COVID-19 Vaccine Safety Monitoring Studies in Low- and Middle-Income Countries (LMICs)—A Systematic Review of Study Designs and Methods
Abstract
:1. Key Points
- Active surveillance studies have been used to monitor COVID-19 vaccine safety in low- and middle-income countries.
- Most studies were cross-sectional with limited outcome validation and no temporal assessment.
- Major vaccination data sources were medical charts or self-reported cases based on clinical signs or symptoms.
- Only one-third of the studies employed parametric models, such as logistic regression (n = 17, 29.3%) and Cox regression (n = 3, 5.2%).
2. Background
3. Methods
3.1. Search Strategy
3.2. Eligibility Criteria
3.3. Data Extraction and Quality Assessment
3.4. Quality Assessment
3.5. Data Synthesis
4. Results
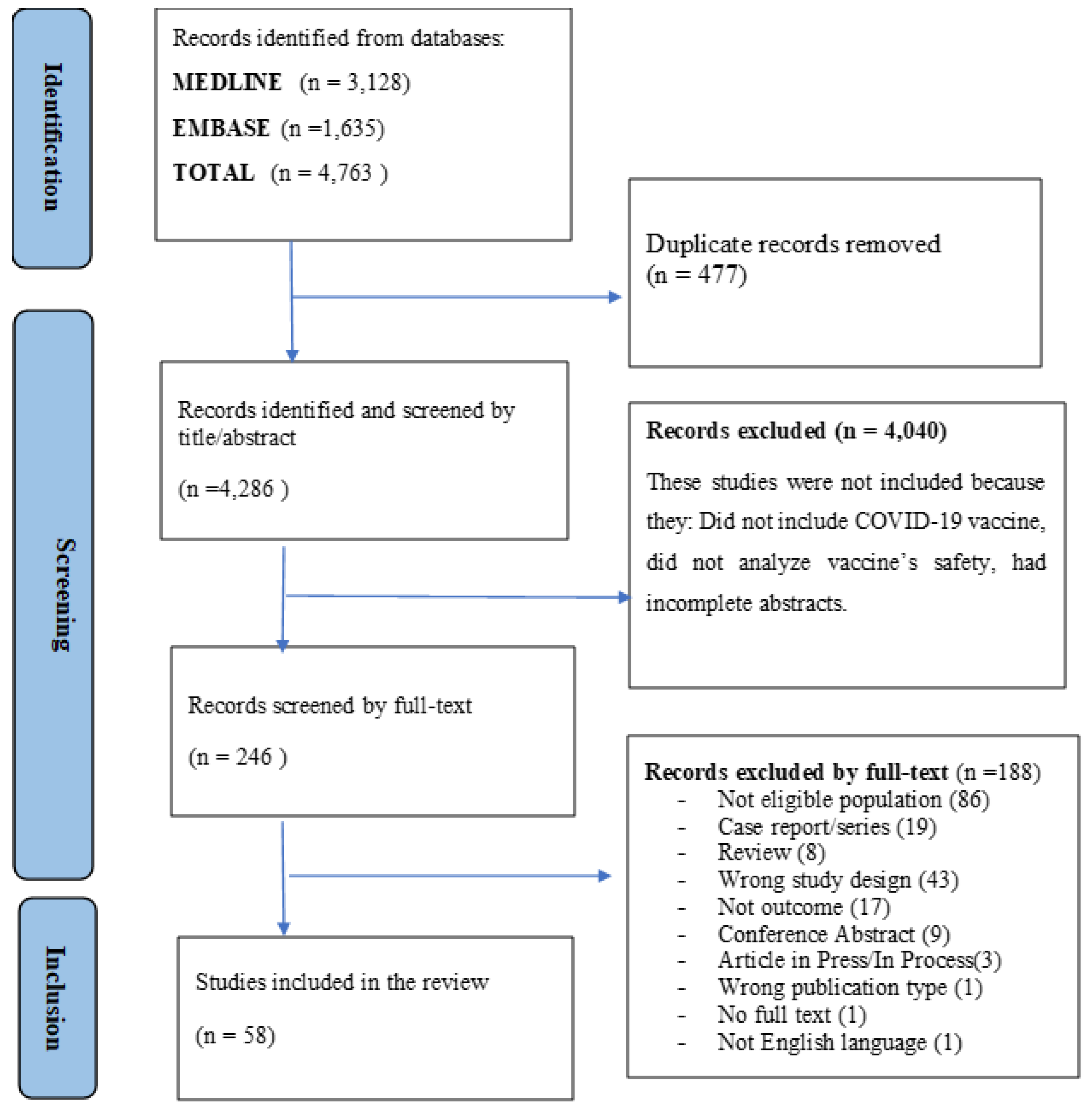
4.1. Study Selection
4.2. Study Characteristics
4.3. Methodological Quality
4.4. Vaccines Studied
4.5. Characteristics of the Reported Safety Data
4.6. Study Designs Employed and Signal Detection Method
4.7. Statistical Analysis for Safety Data Analysis
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Coronavirus (COVID-19); World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Watson, O.J.; Barnsley, G.; Toor, J.; Hogan, A.B.; Winskill, P.; Ghani, A.C. Global impact of the first year of COVID-19 vaccination: A mathematical modelling study. Lancet Infect. Dis. 2022, 22, 1293–1302. [Google Scholar] [CrossRef]
- Suthar, A.B.; Wang, J.; Seffren, V.; Wiegand, R.E.; Griffing, S.; Zell, E. Public health impact of COVID-19 vaccines in the US: Observational study. BMJ 2022, 377, e069317. [Google Scholar] [CrossRef]
- Wiegand, R.E.; Burke, R.M.; Sharma, A.J.; Sheppard, M.; Adjemian, J.; Ahmad, F.B.; Anderson, R.N.; Barbour, K.E.; Binder, A.M.; Dasgupta, S.; et al. Estimating the early impact of the US COVID-19 vaccination programme on COVID-19 cases, emergency department visits, hospital admissions, and deaths among adults aged 65 years and older: An ecological analysis of national surveillance data. Lancet 2022, 399, 152–160. [Google Scholar]
