Epidemiological Characterization of African Swine Fever Dynamics in Ukraine, 2012–2023
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Sources
2.2. Spatio-Temporal Epidemiology and Geographic Information System (GIS) Analyses
3. Results
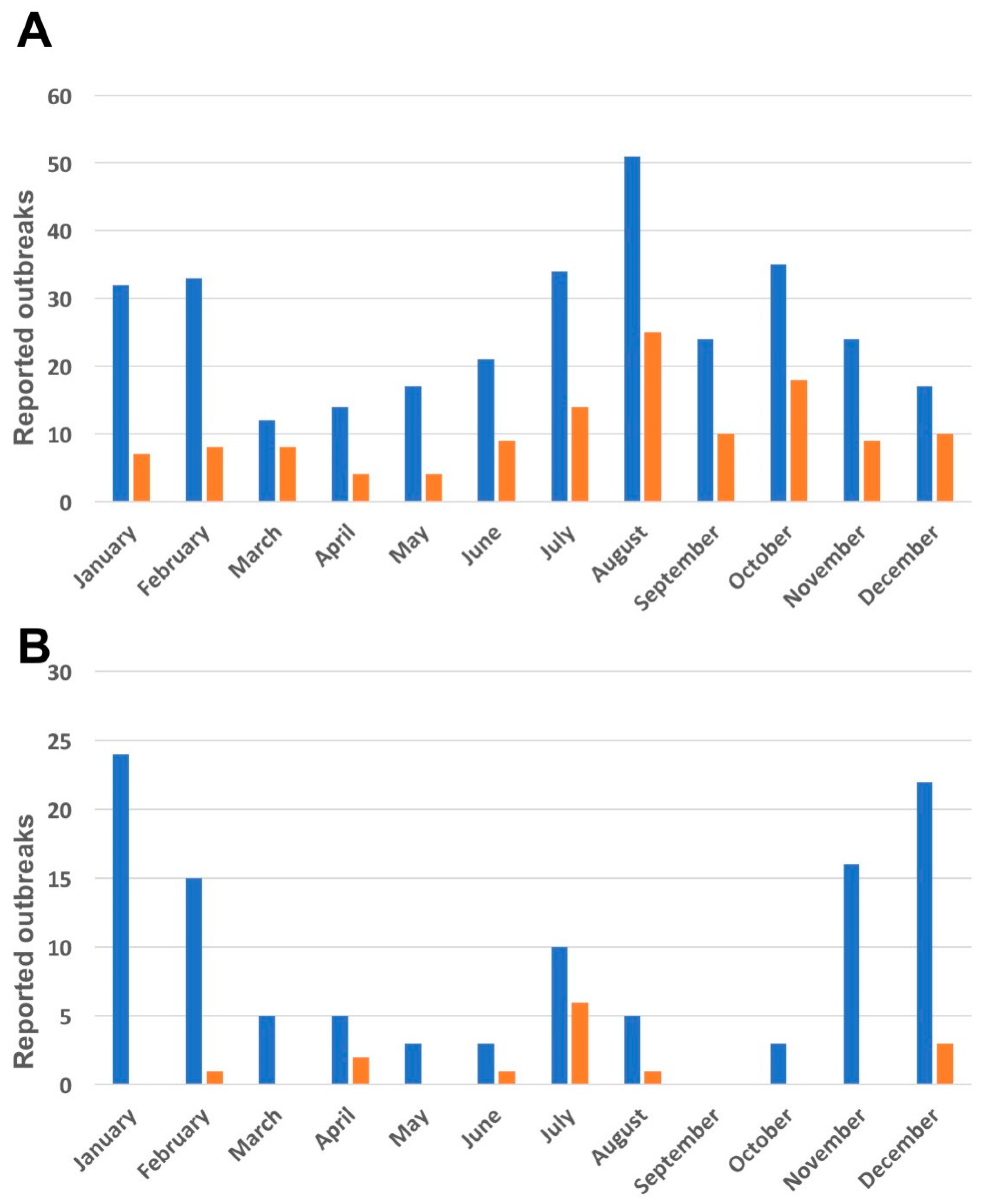
3.1. Seasonal Trends in ASF Outbreaks in Ukraine
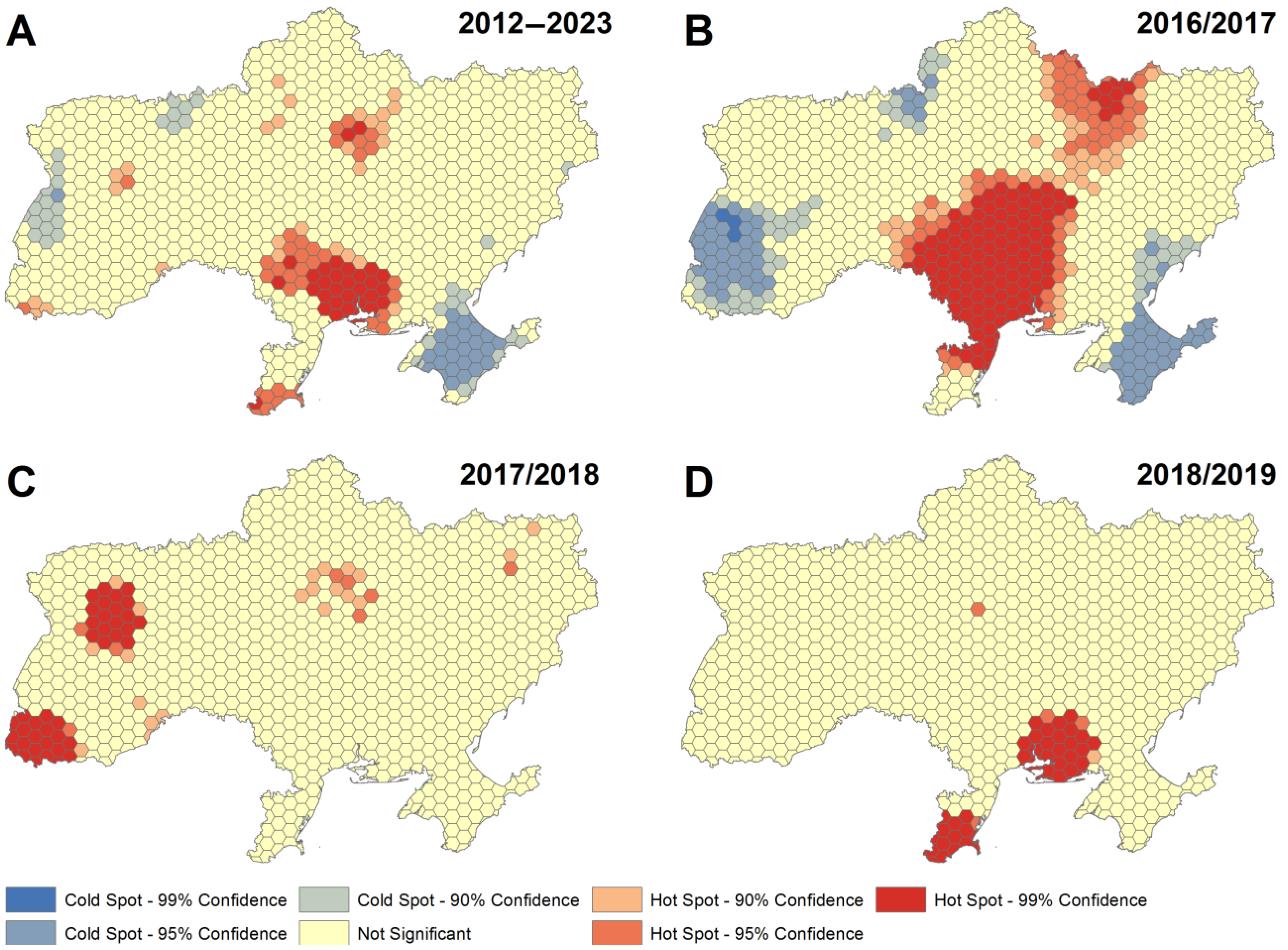
3.2. Spatio-Temporal Analysis of ASF Outbreak Clusters, Velocity of Spread and Geographic Patterns
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sánchez-Vizcaíno, J.M.; Mur, L.; Gomez-Villamandos, J.C.; Carrasco, L. An Update on the Epidemiology and Pathology of African Swine Fever. J. Comp. Pathol. 2015, 152, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, R.E. On A Form of Swine Fever Occurring in British East Africa (Kenya Colony). J. Comp. Pathol. Ther. 1921, 34, 159–191. [Google Scholar] [CrossRef] [Green Version]
- DeTray, D.E. African swine fever. Adv. Vet. Sci. 1963, 8, 299–333. [Google Scholar] [PubMed]
