Sulfated Lactosyl Archaeol Archaeosome-Adjuvanted Vaccine Formulations Targeting Rabbit Hemorrhagic Disease Virus Are Immunogenic and Efficacious
Abstract
:1. Introduction
2. Materials and Methods
2.1. Vaccine Components
2.2. Immunogenicity Studies in Mice and Rabbits
2.3. Antibody ELISA
2.4. ELISpot
2.5. RHDV Challenge Studies in Rabbits
2.6. Statistical Analysis
3. Results
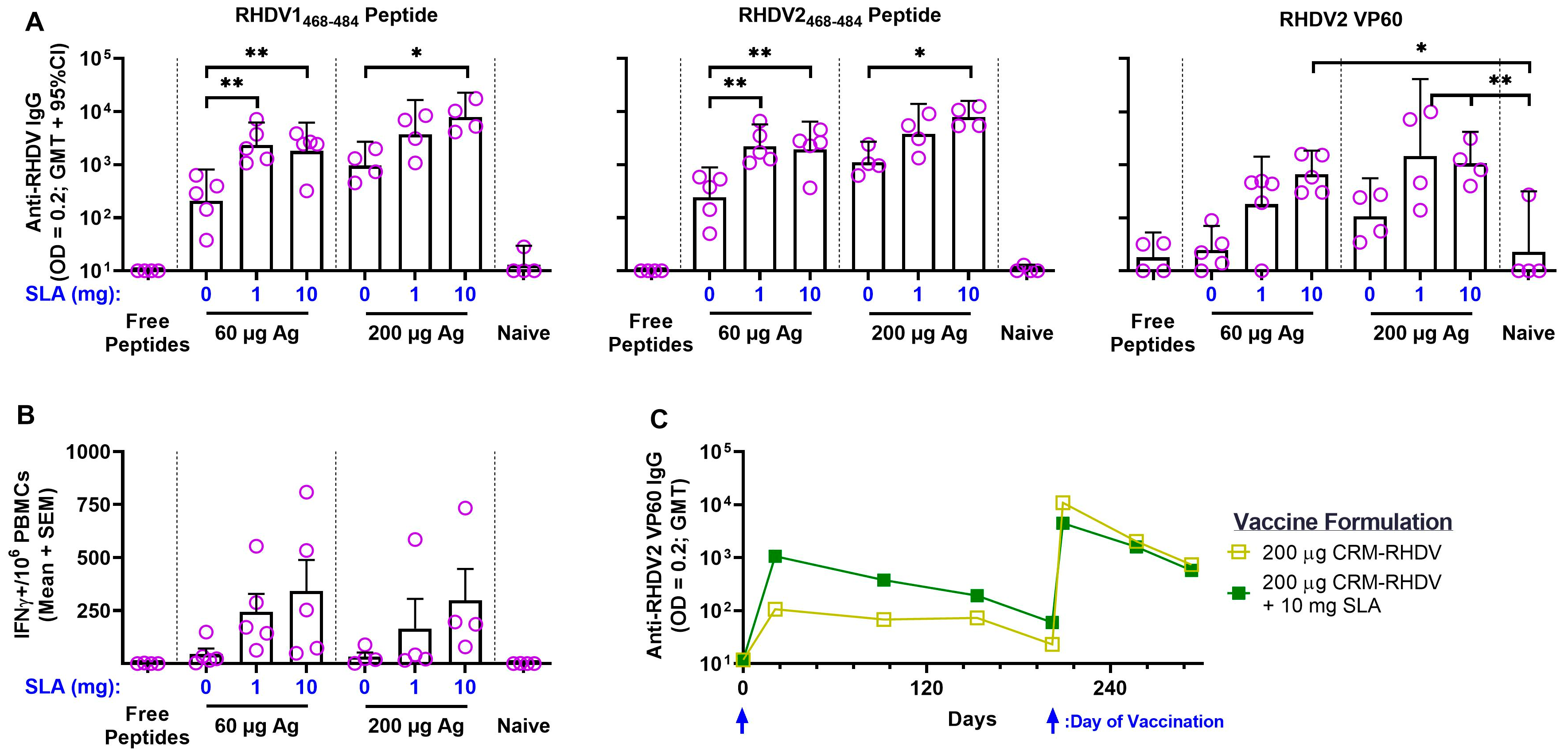
3.1. RHDV–CRM197 Peptide Conjugates
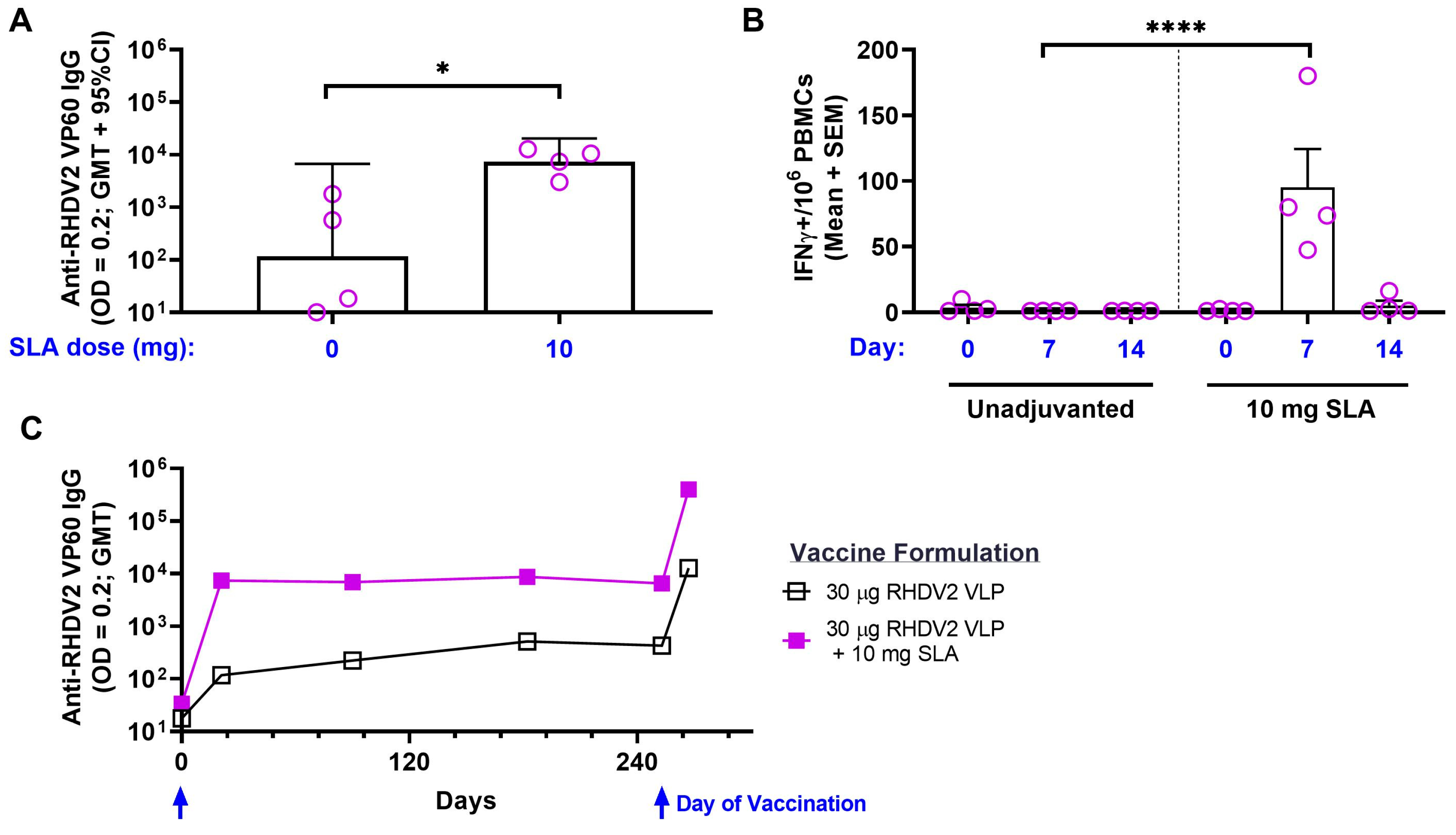
3.2. RHDV2 VLPs
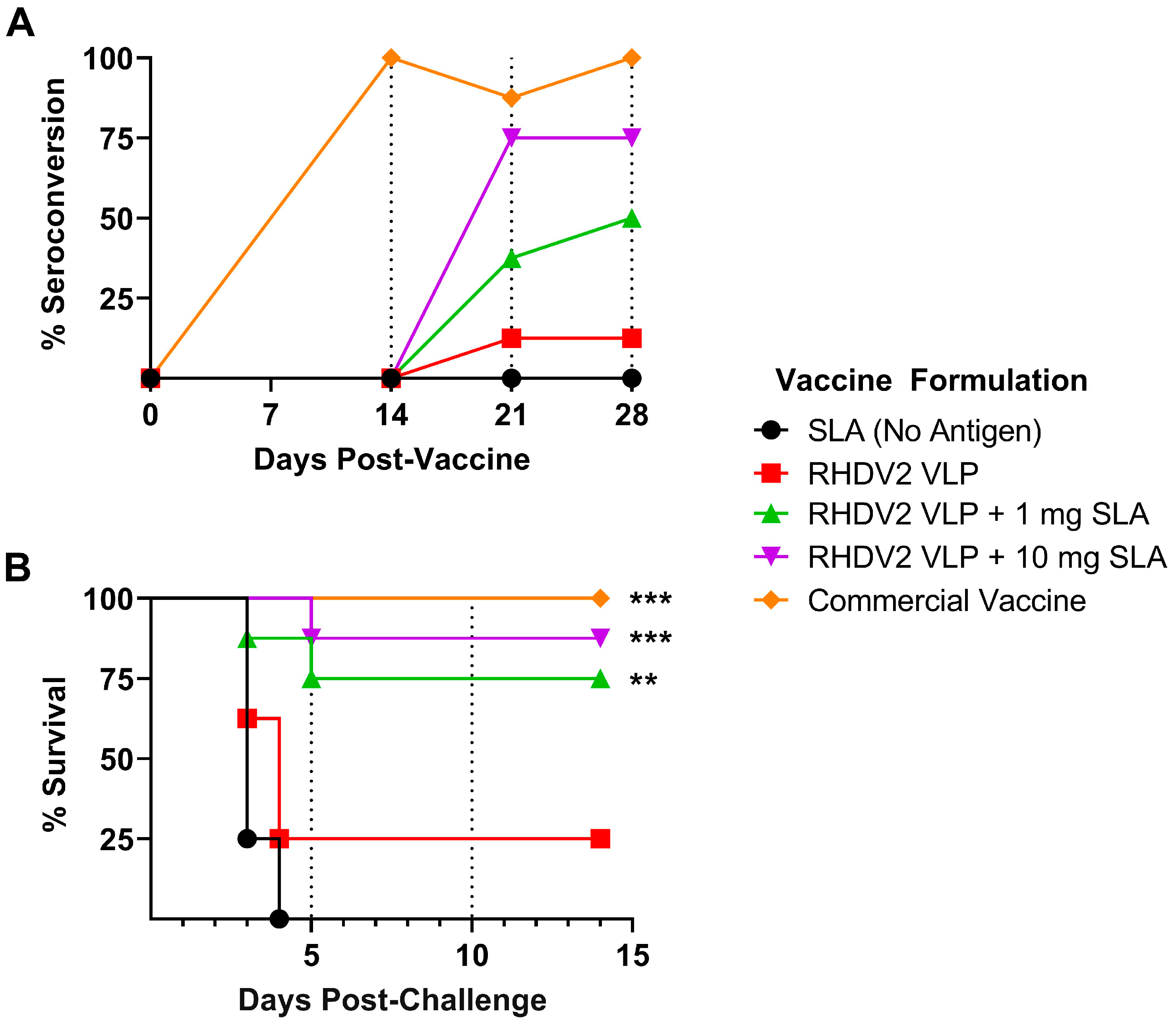
3.3. Efficacy of SLA-Adjuvanted Vaccine Formulations in RHDV2 Challenge Model
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Akache, B.; Stark, F.C.; Agbayani, G.; Renner, T.M.; McCluskie, M.J. Adjuvants: Engineering Protective Immune Responses in Human and Veterinary Vaccines. Vaccine Des. 2022, 2412, 179–231. [Google Scholar] [CrossRef]
- Facciolà, A.; Visalli, G.; Laganà, A.; Di Pietro, A. An Overview of Vaccine Adjuvants: Current Evidence and Future Perspectives. Vaccines 2022, 10, 819. [Google Scholar] [CrossRef] [PubMed]
- Heidary, M.; Kaviar, V.H.; Shirani, M.; Ghanavati, R.; Motahar, M.; Sholeh, M.; Ghahramanpour, H.; Khoshnood, S. A Comprehensive Review of the Protein Subunit Vaccines Against COVID-19. Front. Microbiol. 2022, 13, 927306. [Google Scholar] [CrossRef] [PubMed]
- Chong, P.L.-G.; Chang, A.; Yu, A.; Mammedova, A. Vesicular and Planar Membranes of Archaea Lipids: Unusual Physical Properties and Biomedical Applications. Int. J. Mol. Sci. 2022, 23, 7616. [Google Scholar] [CrossRef]
- Adamiak, N.; Krawczyk, K.T.; Locht, C.; Kowalewicz-Kulbat, M. Archaeosomes and Gas Vesicles as Tools for Vaccine Development. Front. Immunol. 2021, 12, 746235. [Google Scholar] [CrossRef] [PubMed]
- Haq, K.; Jia, Y.; Krishnan, L. Archaeal lipid vaccine adjuvants for induction of cell-mediated immunity. Expert Rev. Vaccines 2016, 15, 1557–1566. [Google Scholar] [CrossRef]