- Savinkina A, Bilinski A, Fitzpatrick M, et al Estimating deaths averted and cost per life saved by scaling up mRNA COVID-19 vaccination in low-income and lower-middle-income countries in the COVID-19 Omicron variant era: A modelling study. BMJ Open 2022, 12, e061752. [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2020, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- OU and WP4 Risk Working Group. ADVANCE: Methods for burden of disease, vaccination coverage, vaccine safety & effectiveness, impact and benefit risk monitoring. In Accelerated Development of VAccine Benefit-Risk Collaboration in Europe. ADVNCE Consortium; European Union: Leuven, Belgium, 2014; Available online: www.advance-vaccines.eu (accessed on 17 April 2023).
- Gavi the Vaccine Alliance. COVID-19: Gavi Steps Up Response to Pandemic; Gavi the Vaccine Alliance: Geneva, Switzerland, 2020. [Google Scholar]
- Kaddar, M.; Schmitt, S.; Makinen, M.; Milstien, J. Global support for new vaccine implementation in middle-income countries. Vaccine 2013, 31 (Suppl. S2), B81–B96. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. COVID-19 Vaccines: Safety Surveillance Manual, 2nd ed.; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- European Medicines Agency (EMA). COVID-19 Guidance: Research and Development. 2020. Available online: https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/guidance-developers-companies/covid-19-guidance-research-development (accessed on 17 April 2023).
- Centers Disease Control (CDC). Protocols and Standard Operating Procedures for COVID-19; Centers for Disease Control and Prevention, Atlanta, USA; 2020. Available online: https://www.cdc.gov/vaccinesafety/ensuringsafety/monitoring/emergencypreparedness/index.html#anchor_1607961664745 (accessed on 17 April 2023).
- Guignard, A.; Praet, N.; Jusot, V.; Bakker, M.; Baril, L. Introducing new vaccines in low- and middle-income countries: Challenges and approaches. Expert Rev. Vaccines 2019, 18, 119–131. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Landscape of Observational Studies on the Effectiveness of COVID-19 vac Cination; World Health Organization: Geneva, Switzerland, 2022; Available online: https://www.who.int/publications/m/item/draft-landscape-of-observational-study-designs-on-the-effectiveness-of-covid-19-vaccination (accessed on 17 April 2023).