- Heuschele, W.P.; Coggins, L. Isolation of African swine fever virus from a giant forest hog. Bull. Epizoot. Dis. Afr. 1965, 13, 255–262. [Google Scholar]
- Heuschele, W.P.; Coggins, L. Epizootiology of African swine fever virus in warthogs. Bull. Epizoot. Dis. Afr. 1969, 17, 179–183. [Google Scholar] [PubMed]
- Parker, J.; Plowright, W.; A Pierce, M. The epizootiology of African swine fever in Africa. Veter. Rec. 1969, 85, 668–674. [Google Scholar]
- Thomson, G.R. The epizootiology of African swine fever: The role of the free-living hosts in Africa. Onderstepoort J. Vet. Res. 1985, 52, 201–209. [Google Scholar] [PubMed]
- Anderson, E.; Hutchings, G.; Mukarati, N.; Wilkinson, P. African swine fever virus infection of the bushpig (Potamochoerus porcus) and its significance in the epidemiology of the disease. Veter. Microbiol. 1998, 62, 1–15. [Google Scholar] [CrossRef]
- Kleiboeker, S.B.; Scoles, G.A.; Burrage, T.G.; Sur, J.-H. African Swine Fever Virus Replication in the Midgut Epithelium Is Required for Infection of Ornithodoros Ticks. J. Virol. 1999, 73, 8587–8598. [Google Scholar] [CrossRef] [Green Version]