- Akache, B.; Stark, F.C.; Jia, Y.; Deschatelets, L.; Dudani, R.; Harrison, B.A.; Agbayani, G.; Williams, D.; Jamshidi, M.P.; Krishnan, L.; et al. Sulfated archaeol glycolipids: Comparison with other immunological adjuvants in mice. PLoS ONE 2018, 13, e0208067. [Google Scholar] [CrossRef]
- Akache, B.; Deschatelets, L.; Harrison, B.A.; Dudani, R.; Stark, F.C.; Jia, Y.; Landi, A.; Law, J.L.M.; Logan, M.; Hockman, D.; et al. Effect of Different Adjuvants on the Longevity and Strength of Humoral and Cellular Immune Responses to the HCV Envelope Glycoproteins. Vaccines 2019, 7, 204. [Google Scholar] [CrossRef]
- Stark, F.C.; Akache, B.; Ponce, A.; Dudani, R.; Deschatelets, L.; Jia, Y.; Sauvageau, J.; Williams, D.; Jamshidi, M.P.; Agbayani, G.; et al. Archaeal glycolipid adjuvanted vaccines induce strong influenza-specific immune responses through direct immunization in young and aged mice or through passive maternal immunization. Vaccine 2019, 37, 7108–7116. [Google Scholar] [CrossRef]
- Perera, D.J.; Hassan, A.S.; Jia, Y.; Ricciardi, A.; McCluskie, M.J.; Weeratna, R.D.; Ndao, M. Adjuvanted Schistosoma mansoni-Cathepsin B With Sulfated Lactosyl Archaeol Archaeosomes or AddaVax™ Provides Protection in a Pre-Clinical Schistosomiasis Model. Front. Immunol. 2020, 11, 605288. [Google Scholar] [CrossRef]
- Akache, B.; Renner, T.M.; Tran, A.; Deschatelets, L.; Dudani, R.; Harrison, B.A.; Duque, D.; Haukenfrers, J.; Rossotti, M.A.; Gaudreault, F.; et al. Immunogenic and efficacious SARS-CoV-2 vaccine based on resistin-trimerized spike antigen SmT1 and SLA archaeosome adjuvant. Sci. Rep. 2021, 11, 21849. [Google Scholar] [CrossRef] [PubMed]
- Akache, B.; Renner, T.M.; Stuible, M.; Rohani, N.; Cepero-Donates, Y.; Deschatelets, L.; Dudani, R.; Harrison, B.A.; Gervais, C.; Hill, J.J.; et al. Immunogenicity of SARS-CoV-2 spike antigens derived from Beta & Delta variants of concern. Npj Vaccines 2022, 7, 118. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Akache, B.; Deschatelets, L.; Qian, H.; Dudani, R.; Harrison, B.A.; Stark, F.C.; Chandan, V.; Jamshidi, M.P.; Krishnan, L.; et al. A comparison of the immune responses induced by antigens in three different archaeosome-based vaccine formulations. Int. J. Pharm. 