- Andrews, N. Epidemiological designs for vaccine safety assessment: Methods and pitfalls. Biologicals 2012, 40, 389–392. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.T.; Shimabukuro, T.T.; Martin, D.B.; Zuber, P.L.; Weibel, D.M.; Sturkenboom, M. Enhancing vaccine safety capacity globally: A lifecycle perspective. Vaccine 2015, 33 (Suppl. S4), D46–D54. [Google Scholar] [CrossRef]
- Teerawattananon, Y.; Anothaisintawee, T.; Pheerapanyawaranun, C.; Botwright, S.; Akksilp, K.; Sirichumroonwit, N.; Budtarad, N.; Isaranuwatchai, W. A systematic review of methodological approaches for evaluating real-world effectiveness of COVID-19 vaccines: Advising resource-constrained settings. PLoS ONE 2022, 17, e0261930. [Google Scholar] [CrossRef]
- Luo, C.; Du, J.; Cuker, A.; Lautenbach, E.; Asch, D.A.; Poland, G.A.; Tao, C.; Chen, Y. Comparability of clinical trials and spontaneous reporting data regarding COVID-19 vaccine safety. Sci. Rep. 2022, 12, 10946. [Google Scholar] [CrossRef]
- World Health Organization. WHO ad hoc Consultation: COVID Vaccines Methodological Approaches to Assess Variants Effect on Vaccine Efficacy, Effectiveness and Impact; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Langan, S.M.; Schmidt, S.A.; Wing, K.; Ehrenstein, V.; Nicholls, S.G.; Filion, K.B.; Klungel, O.; Petersen, I.; Sorensen, H.T.; Dixon, W.G.; et al. The reporting of studies conducted using observational routinely collected health data statement for pharmacoepidemiology (RECORD-PE). BMJ 2018, 363, k3532. [Google Scholar] [CrossRef] [PubMed]
- Blonde, L.; Khunti, K.; Harris, S.B.; Meizinger, C.; Skolnik, N.S. Interpretation and Impact of Real-World Clinical Data for the Practicing Clinician. Adv. Ther. 2018, 35, 1763–1774. [Google Scholar] [CrossRef]
- Dragalin, V.; Fedorov, V. Multistage Designs for Vaccine Safety Studies. J. Biopharm. Stat. 2006, 16, 539–553. [Google Scholar] [CrossRef] [PubMed]
- Dragalin, V.; Fedorov, V.; Cheuvart, B. Statistical approaches to establishing vaccine safety. Stat. Med. 2002, 21, 877–893. [Google Scholar] [CrossRef]
- Nauta, J. Statistics in Clinical and Observational Vaccine Studies; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Gilbert, P.B.; Self, S.G.; Ashby, M.A. Statistical methods for assessing differential vaccine protection against human immunodeficiency virus types. Biometrics 1998, 54, 799. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. Ann. Intern. Med. 2009, 151, W-65–W-94. [Google Scholar] [CrossRef] [PubMed]
- The World Bank Group. World Bank Country and Lending Groups← Country Classification; The World Bank Group: Washington, DC, USA, 2022. [Google Scholar]
- Wells, G.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, V.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Studies in Meta-Analyses; Ottawa Hospital Research Institute: Ottawa, ON, Canada. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 20 April 2022).
- McPheeters, M.; Kripalani, S.; Peterson, N.B.; Idowu, R.T.; Jerome, R.N.; Potter, S.A.; Andrews, J.C. Quality Improvement Interventions to Address Health Disparities. Closing the Quality Gap: Revisiting the State of the Science. Evidence Report No. 208. (Prepared by the Vanderbilt University Evidence-based Practice Center under Contract No. 290-2007-10065.) AHRQ Publication No. 12-E009-EF; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2012. Available online: www.effectivehealthcare.ahrq.gov/reports/final.cfm (accessed on 17 April 2023).
