A Methodological Framework for Assessing the Benefit of SARS-CoV-2 Vaccination following Previous Infection: Case Study of Five- to Eleven-Year-Olds
Abstract
:1. Introduction
2. Methods
2.1. Quantifying the Additional Benefit of Vaccination for an Individual Previously Infected Child
2.2. Incorporating Waning
2.3. Notation
2.4. Estimating Additional Benefit for the Six Months Post-Vaccination following Previous Infection with Linear-Approximated Waning
2.5. Parameterising the Framework and Depicting Plausible Benefit
3. Results
3.1. Hospital Admissions Averted
3.2. Cases of Long Covid Averted
4. Discussion
4.1. Benefit
4.2. Strengths and Limitations
4.3. Policy Implications
4.4. Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Walter, E.B.; Talaat, K.R.; Sabharwal, C.; Gurtman, A.; Lockhart, S.; Paulsen, G.C.; Barnett, E.D.; Muñoz, F.M.; Maldonado, Y.; Pahud, B.A.; et al. Evaluation of the BNT162b2 COVID-19 Vaccine in Children 5 to 11 Years of Age. N. Engl. J. Med. 2022, 386, 35–46. [Google Scholar] [CrossRef]
- Sacco, C.; Del Manso, M.; Mateo-Urdiales, A.; Rota, M.C.; Petrone, D.; Riccardo, F.; Bella, A.; Siddu, A.; Battilomo, S.; Proietti, V.; et al. Effectiveness of BNT162b2 Vaccine against SARS-CoV-2 Infection and Severe COVID-19 in Children Aged 5–11 Years in Italy: A Retrospective Analysis of January–April, 2022. Lancet 2022, 400, 97–103. [Google Scholar] [CrossRef]
- Powell, A.A.; Kirsebom, F.; Stowe, J.; Ramsay, M.E.; Lopez-Bernal, J.; Andrews, N.; Ladhani, S.N. Protection against Symptomatic Infection with Delta (B.1.617.2) and Omicron (B.1.1.529) BA.1 and BA.2 SARS-CoV-2 Variants after Previous Infection and Vaccination in Adolescents in England, August, 2021–March, 2022: A National, Observational, Test-Negative, Case-Control Study. Lancet Infect. Dis. 2022, 23, 435–444. [Google Scholar] [CrossRef]
- ACIP Evidence to Recommendations for Use of Pfizer-BioNTech COVID-19 Vaccine under an Emergency Use Authorization|CDC. Available online: https://www.cdc.gov/vaccines/acip/recs/grade/covid-19-pfizer-age-5-11-eua-etr.html (accessed on 26 July 2022).
- Molteni, E.; Canas, L.S.; Kläser, K.; Deng, J.; Bhopal, S.S.; Hughes, R.C.; Chen, L.; Murray, B.; Kerfoot, E.; Antonelli, M.; et al. Post-vaccination infection rates and modification of COVID-19 symptoms in vaccinated UK school-aged children and adolescents: A prospective longitudinal cohort study. Lancet Reg. Health Eur. 2022. [Google Scholar] [CrossRef] [PubMed]
- Zambrano, L.D. Effectiveness of BNT162b2 (Pfizer-BioNTech) MRNA Vaccination Against Multisystem Inflammatory Syndrome in Children among Persons Aged 12–18 Years—United States, July–December 2021. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 52. [Google Scholar] [CrossRef]
- Lin, D.-Y.; Gu, Y.; Xu, Y.; Zeng, D.; Wheeler, B.; Young, H.; Sunny, S.K.; Moore, Z. Effects of Vaccination and Previous Infection on Omicron Infections in Children. N. Engl. J. Med. 2022, 387, 1141–1143. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-L.; Chua, G.T.; Lu, L.; Chan, B.P.-C.; Wong, J.S.-C.; Chow, C.C.-K.; Yu, T.-C.; Leung, A.S.-Y.; Lam, S.-Y.; Wong, T.-W.; et al. Omicron Variant Susceptibility to Neutralizing Antibodies Induced in Children by Natural SARS-CoV-2 Infection or COVID-19 Vaccine. Emerg. Microbes Infect. 2022, 11, 543–547. [Google Scholar] [CrossRef]
- Bobrovitz, N.; Ware, H.; Ma, X.; Li, Z.; Hosseini, R.; Cao, C.; Selemon, A.; Whelan, M.; Premji, Z.; Issa, H.; et al. Protective Effectiveness of Previous SARS-CoV-2 Infection and Hybrid Immunity against the Omicron Variant and Severe Disease: A Systematic Review and Meta-Regression. Lancet Infect. Dis. 2023, 23, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Andeweg, S.P.; de Gier, B.; Eggink, D.; Ali, L.; Vlaemynck, B.; Schepers, R.; Covid, R.; Hahné, S.J.M. Protection of COVID-19 Vaccination and Previous Infection against Omicron BA.1, BA.2 and Delta SARS-CoV-2 Infections. Nat. Commun. 2022, 13, 4738. [Google Scholar] [CrossRef]