2019, 561, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Akache, B.; Stark, F.C.; Iqbal, U.; Chen, W.; Jia, Y.; Krishnan, L.; McCluskie, M.J. Safety and biodistribution of sulfated archaeal glycolipid archaeosomes as vaccine adjuvants. Hum. Vaccines Immunother. 2018, 14, 1746–1759. [Google Scholar] [CrossRef] [PubMed]
- Agbayani, G.; Jia, Y.; Akache, B.; Chandan, V.; Iqbal, U.; Stark, F.C.; Deschatelets, L.; Lam, E.; Hemraz, U.D.; Régnier, S.; et al. Mechanistic insight into the induction of cellular immune responses by encapsulated and admixed archaeosome-based vaccine formulations. Hum. Vaccines Immunother. 2020, 16, 2183–2195. [Google Scholar] [CrossRef]
- Régnier, S.; Lam, E.; Vasquez, V.; Martinez-Farina, C.F.; Stark, F.C.; Agbayani, G.; Deschatelets, L.; Dudani, R.; Harrison, B.A.; Akache, B.; et al. Effect of Chiral Purity on Adjuvanticity of Archaeol-Based Glycolipids. J. Med. Chem. 2022, 65, 8332–8344. [Google Scholar] [CrossRef]
- Carpenter, A.; Waltenburg, M.A.; Hall, A.; Kile, J.; Killerby, M.; Knust, B.; Negron, M.; Nichols, M.; Wallace, R.M.; Behravesh, C.B.; et al. Vaccine Preventable Zoonotic Diseases: Challenges and Opportunities for Public Health Progress. Vaccines 2022, 10, 993. [Google Scholar] [CrossRef]
- Müller, C.; Hrynkiewicz, R.; Bębnowska, D.; Maldonado, J.; Baratelli, M.; Köllner, B.; Niedźwiedzka-Rystwej, P. Immunity against Lagovirus europaeus and the Impact of the Immunological Studies on Vaccination. Vaccines 2021, 9, 255. [Google Scholar] [CrossRef]
- Meyers, G.; Wirblich, C.; Thiel, H.-J.; Thumfart, J. Rabbit Hemorrhagic Disease Virus: Genome Organization and Polyprotein Processing of a Calicivirus Studied after Transient Expression of cDNA Constructs. Virology 2000, 276, 349–363. [Google Scholar] [CrossRef]
- Reemers, S.; Peeters, L.; Van Schijndel, J.; Bruton, B.; Sutton, D.; Van Der Waart, L.; Van De Zande, S. Novel Trivalent Vectored Vaccine for Control of Myxomatosis and Disease Caused by Classical and a New Genotype of Rabbit Haemorrhagic Disease Virus. Vaccines 2020, 8, 441. [Google Scholar] [CrossRef]