- Schulz, K.; Staubach, C.; Blome, S.; Nurmoja, I.; Viltrop, A.; Conraths, F.J.; Kristian, M.; Sauter-Louis, C. How to Demonstrate Freedom from African Swine Fever in Wild Boar—Estonia as an Example. Vaccines 2020, 8, 336. [Google Scholar] [CrossRef]
- Costard, S.; Mur, L.; Lubroth, J.; Sanchez-Vizcaino, J.M.; Pfeiffer, D.U. Epidemiology of African swine fever virus. Virus Res. 2013, 173, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Mur, L.; Atzeni, M.; Martínez-López, B.; Feliziani, F.; Rolesu, S.; Sanchez-Vizcaino, J.M. Thirty-Five-Year Presence of African Swine Fever in Sardinia: History, Evolution and Risk Factors for Disease Maintenance. Transbound. Emerg. Dis. 2016, 63, e165–e177. [Google Scholar] [CrossRef] [PubMed]
- Beltran Alcrudo, D.; Lubroth, J.; Depner, K.; De La Rocque, S. African Swine Fever in the Caucasus. EMPRES Watch 2008 (April), 1–8. Available online: http://www.fao.org/docs/eims/upload/242232/ew_caucasus_apr08.pdf (accessed on 1 May 2023).