- Aikawa, N.E.; Kupa, L.V.; Pasoto, S.G.; Medeiros-Ribeiro, A.C.; Yuki, E.F.; Saad, C.G.; Pedrosa, T.; Fuller, R.; Shinjo, S.K.; Sampaio-Barros, P.D.; et al. Immunogenicity and safety of two doses of the CoronaVac SARS-CoV-2 vaccine in SARS-CoV-2 seropositive and seronegative patients with autoimmune rheumatic diseases in Brazil: A subgroup analysis of a phase 4 prospective study. Lancet Rheumatol. 2022, 4, e113–e124. [Google Scholar] [CrossRef]
- Pagotto, V.; Ferloni, A.; Soriano, M.M.; Díaz, M.; Golde, N.B.; González, M.I.; Asprea, V.; Staneloni, M.I.; Zingoni, P.; Vidal, G.; et al. Active monitoring of early safety of Sputnik V vaccine in Buenos Aires, Argentina. Medicina 2021, 81, 408–414. [Google Scholar]
- Ariamanesh, M.; Porouhan, P.; PeyroShabany, B.; Fazilat-Panah, D.; Dehghani, M.; Nabavifard, M.; Hatami, F.; Fereidouni, M.; Welsh, J.S.; Javadinia, S.A. Immunogenicity and Safety of the Inactivated SARS-CoV-2 Vaccine (BBIBP-CorV) in Patients with Malignancy. Cancer Investig. 2022, 40, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Basavaraja, C.K.; Sebastian, J.; Ravi, M.D.; John, S.B. Adverse events following COVID-19 vaccination: First 90 days of experience from a tertiary care teaching hospital in South India. Ther. Adv. Vaccines Immunother. 2021, 9, 1055833. [Google Scholar] [CrossRef] [PubMed]
- García-Grimshaw, M.; Ceballos-Liceaga, S.E.; Hernández-Vanegas, L.E.; Núñez, I.; Hernández-Valdivia, N.; Carrillo-García, D.A.; Michel-Chávez, A.; Galnares-Olalde, J.A.; Carbajal-Sandoval, G.; Saniger-Alba, M.D.M.; et al. Neurologic adverse events among 704,003 first-dose recipients of the BNT162b2 mRNA COVID-19 vaccine in Mexico: A nationwide descriptive study. Clin. Immunol. 2021, 229, 108786. [Google Scholar] [CrossRef] [PubMed]
- Haslak, F.; Gunalp, A.; Cebi, M.N.; Yildiz, M.; Adrovic, A.; Sahin, S.; Barut, K.; Kasapcopur, O. Early experience of COVID-19 vaccine-related adverse events among adolescents and young adults with rheumatic diseases: A single-center study. Int. J. Rheum. Dis. 2022, 25, 353–363. [Google Scholar] [CrossRef]
- Karattuthodi, M.S.; Chandrasekher, D.; Panakkal, L.M.; Salman, M.; Shinu, C.; Megha; Swabeech, E.M.; Fasil, M.; Mohammad, A.M.; Reji, M. Pharmacist-directed Sputnik V (GAM-COVID-VAC) surveillance program: A prospective observational study in Southern India. J. Basic Clin. Physiol. Pharmacol. 2022. [Google Scholar] [CrossRef]
- Konda, V.C.R.; Gokul, T.; Poojitha, M.P.M.; Rao, K.U. Adverse Events Following Immunization to COVID-19 vaccines in A Tertiary Care Hospital—A descriptive Study. Biomed. Pharmacol. J. 2021, 14, 2149–2156. [Google Scholar] [CrossRef]
- Clark, A.; Jit, M.; Warren-Gash, C.; Guthrie, B.; Wang, H.H.X.; Mercer, S.W.; Sanderson, C.; McKee, M.; Troeger, C.; Ong, K.L.; et al. Global, regional, and national estimates of the population at increased risk of severe COVID-19 due to underlying health conditions in 2020: A modelling study. Lancet Glob. Health 2020, 8, e1003–e1017. [Google Scholar] [CrossRef]
- Barda, N.; Dagan, N.; Ben-Shlomo, Y.; Kepten, E.; Waxman, J.; Ohana, R.; Hernán, M.A.; Lipsitch, M.; Kohane, I.; Netzer, D.; et al. Safety of the BNT162b2 mRNA COVID-19 Vaccine in a Nationwide Setting. N. Engl. J. Med. 2021, 385, 1078–1090. [Google Scholar] [CrossRef]
- Li, X.; Lai, L.Y.; Ostropolets, A.; Arshad, F.; Tan, E.H.; Casajust, P.; Alshammari, T.M.; Duarte-Salles, T.; Minty, E.P.; Areia, C.; et al. Bias, Precision and Timeliness of Historical (Background) Rate Comparison Methods for Vaccine Safety Monitoring: An Empirical Multi-Database Analysis. Front. Pharmacol. 2021, 12, 773875. [Google Scholar] [CrossRef]
- Rudolph, A.; Mitchell, J.; Barrett, J.; Sköld, H.; Taavola, H.; Erlanson, N.; Melgarejo-González, C.; Yue, Q.-Y. Global safety monitoring of COVID-19 vaccines: How pharmacovigilance rose to the challenge. Ther. Adv. Drug Saf. 2022, 13, 1–14. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Iwagami, M.; Ishiguro, C.; Fujii, D.; Yamamoto, N.; Narisawa, M.; Tsuboi, T.; Umeda, H.; Kinoshita, N.; Iguchi, T.; et al. Safety monitoring of COVID-19 vaccines in Japan. Lancet Reg. Health West. Pac. 2022, 23, 100442. [Google Scholar] [CrossRef] [PubMed]
