.D-12 | [152] |
| KIFGSLAFL | HER2 | Tubo tumor | Lambda | [153,154] |
| GLYDGMEHL, FLWGPRALV | MAGE-A10, MAGE A3 | Antitumor | Fd | [155] |
| LSPPRYP | FGFR | Melanoma | Ph.D-7 | [156] |
| VYMSPF | FGFR1 | Anti-angiogenesis | 6-mer phage | [157] |
| ACSLNHTVNC, ACSAKTTSAC | FGFR1 | Anti-angiogenesis | Ph.D-C7C | [158] |
| HSQAAVP | FGF8b | Prostate cancer | Ph.D-7 | [159] |
| QHYNIVNTQSRV | EGFR | Lung carcinoma | M13 | [160] |
| TRTKLPRLHLQS | TAA | Antitumor | M13 | [161] |
| KRTGQYKL | FGFR | Anti-angiogenesis | M13 | [162] |
| Patent Number | Inventors | Title | Published Year |
|---|---|---|---|
| WO2006008312A1 | Udo Blasi, Christine Hohenadl | Bacteriophage and prophage proteins in cancer gene therapy | 2006 |
| US20060058228A1 | Kimberly Kelly, David Jones | Colon tumor specific binding peptides | 2006 |
| US8137693B2 | Valery A. Petrenko | Drug delivery nanocarriers targeted by landscape phage | 2012 |
| US8088887B2 | Han-Chung, WuChin-Tarng Lin, Tong-Young Lee, Szu-Yao Kuo | Peptide-conjugates that bind to VEGF-stimulated or tumor vasculature and methods of treatment | 2012 |
| US8415131B2 | Qian Wang, Kai Li, Charlene Mello | M13 bacteriophage as a chemo-addressable nanoparticle for biological and medical applications | 2013 |
| US9387257B2 | Han-Chung Wu, Yi-Hsuan CHI | Lung cancer specific peptides for targeted drug delivery and molecular imaging | 2016 |
| CN105267973A | Wu Hanzhong, Wu Jianxun | Cancer targeting peptides for enhancing anti-cancer drug delivery and therapeutic efficiencies | 2016 |
| US9226972B2 | Valery A. Petrenko, Deepa Bedi, Olusegun A. Fagbohun, James W. Gillespie | Targeted particles comprising landscape phage fusion proteins and heterologous nucleic acid | 2016 |
| US9744223B2 | Biswajit Biswas, Carl R. Merril, Hossein A. Ghanbari | Therapeutic cancer vaccine targeted to aspartyl-(asparaginyl)-beta-hydroxylase (HAAH) | 2017 |
| Advantages | Disadvantages |
|---|---|
| Phage-based nanomedicine can be used for precision medicine | Phages may cause allergic reactions in some individuals |
| Manipulating phages to target specific areas can solve the problem of imprecise treatment | Limitation in displaying large antigens on phage particles |
| Phage therapy can potentially reduce the development of antibiotic resistance | Challenging to correctly display a molecule on the phage surface |
| Strong humoral and cellular immune response without the need for adjuvants | Genome length must be within virion packaging limits for phage DNA vaccines |
| Can be applied to oral vaccination due to physical stability in the gastrointestinal tract | Efficacy of phages in entering the body depends on the administration method |
| Phage therapy has the potential to be less expensive than traditional antibiotics | Phage therapy may not be suitable for all patients, such as those with compromised immune systems |
| Combination therapy with phages is possible | Might be challenging to reach certain organs and tumors to provide effective treatment |
| Remarkably stable in many challenging environmental conditions | Requires specialized storage and handling |
| Easy to genetically modify and mass-produce using basic bacteriological media | Endotoxin contamination during phage production |
| Significant potential for use as vaccine carriers for various illnesses including cancer and infectious diseases | More clinical trials are needed to obtain regulatory approval for phage therapy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ragothaman, M.; Yoo, S.Y. Engineered Phage-Based Cancer Vaccines: Current Advances and Future Directions. Vaccines 2023, 11, 919. https://doi.org/10.3390/vaccines11050919
Ragothaman M, Yoo SY. Engineered Phage-Based Cancer Vaccines: Current Advances and Future Directions. Vaccines. 2023; 11(5):919. https://doi.org/10.3390/vaccines11050919
Chicago/Turabian StyleRagothaman, Murali, and So Young Yoo. 2023. "Engineered Phage-Based Cancer Vaccines: Current Advances and Future Directions" Vaccines 11, no. 5: 919. https://doi.org/10.3390/vaccines11050919
APA StyleRagothaman, M., & Yoo, S. Y. (2023). Engineered Phage-Based Cancer Vaccines: Current Advances and Future Directions. Vaccines, 11(5), 919. https://doi.org/10.3390/vaccines11050919