- Rocchi, M.S.; Dagleish, M.P. Diagnosis and prevention of rabbit viral haemorrhagic disease 2. Practice 2018, 40, 11–16. [Google Scholar] [CrossRef]
- Hanley, K.A. The Double-Edged Sword: How Evolution Can Make or Break a Live-Attenuated Virus Vaccine. Evol. Educ. Outreach 2011, 4, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Zhu, J.; Tan, Y.; Li, C.; Chen, Z.; Sun, S.; Liu, G. Self-assembly of virus-like particles of rabbit hemorrhagic disease virus capsid protein expressed in Escherichia coli and their immunogenicity in rabbits. Antivir. Res. 2016, 131, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Nagesha, H.S.; Wang, L.F.; Hyatt, A.D.; Morrissy, C.J.; Lenghaus, C.; Westbury, H.A. Self-assembly, antigenicity, and immunogenicity of the rabbit haemorrhagic disease virus (Czechoslovakian strain V-351) capsid protein expressed in baculovirus. Arch. Virol. 1995, 140, 1095–1108. [Google Scholar] [CrossRef]
- Yang, N.-K.; Kim, H.-H.; Nah, J.-J.; Song, J.-Y. Rabbit Hemorrhagic Disease Virus Variant Recombinant VP60 Protein Induces Protective Immunogenicity. J. Microbiol. Biotechnol. 2015, 25, 1960–1965. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.; Ulrich, R.; Schinköthe, J.; Müller, M.; Köllner, B. Characterization of protective humoral and cellular immune responses against RHDV2 induced by a new vaccine based on recombinant baculovirus. Vaccine 2019, 37, 4195–4203. [Google Scholar] [CrossRef] [PubMed]
- Miao, Q.; Qi, R.; Veldkamp, L.; Ijzer, J.; Kik, M.L.; Zhu, J.; Tang, A.; Dong, D.; Shi, Y.; van Oers, M.M.; et al. Immunogenicity in Rabbits of Virus-Like Particles from a Contemporary Rabbit Haemorrhagic Disease Virus Type 2 (GI.2/RHDV2/b) Isolated in The Netherlands. Viruses 2019, 11, 553. [Google Scholar] [CrossRef]
- Zhu, J.; Miao, Q.; Tang, J.; Wang, X.; Dong, D.; Liu, T.; Qi, R.; Yang, Z.; Liu, G. Nucleolin mediates the internalization of rabbit hemorrhagic disease virus through clathrin-dependent endocytosis. PLoS Pathog. 2018, 14, e1007383. [Google Scholar] [CrossRef] [PubMed]