- Price, A.M.; Olson, S.M.; Newhams, M.M.; Halasa, N.B.; Boom, J.A.; Sahni, L.C.; Pannaraj, P.S.; Irby, K.; Bline, K.E.; Maddux, A.B.; et al. BNT162b2 Protection against the Omicron Variant in Children and Adolescents. N. Engl. J. Med. 2022, 386, 1899–1909. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.H.X.; Cook, A.R.; Heng, D.; Ong, B.; Lye, D.C.; Tan, K.B. Effectiveness of BNT162b2 Vaccine against Omicron in Children 5 to 11 Years of Age. N. Engl. J. Med. 2022, 387, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Fleming-Dutra, K.E.; Britton, A.; Shang, N.; Derado, G.; Link-Gelles, R.; Accorsi, E.K.; Smith, Z.R.; Miller, J.; Verani, J.R.; Schrag, S.J. Association of Prior BNT162b2 COVID-19 Vaccination with Symptomatic SARS-CoV-2 Infection in Children and Adolescents During Omicron Predominance. JAMA 2022, 327, 2210–2219. [Google Scholar] [CrossRef]
- Dorabawila, V.; Hoefer, D.; Bauer, U.E.; Bassett, M.T.; Lutterloh, E.; Rosenberg, E.S. Risk of Infection and Hospitalization among Vaccinated and Unvaccinated Children and Adolescents in New York after the Emergence of the Omicron Variant. JAMA 2022, 327, 2242–2244. [Google Scholar] [CrossRef]
- Buonsenso, D.; Cusenza, F.; Passadore, L.; Bonanno, F.; De Guido, C.; Esposito, S. Duration of Immunity to SARS-CoV-2 in Children after Natural Infection or Vaccination in the Omicron and Pre-Omicron Era: A Systematic Review of Clinical and Immunological Studies. Front. Immunol. 2023, 13, 7883. [Google Scholar] [CrossRef]
- Stich, M.; Benning, L.; Speer, C.; Garbade, S.F.; Bartenschlager, M.; Kim, H.; Jeltsch, K.; Tabatabai, J.; Niesert, M.; Janda, A.; et al. Live-Virus Neutralization of the Omicron Variant in Children and Adults 14 Months after SARS-CoV-2 Wild-Type Infection. J. Med. Virol. 2023, 95, e28582. [Google Scholar] [CrossRef]
- Coronavirus (COVID-19) Infection Survey, UK Statistical Bulletins—Office for National Statistics. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/coronaviruscovid19infectionsurveypilot/previousReleases (accessed on 18 March 2021).
- COVID-19 Schools Infection Survey, England: Pupil Antibody Data, January to February 2022—Office for National Statistics. Available online: https://www.ons.gov.uk/releases/covid19schoolsinfectionsurveyenglandpupilantibodydatajanuarytofebruary2022 (accessed on 1 August 2022).
- Toh, Z.Q.; Anderson, J.; Mazarakis, N.; Neeland, M.; Higgins, R.A.; Rautenbacher, K.; Dohle, K.; Nguyen, J.; Overmars, I.; Donato, C.; et al. Comparison of Seroconversion in Children and Adults with Mild COVID-19. JAMA Netw. Open 2022, 5, e221313. [Google Scholar] [CrossRef]
- Real-Time Epidemiological Estimates from ONS Community Infection Survey Data. Available online: https://epiforecasts.io/inc2prev/report (accessed on 5 October 2022).
- Coronavirus (COVID-19) Infection Survey Technical Article: Cumulative Incidence of the Percentage of People Who Have Been Infected with COVID-19 by Variant and Age, England—Office for National Statistics. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/articles/coronaviruscovid19infectionsurveytechnicalarticlecumulativeincidenceofthenumberofpeoplewhohavebeeninfectedwithcovid19byvariantandageengland/9february2023#data-sources-and-quality (accessed on 11 February 2023).
- Commissioner, O. of the FDA Authorizes Pfizer-BioNTech COVID-19 Vaccine for Emergency Use in Children 5 through 11 Years of Age. Available online: https://www.fda.gov/news-events/press-announcements/fda-authorizes-pfizer-biontech-covid-19-vaccine-emergency-use-children-5-through-11-years-age (accessed on 22 December 2021).
- Reuters Israel Says Children Aged 5-11 Can Receive COVID-19 Vaccine. Reuters 2021.
- EMA. Comirnaty COVID-19 Vaccine: EMA Recommends Approval for Children Aged 5 to 11. Available online: https://www.ema.europa.eu/en/news/comirnaty-covid-19-vaccine-ema-recommends-approval-children-aged-5-11 (accessed on 11 October 2022).
- UK Regulator Approves Use of Pfizer/BioNTech Vaccine in 5 to 11-Year Olds. Available online: https://www.gov.uk/government/news/uk-regulator-approves-use-of-pfizerbiontech-vaccine-in-5-to-11-year-olds (accessed on 11 October 2022).