- Saita, M.; Yan, Y.; Ito, K.; Sasano, H.; Seyama, K.; Naito, T. Reactogenicity following two doses of the BNT162b2 mRNA COVID-19 vaccine: Real-world evidence from healthcare workers in Japan. J. Infect. Chemother. 2022, 28, 116–119. [Google Scholar] [CrossRef] [PubMed]
- Mahallawi, W.H.; Mumena, W.A. Reactogenicity and Immunogenicity of the Pfizer and AstraZeneca COVID-19 Vaccines. Front. Immunol. 2021, 12, 5169. [Google Scholar] [CrossRef] [PubMed]
- Chapin-Bardales, J.; Gee, J.; Myers, T. Reactogenicity following receipt of mRNA-based COVID-19 vaccines. JAMA 2021, 325, 2201–2202. [Google Scholar] [CrossRef]
- Ganesan, S.; Al Ketbi, L.M.B.; Al Kaabi, N.; Al Mansoori, M.; Al Maskari, N.N.; Al Shamsi, M.S.; Alderei, A.S.; El Eissaee, H.N.; Al Ketbi, R.M.; Al Shamsi, N.S.; et al. Vaccine Side Effects Following COVID-19 Vaccination Among the Residents of the UAE—An Observational Study. Front. Public Health 2022, 10, 876336. [Google Scholar] [CrossRef]
- Lee, A.R.Y.B.; Wong, S.Y.; Chai, L.Y.A.; Lee, S.C.; Lee, M.X.; Muthiah, M.D.; Tay, S.H.; Teo, C.B.; Tan, B.K.J.; Chan, Y.H.; et al. Efficacy of COVID-19 vaccines in immunocompromised patients: Systematic review and meta-analysis. BMJ 2022, 376, e068632. [Google Scholar] [CrossRef] [PubMed]
- Al Khames Aga, Q.A.; Alkhaffaf, W.H.; Hatem, T.H.; Nassir, K.F.; Batineh, Y.; Dahham, A.T.; Shaban, D.; Al Khames Aga, L.A.; Agha, M.Y.R.; Traqchi, M. Safety of COVID-19 vaccines. J. Med. Virol. 2021, 93, 6588–6594. [Google Scholar] [CrossRef] [PubMed]
- Assadi, M.; Kiani, M.; Gooshki, E.S.; Aryanian, Z.; Afshar, Z.M.; Hatami, P. COVID-19 vaccination in children as a global dilemma through an ethical lens: A retrospective review. Health Sci. Rep. 2023, 6, e976. [Google Scholar] [CrossRef]
- Sisa, I.; Fornasini, M.; Teran, E. COVID-19 research in LMICs. Lancet 2021, 398, 1212–1213. [Google Scholar] [CrossRef]
- Beccia, F.; Rossi, M.F.; Amantea, C.; Villani, L.; Daniele, A.; Tumminello, A.; Aristei, L.; Santoro, P.E.; Borrelli, I.; Ricciardi, W.; et al. COVID-19 Vaccination and Medical Liability: An International Perspective in 18 Countries. Vaccines 2022, 10, 1275. [Google Scholar] [CrossRef]
- Crum, T.; Mooney, K.; Tiwari, B.R. Current situation of vaccine injury compensation program and a future perspective in light of COVID-19 and emerging viral diseases. F1000Research 2021, 10, 652. [Google Scholar] [CrossRef] [PubMed]
- D’errico, S.; Zanon, M.; Concato, M.; Peruch, M.; Scopetti, M.; Frati, P.; Fineschi, V. “First Do No Harm”. No-Fault Compensation Program for COVID-19 Vaccines as Feasibility and Wisdom of a Policy Instrument to Mitigate Vaccine Hesitancy. Vaccines 2021, 9, 1116. [Google Scholar] [CrossRef] [PubMed]
- Spiera, E.; Ganjian, D.Y.; Zhang, X.; Brenner, E.J.; Agrawal, M.; Colombel, J.-F.; Kappelman, M.D.; Kornbluth, A.; Ungaro, R.C. Outcomes of COVID-19 Infections in Vaccinated Patients with Inflammatory Bowel Disease: Data From an International Registry. Inflamm. Bowel Dis. 2022, 28, 1126–1129. [Google Scholar] [CrossRef] [PubMed]
- Nampota-Nkomba, N.; Nyirenda, O.M.; Khonde, L.; Mapemba, V.; Mbewe, M.; Ndaferankhande, J.M.; Msuku, H.; Masesa, C.; Misiri, T.; Mwakiseghile, F.; et al. Safety and immunogenicity of a typhoid conjugate vaccine among children aged 9 months to 12 years in Malawi: A nested substudy of a double-blind, randomised controlled trial. Lancet Glob. Health 2022, 10, e1326–e1335. [Google Scholar] [CrossRef]
- European Medicines Agency (EMA). The European Network of Centres for Pharmacoepidemiology and Pharmacovigilance (ENCePP). Guide on Methodological Standards in Pharmacoepidemiology (Revision 10). EMA/95098/2010. 2020. Available online: http://www.encepp.eu/standards_and_guidance (accessed on 17 April 2023).