- Khatuntseva, E.; Nifantiev, N. Cross reacting material (CRM197) as a carrier protein for carbohydrate conjugate vaccines targeted at bacterial and fungal pathogens. Int. J. Biol. Macromol. 2022, 218, 775–798. [Google Scholar] [CrossRef]
- Sprott, G.D.; Yeung, A.; Dicaire, C.J.; Yu, S.H.; Whitfield, D.M. Synthetic Archaeosome Vaccines Containing Triglycosylarchaeols Can Provide Additive and Long-Lasting Immune Responses That Are Enhanced by Archaetidylserine. Archaea 2012, 2012, 513231. [Google Scholar] [CrossRef]
- Whitfield, D.M.; Yu, S.H.; Dicaire, C.J.; Sprott, G.D. Development of new glycosylation methodologies for the synthesis of archaeal-derived glycolipid adjuvants. Carbohydr. Res. 2010, 345, 214–229. [Google Scholar] [CrossRef] [PubMed]
- Akache, B.; Jia, Y.; Chandan, V.; Deschatelets, L.; McCluskie, M.J. Generation of a Liposomal Vaccine Adjuvant Based on Sulfated S-Lactosylarchaeol (SLA) Glycolipids. Methods Mol. Biol. 2022, 2412, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Chandan, V.; Akache, B.; Qian, H.; Jakubek, Z.J.; Vinogradov, E.; Dudani, R.; Harrison, B.A.; Jamshidi, M.P.; Stark, F.C.; et al. Assessment of stability of sulphated lactosyl archaeol archaeosomes for use as a vaccine adjuvant. J. Liposome Res. 2021, 31, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Akache, B.; Stark, F.C.; McCluskie, M.J. Measurement of Antigen-Specific IgG Titers by Direct ELISA. Methods Mol. Biol. 2021, 2183, 537–547. [Google Scholar] [CrossRef]
- Akache, B.; McCluskie, M.J. The Quantification of Antigen-Specific T Cells by IFN-γ ELISpot. Methods Mol. Biol. 2021, 2183, 525–536. [Google Scholar] [CrossRef]
- O’connor, T.W.; Read, A.J.; Hall, R.N.; Strive, T.; Kirkland, P.D. Immunological Cross-Protection between Different Rabbit Hemorrhagic Disease Viruses—Implications for Rabbit Biocontrol and Vaccine Development. Vaccines 2022, 10, 666. [Google Scholar] [CrossRef]
- Read, A.; Kirkland, P. Efficacy of a commercial vaccine against different strains of rabbit haemorrhagic disease virus. Aust. Vet. J. 2017, 95, 223–226. [Google Scholar] [CrossRef]