- COVID-19: The Green Book, Chapter 14a. Available online: https://www.gov.uk/government/publications/covid-19-the-green-book-chapter-14a (accessed on 27 February 2023).
- Vaccinations in England|Coronavirus in the UK. Available online: https://coronavirus.data.gov.uk/details/vaccinations?areaType=nation&areaName=England (accessed on 3 April 2023).
- Cohen-Stavi, C.J.; Magen, O.; Barda, N.; Yaron, S.; Peretz, A.; Netzer, D.; Giaquinto, C.; Judd, A.; Leibovici, L.; Hernán, M.A.; et al. BNT162b2 Vaccine Effectiveness against Omicron in Children 5 to 11 Years of Age. N. Engl. J. Med. 2022, 387, 227–236. [Google Scholar] [CrossRef]
- Davis, N.; Correspondent, N.D.S. Rising UK Covid Levels: What’s Driving It and What Will Happen next? Guardian 2022. [Google Scholar]
- CovSPECTRUM. Available online: https://cov-spectrum.org (accessed on 3 April 2023).
- Tuekprakhon, A.; Nutalai, R.; Dijokaite-Guraliuc, A.; Zhou, D.; Ginn, H.M.; Selvaraj, M.; Liu, C.; Mentzer, A.J.; Supasa, P.; Duyvesteyn, H.M.E.; et al. Antibody Escape of SARS-CoV-2 Omicron BA.4 and BA.5 from Vaccine and BA.1 Serum. Cell 2022, 185, 2422–2433.e13. [Google Scholar] [CrossRef] [PubMed]
- Wise, J. COVID-19: Sharp Rise in Infections Seen across the UK. BMJ 2022, 378, o1638. [Google Scholar] [CrossRef]
- Bowe, B.; Xie, Y.; Al-Aly, Z. Acute and Postacute Sequelae Associated with SARS-CoV-2 Reinfection. Nat. Med. 2022, 28, 2398–2405. [Google Scholar] [CrossRef] [PubMed]
- New-Onset, Self-Reported Long COVID after Coronavirus (COVID-19) Reinfection—Office for National Statistics. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/datasets/newonsetselfreportedlongcovidaftercoronaviruscovid19reinfection (accessed on 27 February 2023).
- Reynolds, C.J.; Pade, C.; Gibbons, J.M.; Otter, A.D.; Lin, K.-M.; Sandoval, D.M.; Pieper, F.P.; Butler, D.K.; Liu, S.; Joy, G.; et al. Immune Boosting by B.1.1.529 (Omicron) Depends on Previous SARS-CoV-2 Exposure. Science 2022, 377, eabq1841. [Google Scholar] [CrossRef] [PubMed]
- Borchering, R.K.; Mullany, L.C.; Howerton, E.; Chinazzi, M.; Smith, C.P.; Qin, M.; Reich, N.G.; Contamin, L.; Levander, J.; Kerr, J.; et al. Impact of SARS-CoV-2 Vaccination of Children Ages 5–11 Years on COVID-19 Disease Burden and Resilience to New Variants in the United States, November 2021–March 2022: A Multi-Model Study. Lancet Reg. Health Am. 2023, 17, 100398. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, M.T.; Good, M.F. Vaccinating Children against COVID-19: Commentary and Mathematical Modeling. mBio 2022, 13, e03789-21. [Google Scholar] [CrossRef] [PubMed]
- Keeling, M.J.; Moore, S.E. An Assessment of the Vaccination of School-Aged Children in England against SARS-CoV-2. BMC Med. 2022, 20, 196. [Google Scholar] [CrossRef]
- Moore, S.; Hill, E.M.; Tildesley, M.J.; Dyson, L.; Keeling, M.J. Vaccination and Non-Pharmaceutical Interventions for COVID-19: A Mathematical Modelling Study. Lancet Infect. Dis. 2021, 21, 793–802. [Google Scholar] [CrossRef]
- Keeling, M.J.; Tildesley, M.J.; Atkins, B.D.; Penman, B.; Southall, E.; Guyver-Fletcher, G.; Holmes, A.; McKimm, H.; Gorsich, E.E.; Hill, E.M.; et al. The Impact of School Reopening on the Spread of COVID-19 in England. Philos. Trans. R. Soc. B Biol. Sci. 2021, 376, 20200261. [Google Scholar] [CrossRef]
- JCVI. Statement on Vaccination of Children Aged 5 to 11 Years Old. Available online: https://www.gov.uk/government/publications/jcvi-update-on-advice-for-covid-19-vaccination-of-children-aged-5-to-11/jcvi-statement-on-vaccination-of-children-aged-5-to-11-years-old (accessed on 26 July 2022).