- Lai, L.Y.; Arshad, F.; Areia, C.; Alshammari, T.M.; Alghoul, H.; Casajust, P.; Li, X.; Dawoud, D.; Nyberg, F.; Pratt, N.; et al. Current Approaches to Vaccine Safety Using Observational Data: A Rationale for the EUMAEUS (Evaluating Use of Methods for Adverse Events Under Surveillance-for Vaccines) Study Design. Front. Pharmacol. 2022, 13, 554. [Google Scholar] [CrossRef]
- Straus, W.; Rubin, H. COVID-19 vaccine safety monitoring in low and middle income countries—Time for a bold new approach. Vaccine 2022, 40, 4301–4302. [Google Scholar] [CrossRef]
- Zewude, B.; Habtegiorgis, T.; Hizkeal, A.; Dela, T.; Siraw, G. Perceptions and Experiences of COVID-19 Vaccine Side-Effects Among Healthcare Workers in Southern Ethiopia: A Cross-Sectional Study. Pragmatic Obs. Res. 2021, 12, 131–145. [Google Scholar] [CrossRef]
- Serwaa, D.; Osei-Boakye, F.; Nkansah, C.; Ahiatrogah, S.; Lamptey, E.; Abdulai, R.; Antwi, M.H.; Wirekoh, E.Y.; Owusu, E.; Buckman, T.A.; et al. Non-life-threatening adverse reactions from COVID-19 vaccine; a cross-sectional study with self-reported symptoms among Ghanaian healthcare workers. Hum. Vaccines Immunother. 2021, 17, 3881–3886. [Google Scholar] [CrossRef]
- Jahan, N.; Rahman, F.I.; Saha, P.; Ether, S.A.; Roknuzzaman, A.S.M.; Sarker, R.; Kalam, K.T.; Haq, K.; Nyeen, J.; Himi, H.Z.; et al. Side Effects Following Administration of the First Dose of Oxford-AstraZeneca’s Covishield Vaccine in Bangladesh: A Cross-Sectional Study. Infect. Dis. Rep. 2021, 13, 80. [Google Scholar] [CrossRef]
- Subedi, P.; Yadav, G.K.; Paudel, B.; Regmi, A.; Pyakurel, P. Adverse events following the first dose of Covishield (ChAdOx1 nCoV-19) vaccination among health workers in selected districts of central and western Nepal: A cross-sectional study. PLoS ONE 2021, 16, e0260638. [Google Scholar] [CrossRef]
- Wang, Q.; Lv, C.; Han, X.; Shen, M.; Kuang, Y. A Web-Based Survey on Factors for Unvaccination and Adverse Reactions of SARS-CoV-2 Vaccines in Chinese Patients with Psoriasis. J. Inflamm. Res. 2021, 14, 6265–6273. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, S.; Nagpal, R.; Marya, C.; Taneja, P.; Kataria, S. Adverse events occurring post-COVID-19 vaccination among healthcare professionals—A mixed method study. Int. Immunopharmacol. 2021, 100, 108136. [Google Scholar] [CrossRef] [PubMed]
- Christoffels, A.; Mboowa, G.; van Heusden, P.; Makhubela, S.; Githinji, G.; Mwangi, S.; Onywera, H.; Nnaemeka, N.; Amoako, D.G.; Olawoye, I.; et al. A pan-African pathogen genomics data sharing platform to support disease outbreaks. Nat. Med. 2023, 29, 1052–1055. [Google Scholar] [CrossRef] [PubMed]
- Reisinger, S.J.; Ryan, P.B.; O’Hara, D.J.; Powell, G.E.; Painter, J.L.; Pattishall, E.N.; Morris, J.A. Development and evaluation of a common data model enabling active drug safety surveillance using disparate healthcare databases. J. Am. Med. Informatics. Assoc. 2010, 17, 652–662. [Google Scholar] [CrossRef]
- Xiao, C.; Li, Y.; Baytas, I.M.; Zhou, J.; Wang, F. An MCEM Framework for Drug Safety Signal Detection and Combination from Heterogeneous Real World Evidence. Sci. Rep. 2018, 8, 1806. [Google Scholar] [CrossRef]
- World Health Organization. The Oxford/AstraZeneca COVID-19 Vaccine: What You Need to Know. 2021. Available online: https://www.who.int/news-room/feature-stories/detail/the-oxford-astrazeneca-covid-19-vaccine-what-you-need-to-know (accessed on 17 April 2023).