- Rowlands, R.J.; Michaud, V.; Heath, L.; Hutchings, G.; Oura, C.; Vosloo, W.; Dwarka, R.; Onashvili, T.; Albina, E.; Dixon, L.K. African Swine Fever Virus Isolate, Georgia, 2007. Emerg. Infect. Dis. 2008, 14, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Śmietanka, K.; Woźniakowski, G.; Kozak, E.; Niemczuk, K.; Frączyk, M.; Bocian, Ł.; Kowalczyk, A.; Pejsak, Z. African Swine Fever Epidemic, Poland, 2014–2015. Emerg. Infect. Dis. 2016, 22, 1201–1207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolbasov, D.; Titov, I.; Tsybanov, S.; Gogin, A.; Malogolovkin, A. African Swine Fever Virus, Siberia, Russia, 2017. Emerg. Infect. Dis. 2018, 24, 796–798. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Li, N.; Luo, Y.; Liu, Y.; Miao, F.; Chen, T.; Zhang, S.; Cao, P.; Li, X.; Tian, K.; et al. Emergence of African Swine Fever in China, 2018. Transbound. Emerg. Dis. 2018, 65, 1482–1484. [Google Scholar] [CrossRef] [Green Version]
- Kovalenko, G.; Ducluzeau, A.-L.; Ishchenko, L.; Sushko, M.; Sapachova, M.; Rudova, N.; Solodiankin, O.; Gerilovych, A.; Dagdag, R.; Redlinger, M.; et al. Complete Genome Sequence of a Virulent African Swine Fever Virus from a Domestic Pig in Ukraine. Genome Announc. 2019, 8, e00883-19. [Google Scholar] [CrossRef] [Green Version]
- Cwynar, P.; Stojkov, J.; Wlazlak, K. African Swine Fever Status in Europe. Viruses 2019, 11, 310. [Google Scholar] [CrossRef] [Green Version]
- Le, V.P.; Jeong, D.G.; Yoon, S.-W.; Kwon, H.-M.; Trinh, T.B.N.; Nguyen, T.L.; Bui, T.T.N.; Oh, J.; Kim, J.B.; Cheong, K.M.; et al. Outbreak of African Swine Fever, Vietnam, 2019. Emerg. Infect. Dis. 2019, 25, 1433–1435. [Google Scholar] [CrossRef]
- Kim, H.-J.; Cho, K.-H.; Lee, S.-K.; Kim, D.-Y.; Nah, J.-J.; Kim, H.-J.; Kim, H.-J.; Hwang, J.-Y.; Sohn, H.-J.; Choi, J.G.; et al. Outbreak of African swine fever in South Korea. Transbound. Emerg. Dis. 2020, 67, 473–475. [Google Scholar] [CrossRef]
- Ankhanbaatar, U.; Sainnokhoi, T.; Khanui, B.; Ulziibat, G.; Jargalsaikhan, T.; Purevtseren, D.; Settypalli, T.B.K.; Flannery, J.; Dundon, W.G.; Basan, G.; et al. African swine fever virus genotype II in Mongolia, 2019. Transbound. Emerg. Dis. 2021, 68, 2787–2794. [Google Scholar] [CrossRef] [PubMed]
- Hyeon, J.-Y.; Tseren-Ochir, E.-O.; Lee, D.-H.; Nahm, S.-S.; Gladue, D.P.; Borca, M.V.; Song, C.-S.; Risatti, G.R. Whole genome sequencing and phylogenetic analysis of African swine fever virus detected in a backyard pig in Mongolia, 2019. Front. Veter. Sci. 2023, 10, 1094052. [Google Scholar] [CrossRef] [PubMed]
- Richter, M.; Schulz, K.; Elflein, T.; Achterberg, J.; Oļševskis, E.; Seržants, M.; Lamberga, K.; Conraths, F.J.; Sauter-Louis, C. The First Eighteen Months of African Swine Fever in Wild Boar in Saxony, Germany and Latvia—A Comparison. Pathogens 2023, 12, 87. [Google Scholar] [CrossRef]
- Forth, J.H.; Calvelage, S.; Fischer, M.; Hellert, J.; Sehl-Ewert, J.; Roszyk, H.; Deutschmann, P.; Reichold, A.; Lange, M.; Thulke, H.-H.; et al. African swine fever virus—variants on the rise. Emerg. Microbes Infect. 2023, 12. [Google Scholar] [CrossRef] [PubMed]
- Probst, C.; Globig, A.; Knoll, B.; Conraths, F.J.; Depner, K. Behaviour of free ranging wild boar towards their dead fellows: Potential implications for the transmission of African swine fever. R. Soc. Open Sci. 2017, 4, 170054. [Google Scholar] [CrossRef] [Green Version]