- Molloy, E.J.; Nakra, N.; Gale, C.; Dimitriades, V.R.; Lakshminrusimha, S. Multisystem Inflammatory Syndrome in Children (MIS-C) and Neonates (MIS-N) Associated with COVID-19: Optimizing Definition and Management. Pediatr. Res. 2022, 93, 1499–1508. [Google Scholar] [CrossRef]
- Levy, M.; Recher, M.; Hubert, H.; Javouhey, E.; Fléchelles, O.; Leteurtre, S.; Angoulvant, F. Multisystem Inflammatory Syndrome in Children by COVID-19 Vaccination Status of Adolescents in France. JAMA 2021, 327, 281–283. [Google Scholar] [CrossRef]
- Wilde, H.; Tomlinson, C.; Mateen, B.; Selby, D.; Kanthimathinathan, H.K.; Ramnarayan, P.; du Pré, P.; Johnson, M.; Pathan, N.; Gonzalez-Izquierdo, A.; et al. Hospital Admissions Linked to SARS-CoV-2 Infection in Children: A Cohort Study of 3.2 Million First Ascertained Infections in England. BMJ Br. Med. J. 2023. [Google Scholar]
- Estimates of the Population for the UK, England, Wales, Scotland and Northern Ireland—Office for National Statistics. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates/datasets/populationestimatesforukenglandandwalesscotlandandnorthernireland (accessed on 9 February 2023).
- Watanabe, A.; Kani, R.; Iwagami, M.; Takagi, H.; Yasuhara, J.; Kuno, T. Assessment of Efficacy and Safety of MRNA COVID-19 Vaccines in Children Aged 5 to 11 Years: A Systematic Review and Meta-Analysis. JAMA Pediatr. 2023, 177, 384–394. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Lee, G.M.; Razzaghi, H.; Lorman, V.; Mejias, A.; Pajor, N.M.; Thacker, D.; Webb, R.; Dickinson, K.; Bailey, L.C.; et al. Clinical Features and Burden of Postacute Sequelae of SARS-CoV-2 Infection in Children and Adolescents. JAMA Pediatr. 2022, 176, 1000–1009. [Google Scholar] [CrossRef] [PubMed]
- Kikkenborg Berg, S.; Palm, P.; Nygaard, U.; Bundgaard, H.; Petersen, M.N.S.; Rosenkilde, S.; Thorsted, A.B.; Ersbøll, A.K.; Thygesen, L.C.; Nielsen, S.D.; et al. Long COVID Symptoms in SARS-CoV-2-Positive Children Aged 0–14 Years and Matched Controls in Denmark (LongCOVIDKidsDK): A National, Cross-Sectional Study. Lancet Child Adolesc. Health 2022, 6, 614–623. [Google Scholar] [CrossRef] [PubMed]
- Post-COVID-19 Conditions in Children and Adolescents. Available online: http://www.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/clinical-guidance/post-covid-19-conditions-in-children-and-adolescents/ (accessed on 3 October 2022).
- Funk, A.L.; Kuppermann, N.; Florin, T.A.; Tancredi, D.J.; Xie, J.; Kim, K.; Finkelstein, Y.; Neuman, M.I.; Salvadori, M.I.; Yock-Corrales, A.; et al. Post–COVID-19 Conditions among Children 90 Days after SARS-CoV-2 Infection. JAMA Netw. Open 2022, 5, e2223253. [Google Scholar] [CrossRef]
- Dowell, A.C.; Lancaster, T.; Bruton, R.; Ireland, G.; Bentley, C.; Sylla, P.; Zuo, J.; Scott, S.; Jadir, A.; Begum, J.; et al. Primary Omicron Infection Elicits Weak Antibody Response but Robust Cellular Immunity in Children. In Immunology; Wiley: Hoboken, NJ, USA, 2022. [Google Scholar]
- COVID-19 Vaccination Protects against Long COVID. Available online: https://www.gavi.org/vaccineswork/covid-19-vaccination-protects-against-long-covid (accessed on 24 October 2022).
- Byambasuren, O.; Stehlik, P.; Clark, J.; Alcorn, K.; Glasziou, P. Effect of COVID-19 Vaccination on Long Covid: Systematic Review. BMJ Med. 2023, 2, e000385. [Google Scholar] [CrossRef] [PubMed]
- Mizrahi, B.; Sudry, T.; Flaks-Manov, N.; Yehezkelli, Y.; Kalkstein, N.; Akiva, P.; Ekka-Zohar, A.; David, S.S.B.; Lerner, U.; Bivas-Benita, M.; et al. Long Covid Outcomes at One Year after Mild SARS-CoV-2 Infection: Nationwide Cohort Study. BMJ 2023, 380, e072529. [Google Scholar] [CrossRef]
- Tehrani, S.; Killander, A.; Åstrand, P.; Jakobsson, J.; Gille-Johnson, P. Risk Factors for Death in Adult COVID-19 Patients: Frailty Predicts Fatal Outcome in Older Patients. Int. J. Infect. Dis. 2021, 102, 415–421. [Google Scholar] [CrossRef]
- Tran, V.-T.; Perrodeau, E.; Saldanha, J.; Pane, I.; Ravaud, P. Efficacy of First Dose of COVID-19 Vaccine versus No Vaccination on Symptoms of Patients with Long Covid: Target Trial Emulation Based on ComPaRe e-Cohort. BMJ Med. 2023, 2, e000229. [Google Scholar] [CrossRef]
- Attendance in Education and Early Years Settings during the Coronavirus (COVID-19) Pandemic, Week 50 2021. Available online: https://explore-education-statistics.service.gov.uk/find-statistics/attendance-in-education-and-early-years-settings-during-the-coronavirus-covid-19-outbreak (accessed on 22 December 2021).