| Study Characteristics | Classification | Number (%) |
|---|---|---|
| Study Designs | Cross-Sectional Studies/Descriptive studies | 41 (70.69) |
| Cohort Studies | 13 (22.41) | |
| Retrospective | 2 (3.45) | |
| Both Cross-sectional and Cohort | 1 (1.72) | |
| Cross-sectional—Sequential mixed-method | 1 (1.72) | |
| Country world bank classification | Low-income economies | 4 (7.00) |
| Lower-middle-income economies | 26 (45.00) | |
| Upper-middle-income economies | 28(48.00) | |
| Data sources | Primary data | 51 (87.93) |
| Secondary data | 5 (8.62) | |
| Mixed | 2 (3.45) | |
| Source of vaccination data | Spontaneous reporting | 3 (5.17) |
| Registry in Epidemiological Surveillance System | 2 (3.45) | |
| Self-reported (Primary data collection) | 52 (89.66) | |
| Active surveillance | 1 (1.72) | |
| Populations of interest | High-risk population (e.g., healthcare workers, immunocompromised hosts) | 37 (63.79) |
| Children | 1 (1.72) | |
| Adults | 15 (25.86) | |
| All group | 5 (8.62) | |
| Analysis method | Statistical tests (association)—No adjustment for confounder | 47 (82.46) |
| Advanced modeling (e.g., regression analysis)—Adjustment for confounders | 10 (17.54) | |
| Study type | Near real-time surveillance | 57 (98.28) |
| Phase IV observation study | 1 (1.72) | |
| Comparator for safety assessment (e.g., non-exposed, active comparator/vaccine) | Yes | 2 (3.45) |
| No | 56 (96.55) |
| Manufacturer | Name of Vaccine | Platform | Frequency |
|---|---|---|---|
| AstraZeneca, AB or Serum Institute of India Pvt. Ltd., Maharashtra, India | AZD1222 Vaxzevria or Covishield (ChAdOx1_nCoV-19) | Recombinant ChAdOx1 chimpansee adenoviral vector | 29 |
| Sinovac Life Sciences Co., Ltd., Hong Kong, China | COVID-19 Vaccine (Vero Cell), Inactivated/ CoronaVac (Sinopharm or Sinovac or CoronaVac) | Inactivated virus | 28 |
| BioNTech Manufacturing GmbH, Mainz, Germany | BNT162b2/COMIRNATY Tozinameran (INN) | Nucleoside modified mRNA | 18 |
| Russian Direct Investment Fund, Moscow, Russia | Sputnik V | Human Adenovirus Vector | 10 |
| Any type of Vaccine | Any (Not specified) | N/A (Not mentioned) | 7 |
| Bharat Biotech, Telangana, India | SARS-CoV-2 Vaccine, Inactivated (Vero Cell)/ COVAXIN | Whole-Virion Inactivated | 4 |
| CasinoBio Cansino Biologics, Tianjin, China | Ad5-nCoV, Convidecia | Recombinant Novel Coronavirus Vaccine (Adenovirus Type 5 Vector) | 4 |
| Moderna Biotech, Cambridge, MA, USA | mRNA-1273, Spikevax | Nucleoside modified mRNA | 3 |
| Janssen–Cilag International NV, Beerse, Belgium | Ad26.COV2.S, JCOVDEN | Recombinant, replication incompetent adenovirus type 26 (Ad26) vectored vaccine encoding the (SARS-CoV-2) Spike (S) protein | 1 |
| Adverse Events Category | Number of Studies | Adverse Events |
|---|---|---|
| Systemic event reactions | 53 | Fever or hyperthermia or feverish, headaches, fatigue, vomiting, diarrhea, muscle pain, joint pain, cough, nausea, dyspnoea, appetite impaired, dizziness, mucosal abnormality, pruritus, hypersensitivity, syncope, asthenia, rhinorrhoea, malaise, sore throat (throat irritation), pain in the oropharynx (pharyngalgia), hives, nasal congestion. |