- Chenais, E.; Depner, K.; Guberti, V.; Dietze, K.; Viltrop, A.; Ståhl, K. Epidemiological considerations on African swine fever in Europe 2014–2018. Porc. Health Manag. 2019, 5, 6. [Google Scholar] [CrossRef]
- Mazur-Panasiuk, N.; Żmudzki, J.; Woźniakowski, G. African swine fever virus—persistence in different environmental conditions and the possibility of its indirect transmission. J. Veter. Res. 2019, 63, 303–310. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.-J.; Lee, M.-J.; Lee, S.-K.; Kim, D.-Y.; Seo, S.-J.; Kang, H.-E.; Nam, H.-M. African Swine Fever Virus in Pork Brought into South Korea by Travelers from China, August 2018. Emerg. Infect. Dis. 2019, 25, 1231–1233. [Google Scholar] [CrossRef]
- Pepin, K.M.; Golnar, A.J.; Abdo, Z.; Podgórski, T. Ecological drivers of African swine fever virus persistence in wild boar populations: Insight for control. Ecol. Evol. 2020, 10, 2846–2859. [Google Scholar] [CrossRef] [Green Version]
- Kurian, A.; Hall, W.; Neumann, E. African swine fever: A New Zealand perspective on epidemiological risk factors for its occurrence. N. Z. Veter. J. 2021, 69, 135–146. [Google Scholar] [CrossRef]
- Bellini, S.; Rutili, D.; Guberti, V. Preventive measures aimed at minimizing the risk of African swine fever virus spread in pig farming systems. Acta Veter. Scand. 2016, 58, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korennoy, F.I.; Gulenkin, V.M.; Gogin, A.E.; Vergne, T.; Karaulov, A.K. Estimating the Basic Reproductive Number for African Swine Fever Using the Ukrainian Historical Epidemic of 1977. Transbound. Emerg. Dis. 2017, 64, 1858–1866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Getis, A.; Ord, J.K. The Analysis of Spatial Association by Use of Distance Statistics. Geogr. Anal. 1992, 24, 189–206. [Google Scholar] [CrossRef]
- Blome, S.; Gabriel, C.; Beer, M. Pathogenesis of African swine fever in domestic pigs and European wild boar. Virus Res. 2013, 173, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Dixon, L.K.; Stahl, K.; Jori, F.; Vial, L.; Pfeiffer, D.U. African Swine Fever Epidemiology and Control. Annu. Rev. Anim. Biosci. 2020, 8, 221–246. [Google Scholar] [CrossRef] [Green Version]
- Kempeneers, P.; Sedano, F.; Seebach, L.; Strobl, P.; San-Miguel-Ayanz, J. Data Fusion of Different Spatial Resolution Remote Sensing Images Applied to Forest-Type Mapping. IEEE Trans. Geosci. Remote. Sens. 2011, 49, 4977–4986. [Google Scholar] [CrossRef]
- Päivinen, R.; Lehikoinen, M.; Schuck, A.; Häme, T.; Väätäinen, S.; Andersson, K.; Kennedy, P.; Folving, S. Combining Earth Observation Data and Forest Statistics; EFI Research Report 14; European Forest Institute, Joint Research Centre—European Commission, EUR 19911 EN: Joensuu, Finland, 2001; 101p. [Google Scholar]
- Schuck, A.; Van Brusselen, J.; Päivinen, R.; Häme, T.; Kennedy, P.; Folving, S. Compilation of a Calibrated European Forest Map Derived from NOAA-AVHRR Data; EFI Internal Report; European Forest Institute: Joensuu, Finland, 2002; Volume 13, 44p. [Google Scholar]
- Gilbert, M.; Cinardi, G.; Zhao, Q.; Tago, D.; Robinson, T. New Global Pig Data in Support of the African Swine Fever Epidemics. Harvard Dataverse. 2019. V1. Available online: https://dataverse.harvard.edu/dataset.xhtml?persistentId=doi:10.7910/DVN/JEV3WA (accessed on 1 May 2023).