- Liu, J.; Lee, M.; Gershenson, S. The Short- and Long-Run Impacts of Secondary School Absences. J. Public Econ. 2021, 199, 104441. [Google Scholar] [CrossRef]
- New Recommendations for Vaccination against COVID-19—The Public Health Agency of Sweden. Available online: https://www.folkhalsomyndigheten.se/the-public-health-agency-of-sweden/communicable-disease-control/covid-19/vaccination-against-covid-19/order-of-priority-for-covid-19-vaccine/ (accessed on 3 May 2023).
- Vaccination against COVID-19. Available online: https://www.sst.dk/en/english/corona-eng/vaccination-against-covid-19 (accessed on 3 May 2023).
- Callaway, E. COVID’s Future: Mini-Waves Rather than Seasonal Surges. Nature 2023, 617, 229–230. [Google Scholar] [CrossRef] [PubMed]
- Wood, A.; Denholm, R.; Hollings, S.; Cooper, J.; Ip, S.; Walker, V.; Denaxas, S.; Akbari, A.; Banerjee, A.; Whiteley, W.; et al. Linked Electronic Health Records for Research on a Nationwide Cohort of More than 54 Million People in England: Data Resource. BMJ 2021, 373, n826. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, T.; Stephenson, T.; Pereira, S.P.; Shafran, R.; De Stavola, B.; Rojas, N.; McOwat, K.; Simmons, R.; Zavala, M.; O’Mahoney, L.; et al. Long COVID—The Physical and Mental Health of Children and Non-Hospitalised Young People 3 Months after SARS-CoV-2 Infection; a National Matched Cohort Study (The CLoCk) Study. Lancet Child Adolesc. Health 2021, 6, 230–239. [Google Scholar] [CrossRef]
- Molteni, E.; Sudre, C.H.; Canas, L.S.; Bhopal, S.S.; Hughes, R.C.; Antonelli, M.; Murray, B.; Kläser, K.; Kerfoot, E.; Chen, L.; et al. Illness Duration and Symptom Profile in Symptomatic UK School-Aged Children Tested for SARS-CoV-2. Lancet Child Adolesc. Health 2021, 5, 708–718. [Google Scholar] [CrossRef]
- Gurdasani, D.; Akrami, A.; Bradley, V.C.; Costello, A.; Greenhalgh, T.; Flaxman, S.; McKee, M.; Michie, S.; Pagel, C.; Rasmussen, S.; et al. Long COVID in Children. Lancet Child Adolesc. Health 2022, 6, e2. [Google Scholar] [CrossRef] [PubMed]
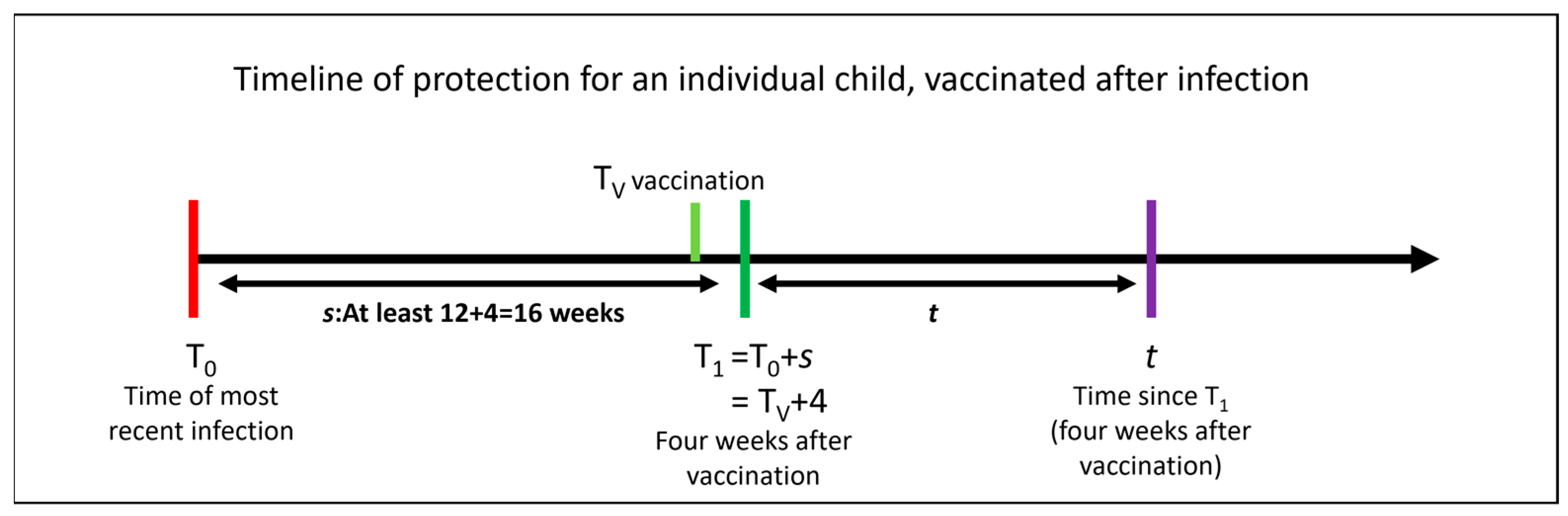


| Variable | Description |
|---|---|
| Adverse outcome of type due to infection | |
| Adverse outcome of type due to vaccination | |
| Time of a child’s most recent infection | |
| Time of vaccination | |
| Time of maximal vaccination efficacy in weeks (while Lin et al. show good effectiveness by two weeks, their reported maximal efficacy is at four weeks, after which waning begins [7]) | |
| Time between last infection and four weeks after vaccination (), where this is at least 16 weeks (allowing 12 weeks between infection and vaccination, as per current UK guidance) | |
| Time since four weeks after vaccination | |
| The degree of protection afforded by previous infection against adverse outcome of type at time after infection in an unvaccinated child. Protection can range from 0 (no protection) to 1 (complete protection) | |
| The degree of protection afforded by vaccination after previous infection against adverse outcome of type at time after administration + four weeks (to allow for maximum efficacy). Protection can range from (same as previous infection alone) to 1 (complete protection) | |
| Probability of adverse event of type for a child infected for the first time | |