| Injection site adverse reactions | 53 | Pain, induration, redness, or erythema, swelling, itch, muscular weakness. |
| Serious vaccine-related adverse event | 3 | Deaths, hospitalization, thrombotic complications. |
| Others | 5 | Reported positive test for COVID-19 and other complications |
| Doses | 58 | Investigated Dose 1 effects |
| 12 | Both dose (1 & 2) | |
| 1 | Booster analysis | |
| Outcome identification methods and validation (by diagnostic codes, …) | 8 | Common Terminology Criteria for Adverse Events (CTCAE) version V5 World Health Organization-Uppsala Monitoring Centre (WHO-UMC) causality assessment scale IgG anti-spike-protein antibodies test and laboratory tests Medically reviewed at in-person visits |
| Statistical Method/Approach | Number of Articles (%) | |
|---|---|---|
| Descriptive statistics | Proportion/count | 57 (98.3) |
| Mean/median | 27 (46.6) | |
| Incidence rate | 5 (8.6) | |
| Inferential methods | Univariate methods | 44 (75.9) |
| Fisher’s exact test/Chi-square | 40 (70) | |
| Mann–Whitney/Wilcoxon | 12 (20.7) | |
| t-test | 8 (13.8) | |
| ANOVA | 5 (8.6) | |
| Multivariable modeling | Binary regression | 17 (29.3) |
| Survival Analysis | 3 (5.2) | |
| Number of inferential methods | One method | 37 (63.8) |
| More than one method | 20 (34.5) | |
| None | 1 (1.7) | |
| Analysis approach | Handling missing data | 0 (0) |
| Imputation | 0 (0) | |
| Model diagnostics and validity checks (e.g., goodness of fit, identification of outliers, and co-linearity) | 12 (20.7) | |
| Results presentation | Tables | 57 (98.3) |
| Point estimate and confidence interval | 55 (94.8) | |
| p value | 55 (94.8) | |
| Graphs | 7 (10.3) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sisay, M.M.; Montesinos-Guevara, C.; Osman, A.K.; Saraswati, P.W.; Tilahun, B.; Ayele, T.A.; Ahmadizar, F.; Durán, C.E.; Sturkenboom, M.C.J.M.; van de Ven, P.; et al. COVID-19 Vaccine Safety Monitoring Studies in Low- and Middle-Income Countries (LMICs)—A Systematic Review of Study Designs and Methods. Vaccines 2023, 11, 1035. https://doi.org/10.3390/vaccines11061035
Sisay MM, Montesinos-Guevara C, Osman AK, Saraswati PW, Tilahun B, Ayele TA, Ahmadizar F, Durán CE, Sturkenboom MCJM, van de Ven P, et al. COVID-19 Vaccine Safety Monitoring Studies in Low- and Middle-Income Countries (LMICs)—A Systematic Review of Study Designs and Methods. Vaccines. 2023; 11(6):1035. https://doi.org/10.3390/vaccines11061035
Chicago/Turabian StyleSisay, Malede Mequanent, Camila Montesinos-Guevara, Alhadi Khogali Osman, Putri Widi Saraswati, Binyam Tilahun, Tadesse Awoke Ayele, Fariba Ahmadizar, Carlos E. Durán, Miriam C. J. M. Sturkenboom, Peter van de Ven, and et al. 2023. "COVID-19 Vaccine Safety Monitoring Studies in Low- and Middle-Income Countries (LMICs)—A Systematic Review of Study Designs and Methods" Vaccines 11, no. 6: 1035. https://doi.org/10.3390/vaccines11061035