- Hall, R.N.; Mahar, J.E.; Read, A.J.; Mourant, R.; Piper, M.; Huang, N.; Strive, T. A strain-specific multiplex RT-PCR for Australian rabbit haemorrhagic disease viruses uncovers a new recombinant virus variant in rabbits and hares. Transbound. Emerg. Dis. 2018, 65, e444–e456. [Google Scholar] [CrossRef]
- Pichichero, M.E. Protein carriers of conjugate vaccines: Characteristics, development and clinical trials. Hum. Vaccines Immunother. 2013, 9, 2505–2523. [Google Scholar] [CrossRef]
- Qi, R.; Miao, Q.; Zhu, J.; Tang, J.; Tang, A.; Wang, X.; Dong, D.; Guo, H.; Liu, G. Construction and immunogenicity of novel bivalent virus-like particles bearing VP60 genes of classic RHDV(GI.1) and RHDV2(GI.2). Vet. Microbiol. 2020, 240, 108529. [Google Scholar] [CrossRef]
- Vecchi, S.; Bufali, S.; Skibinski, D.A.; O’Hagan, D.T.; Singh, M. Aluminum Adjuvant Dose Guidelines in Vaccine Formulation for Preclinical Evaluations. J. Pharm. Sci. 2012, 101, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Garçon, N.; Di Pasquale, A. From discovery to licensure, the Adjuvant System story. Hum. Vaccines Immunother. 2017, 13, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Pon, R.; Marcil, A.; Chen, W.; Gadoury, C.; Williams, D.; Chan, K.; Zhou, H.; Ponce, A.; Paquet, E.; Gurnani, K.; et al. Masking terminal neo-epitopes of linear peptides through glycosylation favours immune responses towards core epitopes producing parental protein bound antibodies. Sci. Rep. 2020, 10, 18497. [Google Scholar] [CrossRef]
- McCluskie, M.J.; Evans, D.M.; Zhang, N.; Benoit, M.; McElhiney, S.P.; Unnithan, M.; DeMarco, S.C.; Clay, B.; Huber, C.; Deora, A.; et al. The effect of preexisting anti-carrier immunity on subsequent responses to CRM197or Qb-VLP conjugate vaccines. Immunopharmacol. Immunotoxicol. 2016, 38, 184–196. [Google Scholar] [CrossRef]

| CRM197 Conjugate | Weight (Da) | Molar Ratio | Peptide % |
|---|---|---|---|
| Untagged | 58,511 | N/A | 0 |
| CRM197–RHDV1 | 64,551 | 3.04 | 9.3 |