| Probability of adverse event of type due to the vaccine | |
| The number of adverse events of type caused by the vaccine if the whole population of children were vaccinated | |
| Number of adverse events of type across the whole population of children, if all infected for the first time | |
| Number of adverse events of type due to the vaccine across the whole population of children, if all vaccinated | |
| The proportion of children infected (again) in the future, over some time period, where the proportion can range from 0 (no new infections) to 1 (all children reinfected) | |
| The rate of waning of protection following infection or vaccination following infection, respectively |
| Parameter for Children Aged 5 to 11 | Value for Those with UHC | Value for Those with no UHC | Comment (Further Detail Provided in Supplementary Materials) |
|---|---|---|---|
| Number in population resident in England | 687,935 | 4,036,891 | We use 2021 UK Office for National Statistics (ONS) estimates for overall population of 5–11-year-olds [45]. We derive a UHC rate of 14.6% based on the proportion of medical records for all of the five- to eleven-year-olds in the NHS Digital Trusted Research Environment where there was evidence of a health condition linked to greater vulnerability to severe disease with SARS-CoV-2 infection by the JCVI [26] as per the method described in Wilde et al. (under review) [44]. This rate is then applied to the overall ONS population estimate. |
| Proportion infected at least once by March 2022 | 82% | We use the UK Office for National Statistics (ONS) Schools Infection Survey antibody study for primary school children from early 2022 reporting that 82% of primary-school-age children had antibodies. The actual proportion infected is likely to be much higher (ONS infection survey estimates cumulative incidence of 123% by March 2022 [21]), so this is a conservative estimate. We assume the same proportion for children with UHC and those without UHC since we do not have a reliable way of estimating difference in infection likelihood between the two populations. | |
| Number of hospital admissions directly associated with first ascertained SARS-CoV-2 infection July 2020 to end February 2022 | 3375 | 3465 | We use the number reported in Wilde et al. (under review) [44] for first hospital admissions associated with first ascertained SARS-CoV-2 infection in children aged five to eleven and excluding admissions that are incidental to SARS-CoV-2 infection (see Wilde et al. for details), from July 2020 to end of February 2022 in England. NOTE: this will be an underestimate of all hospitalisations since it excludes individuals that had an admission prior to July 2020. |
| Number of hospital admissions expected if whole population had been infected for the first time () | 4120 | 4230 | Obtained by dividing the number observed by the proportion of children infected in each group as given in row 2 above, to three significant figures. |
| Maximum protection of previous infection against hospitalisation from reinfection ) | 99.5% | Lin et al. [7] give an estimate of 99.5% efficacy at 1 month post-infection against hospitalisation. | |