- Pejsak, Z.; Truszczyński, M.; Niemczuk, K.; Kozak, E.; Markowska-Daniel, I. Epidemiology of African Swine Fever in Poland since the detection of the first case. Pol. J. Veter. Sci. 2014, 17, 665–672. [Google Scholar] [CrossRef] [Green Version]
- Oļševskis, E.; Guberti, V.; Serzants, M.; Westergaard, J.; Gallardo, C.; Rodze, I.; Depner, K. African swine fever virus introduction into the EU in 2014: Experience of Latvia. Res. Vet. Sci. 2016, 105, 28–30. [Google Scholar] [CrossRef]
- Nurmoja, I.; Schulz, K.; Staubach, C.; Sauter-Louis, C.; Depner, K.; Conraths, F.J.; Viltrop, A. Development of African swine fever epidemic among wild boar in Estonia—Two different areas in the epidemiological focus. Sci. Rep. 2017, 7, 12562. [Google Scholar] [CrossRef] [Green Version]
- Pautienius, A.; Grigas, J.; Pileviciene, S.; Zagrabskaite, R.; Buitkuviene, J.; Pridotkas, G.; Stankevicius, R.; Streimikyte, Z.; Salomskas, A.; Zienius, D.; et al. Prevalence and spatiotemporal distribution of African swine fever in Lithuania, 2014–2017. Virol. J. 2018, 15, 177. [Google Scholar] [CrossRef]
- Frant, M.P.; Gal-Cisoń, A.; Bocian, Ł.; Ziętek-Barszcz, A.; Niemczuk, K.; Szczotka-Bochniarz, A. African Swine Fever (ASF) Trend Analysis in Wild Boar in Poland (2014–2020). Animals 2022, 12, 1170. [Google Scholar] [CrossRef]
- Frant, M.P.; Gal-Cisoń, A.; Bocian, Ł.; Ziętek-Barszcz, A.; Niemczuk, K.; Woźniakowski, G.; Szczotka-Bochniarz, A. African Swine Fever in Wild Boar (Poland 2020): Passive and Active Surveillance Analysis and Further Perspectives. Pathogens 2021, 10, 1219. [Google Scholar] [CrossRef]
- Podgórski, T.; Śmietanka, K. Do wild boar movements drive the spread of African Swine Fever? Transbound. Emerg. Dis. 2018, 65, 1588–1596. [Google Scholar] [CrossRef] [PubMed]
- Gulenkin, V.M.; Korennoy, F.I.; Karaulov, A.K.; Dudnikov, S.A. Cartographical analysis of African swine fever outbreaks in the territory of the Russian Federation and computer modeling of the basic reproduction ratio. Prev. Veter. Med. 2011, 102, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Gogin, A.; Gerasimov, V.; Malogolovkin, A.; Kolbasov, D. African swine fever in the North Caucasus region and the Russian Federation in years 2007–2012. Virus Res. 2013, 173, 198–203. [Google Scholar] [CrossRef]
- Andraud, M.; Bougeard, S.; Chesnoiu, T.; Rose, N. Spatiotemporal clustering and Random Forest models to identify risk factors of African swine fever outbreak in Romania in 2018–2019. Sci. Rep. 2021, 11, 2098. [Google Scholar] [CrossRef] [PubMed]
- Pittiglio, C.; Khomenko, S.; Beltran-Alcrudo, D. Wild boar mapping using population-density statistics: From polygons to high resolution raster maps. PLoS ONE 2018, 13, e0193295. [Google Scholar] [CrossRef] [Green Version]
- Bocian, Ł.; Frant, M.; Ziętek-Barszcz, A.; Niemczuk, K.; Szczotka-Bochniarz, A. Dynamics of the African swine fever spread in Poland. J. Veter. Res. 2022, 66, 459–471. [Google Scholar] [CrossRef]
- Podgórski, T.; Borowik, T.; Łyjak, M.; Woźniakowski, G. Spatial epidemiology of African swine fever: Host, landscape and anthropogenic drivers of disease occurrence in wild boar. Prev. Veter. Med. 2019, 177, 104691. [Google Scholar] [CrossRef]