| CRM197–RHDV2 | 64,675 | 3 | 9.5 |
| Vaccine Formulation | |||||
|---|---|---|---|---|---|
| Animal | SLA | RHDV2 VLP | RHDV2 VLP + 1 mg SLA | RHDV2 VLP + 10 mg SLA | Commercial Vaccine |
| 1 | 8.4 | ND | ND | ND | ND |
| 2 | 10.5 | ND | ND | ND | ND |
| 3 | 10.2 | 11 | ND | ND | ND |
| 4 | 9.7 | 10.2 | ND | ND | ND |
| 5 | 9.1 | 9.8 | 33.1 | ND | ND |
| 6 | 7 | 9.2 | 25.1 | ND | ND |
| 7 | 6.8 | 8.6 | 9.9 | 21.8 | ND |
| 8 | 6.8 | 8.5 | 9.1 | 9 | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akache, B.; Read, A.J.; Dudani, R.; Harrison, B.A.; Williams, D.; Deschatelets, L.; Jia, Y.; Chandan, V.; Stark, F.C.; Agbayani, G.; et al. Sulfated Lactosyl Archaeol Archaeosome-Adjuvanted Vaccine Formulations Targeting Rabbit Hemorrhagic Disease Virus Are Immunogenic and Efficacious. Vaccines 2023, 11, 1043. https://doi.org/10.3390/vaccines11061043
Akache B, Read AJ, Dudani R, Harrison BA, Williams D, Deschatelets L, Jia Y, Chandan V, Stark FC, Agbayani G, et al. Sulfated Lactosyl Archaeol Archaeosome-Adjuvanted Vaccine Formulations Targeting Rabbit Hemorrhagic Disease Virus Are Immunogenic and Efficacious. Vaccines. 2023; 11(6):1043. https://doi.org/10.3390/vaccines11061043
Chicago/Turabian StyleAkache, Bassel, Andrew J. Read, Renu Dudani, Blair A. Harrison, Dean Williams, Lise Deschatelets, Yimei Jia, Vandana Chandan, Felicity C. Stark, Gerard Agbayani, and et al. 2023. "Sulfated Lactosyl Archaeol Archaeosome-Adjuvanted Vaccine Formulations Targeting Rabbit Hemorrhagic Disease Virus Are Immunogenic and Efficacious" Vaccines 11, no. 6: 1043. https://doi.org/10.3390/vaccines11061043
APA StyleAkache, B., Read, A. J., Dudani, R., Harrison, B. A., Williams, D., Deschatelets, L., Jia, Y., Chandan, V., Stark, F. C., Agbayani, G., Makinen, S. R., Hemraz, U. D., Lam, E., Régnier, S., Zou, W., Kirkland, P. D., & McCluskie, M. J. (2023). Sulfated Lactosyl Archaeol Archaeosome-Adjuvanted Vaccine Formulations Targeting Rabbit Hemorrhagic Disease Virus Are Immunogenic and Efficacious. Vaccines, 11(6), 1043. https://doi.org/10.3390/vaccines11061043