| Maximum protection of vaccination in children with previous infection (at least 16 weeks after infection and 4 weeks after vaccination) ) | 100% | Lin et al. [7] do not provide estimates for additional protection from vaccination but just say that it is higher than vaccination or infection alone. Bobrovitz et al. [9] give an efficacy of over 95% at both three and twelve months after two or three doses of vaccine following infection. | |
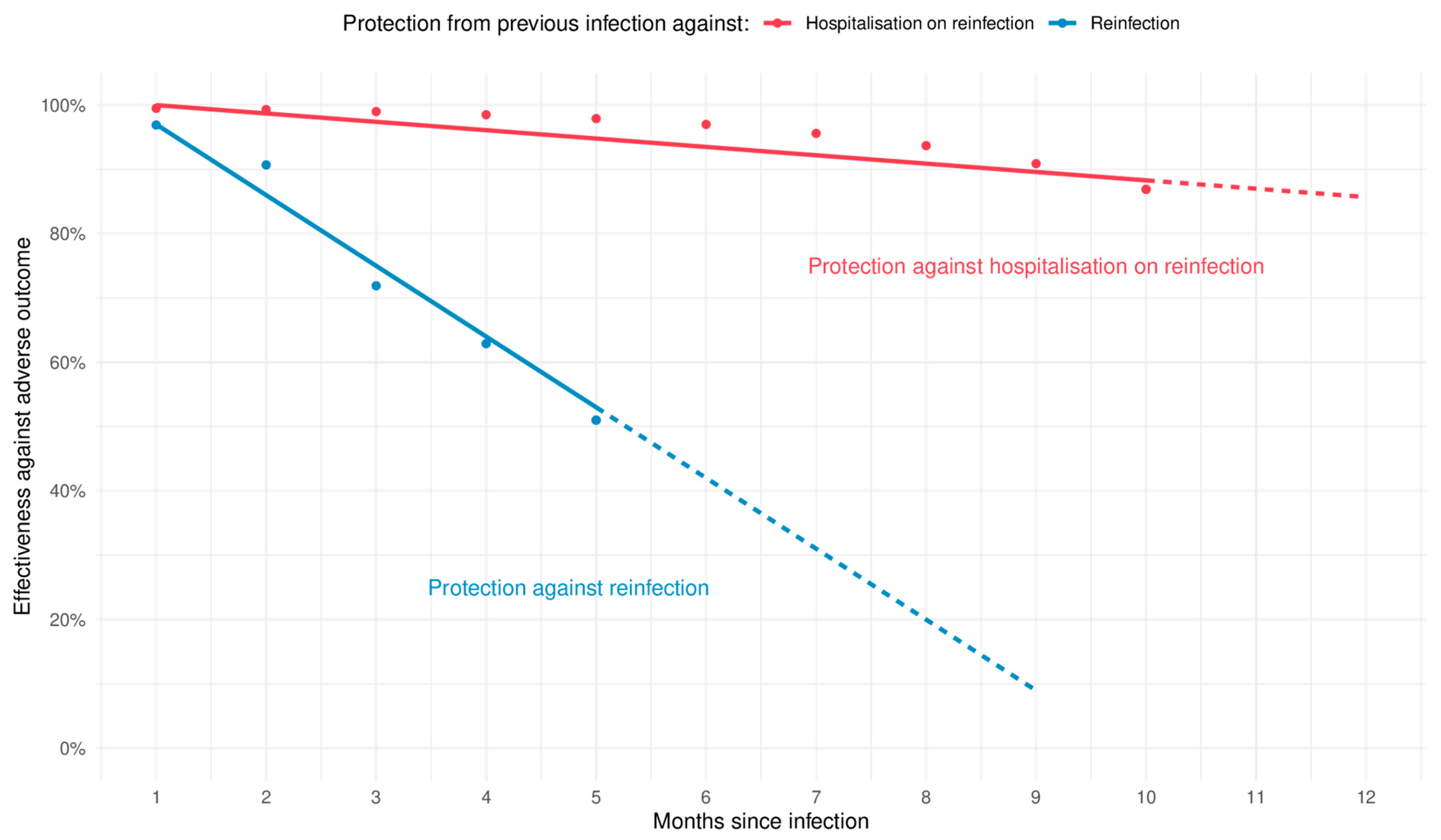
| The waning rate of protection from either vaccination following infection or infection alone () | 1.3 percentage points per month (minimum 0.6 and maximum 1.7) | Estimated using the data in the supplemental material for Lin et al. [7] for protection from previous Omicron infection only. See Supplementary Materials for fits for maximum and minimum ranges and Figure 2 for the central estimate. | |
| The number of hospitalisations due to vaccine adverse events if all children were vaccinated (NVA) | 1 | 7 | A systematic review of vaccination for 5–11-year-olds reported a myocarditis rate of 1.3–1.8/million vaccinations given [46]. Extrapolating this rate to the five to eleven year old population in UK (five million) and conservatively assuming all would be hospitalised gives a central estimate of eight hospital admissions due to the vaccine, which we split across UHC and non UHC children 1:7. We note that Watanabe et al. report no vaccine-caused deaths among 16.6 million injections [46]. |
| Attack Rate (Proportion of Children Reinfected in the 6 Months Post-Vaccination) | Estimated Number of Hospitalisations Averted on Reinfection if All 4 Million Children without Underlying Health Conditions (UHC) Were Vaccinated on Average a Year after Previous Infection | Estimated Number of New Cases of Long Covid Averted on Reinfection if All 4.7 Million Children Were Vaccinated on Average a Year after Previous Infection |
|---|---|---|
| 5% | 25 (10–35) | 3500 (800–6500) |
| 25% | 160 (75–215) | 18,000 (4000–32,000) |
| 40% | 265 (120–350) | 29,000 (6500–51,000) |
| 50% (ONS estimate an incidence rate of 45% in 2- to 11-year-olds July–December 2022) | 330 (160–440) | 36,500 (8000–64,000) |
| 60% | 400 (190–520) | 43,500 (9500–76,500) |
| 75% | 500 (240–660) | 54,500 (12,000–96,000) |
| 95% (ONS estimate an incidence rate of 98% in 2- to 11-year-olds, Dec 2021 to June 2022) | 640 (300–830) | 69,000 (15,000–121,500) |
| Parameter for Children Aged Five to Eleven | Value | Comment |
|---|---|---|
| Number in population resident in England | 4,724,826 | We use 2021 UK Office for National Statistics (ONS) estimates for overall population of five- to eleven-year-olds [45]. |
| Incidence of Long Covid (ongoing symptoms lasting at least 3 months following first infection) | 3.5% | A central estimate from recent studies and the American Academy of Pediatrics [47,48,49,50]. |
| Number of children experiencing Long Covid if whole population had been infected for the first time () | 165,000 | Rounded to three significant figures. |
| (Infection only): Protection of previous infection against new Omicron infection at one year post-infection (representing ) | 16–36% | Bobrovitz et al. [9] give an efficacy of 24.7% at 12 months with CI 16.4% to 35.5%. |