- Frant, M.; Lyjak, M.; Bocian, L.; Barszcz, A.; Niemczuk, K.; Wozniakowski, G. African swine fever virus (ASFV) in Poland:Prevalence in a wild boar population (2017–2018). Vet. Med. 2020, 65, 143–158. [Google Scholar] [CrossRef] [Green Version]
- Muñoz-Gómez, V.; Solodiankin, O.; Rudova, N.; Gerilovych, A.; Nychyk, S.; Hudz, N.; Ukhovska, T.; Sytiuk, M.; Polischuk, V.; Mustra, D.; et al. Supporting control programs on African swine fever in Ukraine through a knowledge, attitudes, and practices survey targeting backyard farmers. Veter. Med. Sci. 2021, 7, 1786–1799. [Google Scholar] [CrossRef] [PubMed]
- Costard, S.; Zagmutt, F.J.; Porphyre, T.; Pfeiffer, D.U. Small-scale pig farmers’ behavior, silent release of African swine fever virus and consequences for disease spread. Sci. Rep. 2015, 5, 17074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danzetta, M.L.; Marenzoni, M.L.; Iannetti, S.; Tizzani, P.; Calistri, P.; Feliziani, F. African Swine Fever: Lessons to Learn From Past Eradication Experiences. A Systematic Review. Front. Veter. Sci. 2020, 7, 296. [Google Scholar] [CrossRef] [PubMed]
- ADNS (Animal Disease Notification System). Available online: https://ec.europa.eu/food/animals/animal-diseases/not-system_en (accessed on 7 August 2020).
- Omelchenko, H.; Avramenko, N.O.; Petrenko, M.O.; Wojciechowski, J.; Pejsak, Z.; Woźniakowski, G. Ten Years of African Swine Fever in Ukraine: An Endemic Form of the Disease in the Wild Boar Population as a Threat to Domestic Pig Production. Pathogens 2022, 11, 1459. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bezymennyi, M.; Tarasov, O.; Kyivska, G.V.; Mezhenska, N.A.; Mandyhra, S.; Kovalenko, G.; Sushko, M.; Hudz, N.; Skorokhod, S.V.; Datsenko, R.; et al. Epidemiological Characterization of African Swine Fever Dynamics in Ukraine, 2012–2023. Vaccines 2023, 11, 1145. https://doi.org/10.3390/vaccines11071145
Bezymennyi M, Tarasov O, Kyivska GV, Mezhenska NA, Mandyhra S, Kovalenko G, Sushko M, Hudz N, Skorokhod SV, Datsenko R, et al. Epidemiological Characterization of African Swine Fever Dynamics in Ukraine, 2012–2023. Vaccines. 2023; 11(7):1145. https://doi.org/10.3390/vaccines11071145
Chicago/Turabian StyleBezymennyi, Maksym, Oleksandr Tarasov, Ganna V. Kyivska, Nataliia A. Mezhenska, Svitlana Mandyhra, Ganna Kovalenko, Mykola Sushko, Nataliia Hudz, Serhii V. Skorokhod, Roman Datsenko, and et al. 2023. "Epidemiological Characterization of African Swine Fever Dynamics in Ukraine, 2012–2023" Vaccines 11, no. 7: 1145. https://doi.org/10.3390/vaccines11071145
APA StyleBezymennyi, M., Tarasov, O., Kyivska, G. V., Mezhenska, N. A., Mandyhra, S., Kovalenko, G., Sushko, M., Hudz, N., Skorokhod, S. V., Datsenko, R., Muzykina, L., Milton, E., Sapachova, M. A., Nychyk, S., Halka, I., Frant, M., Huettmann, F., Drown, D. M., Gerilovych, A., ... Lange, C. E. (2023). Epidemiological Characterization of African Swine Fever Dynamics in Ukraine, 2012–2023. Vaccines, 11(7), 1145. https://doi.org/10.3390/vaccines11071145