| Hybrid): Maximum protection against reinfection of vaccination in children following previous infection. We are interested in efficacy just after vaccination ) | 59–78% | Bobrovitz et al. [9] give an efficacy of 69.0% at 3 months with CI 58.9% to 77.5%, and we use this as a conservative estimate of maximum vaccine benefit following previous infection. Note also that Dowell et al. [51] show that antibodies to SARS-CoV-2 are increased greatly in children with vaccination on top of previous immunity (from Omicron). |
| (Infection only): Minimum and maximum protection of previous infection against Long Covid once reinfected at least one year later | 0–40% | There is a great deal of uncertainty in the protection afforded by previous infection once reinfected. The UK ONS reports no significant difference in reporting Long Covid in 2- to 11-year-olds 20 weeks after reinfection vs. 20 weeks after first infection [34]. We choose a range of 0–40% as a plausible range. |
| (Hybrid): Maximum protection of vaccination following previous infection against Long Covid once reinfected | 30–70% | There is a great deal of uncertainty in the protection afforded by hybrid immunity once reinfected. Protection from vaccination alone is thought to be somewhere between 15–50% [52]. A recent systematic review reported great uncertainty in the scale but likely definite benefit of vaccination in preventing Long Covid [53]. Assuming hybrid protection is better than vaccination alone, we chose a range of 30–70%. |
| Overall effectiveness of previous infection one year earlier against new Long Covid on reinfection | 16–62% | Combining minimum and maximum ranges of respective protections above. |
| Overall effectiveness of vaccination after previous infection against new Long Covid on reinfection | 71–93% | Combining minimum and maximum ranges of respective protections above. |
| The number of Long Covid cases due to vaccination if all children were vaccinated | 0 | There is no mechanism by which vaccination can cause Long Covid. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pagel, C.; Wilde, H.; Tomlinson, C.; Mateen, B.; Brown, K. A Methodological Framework for Assessing the Benefit of SARS-CoV-2 Vaccination following Previous Infection: Case Study of Five- to Eleven-Year-Olds. Vaccines 2023, 11, 988. https://doi.org/10.3390/vaccines11050988
Pagel C, Wilde H, Tomlinson C, Mateen B, Brown K. A Methodological Framework for Assessing the Benefit of SARS-CoV-2 Vaccination following Previous Infection: Case Study of Five- to Eleven-Year-Olds. Vaccines. 2023; 11(5):988. https://doi.org/10.3390/vaccines11050988
Chicago/Turabian StylePagel, Christina, Harrison Wilde, Christopher Tomlinson, Bilal Mateen, and Katherine Brown. 2023. "A Methodological Framework for Assessing the Benefit of SARS-CoV-2 Vaccination following Previous Infection: Case Study of Five- to Eleven-Year-Olds" Vaccines 11, no. 5: 988. https://doi.org/10.3390/vaccines11050988
APA StylePagel, C., Wilde, H., Tomlinson, C., Mateen, B., & Brown, K. (2023). A Methodological Framework for Assessing the Benefit of SARS-CoV-2 Vaccination following Previous Infection: Case Study of Five- to Eleven-Year-Olds. Vaccines, 11(5), 988. https://doi.org/10.3390/vaccines11050988






