Recipient-Reported Reactogenicity of Different SARS-CoV-2 Vaccination Regimens among Healthcare Professionals and Police Staff in Germany
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Design and Data Collection Procedures
2.3. Questionnaire
2.4. Statistical Analysis
3. Results
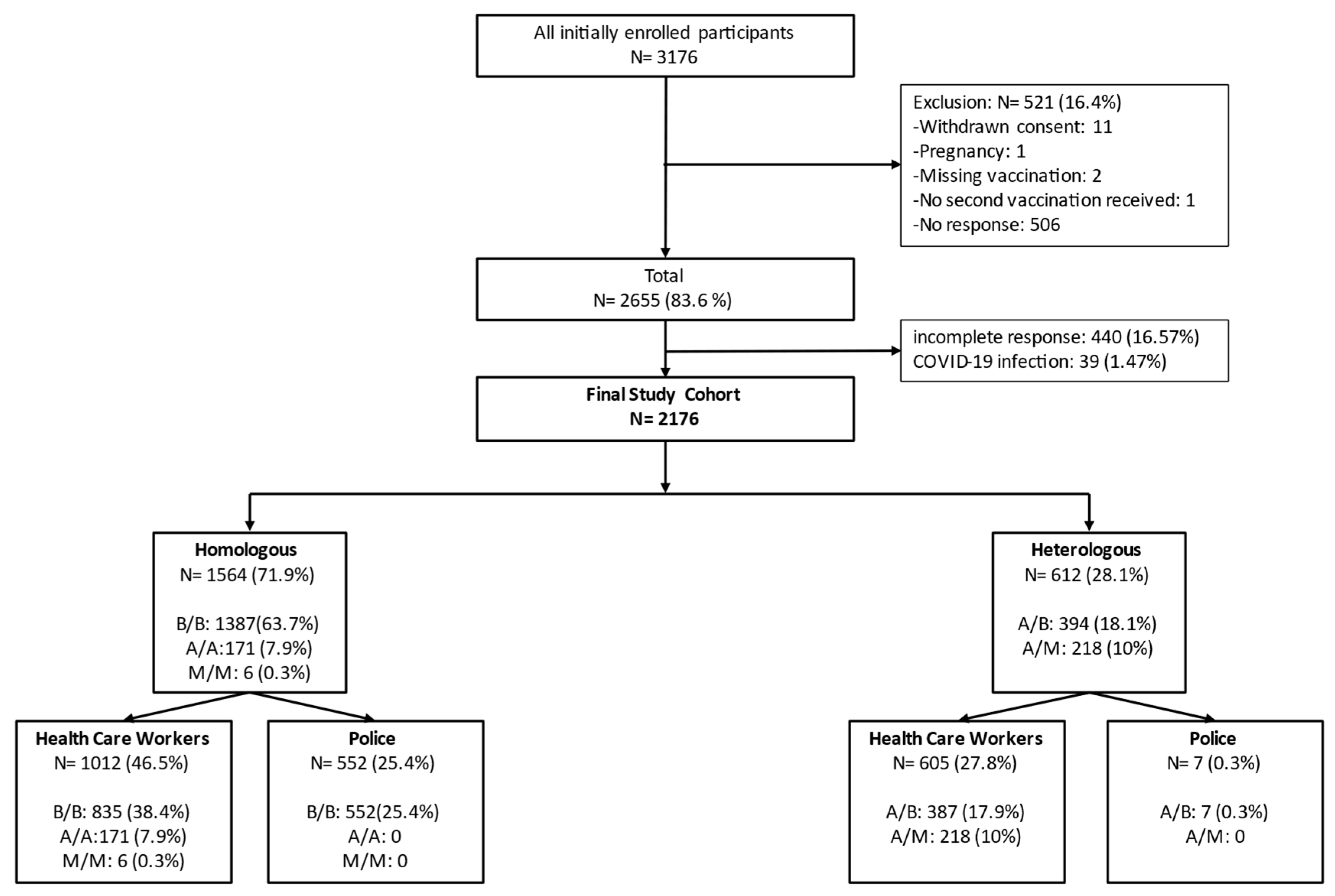
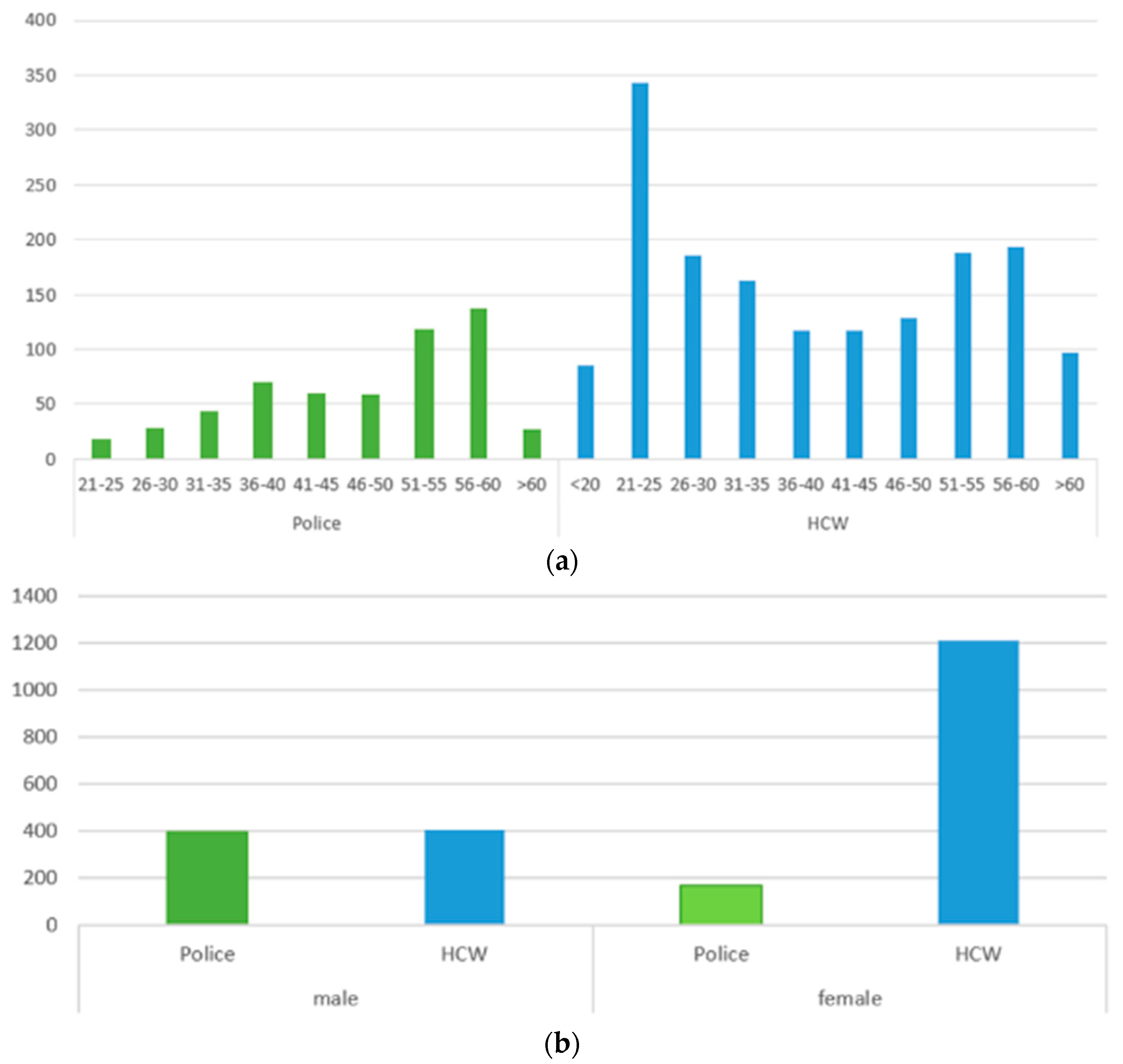
3.1. Study Profile and Epidemiological Characteristics
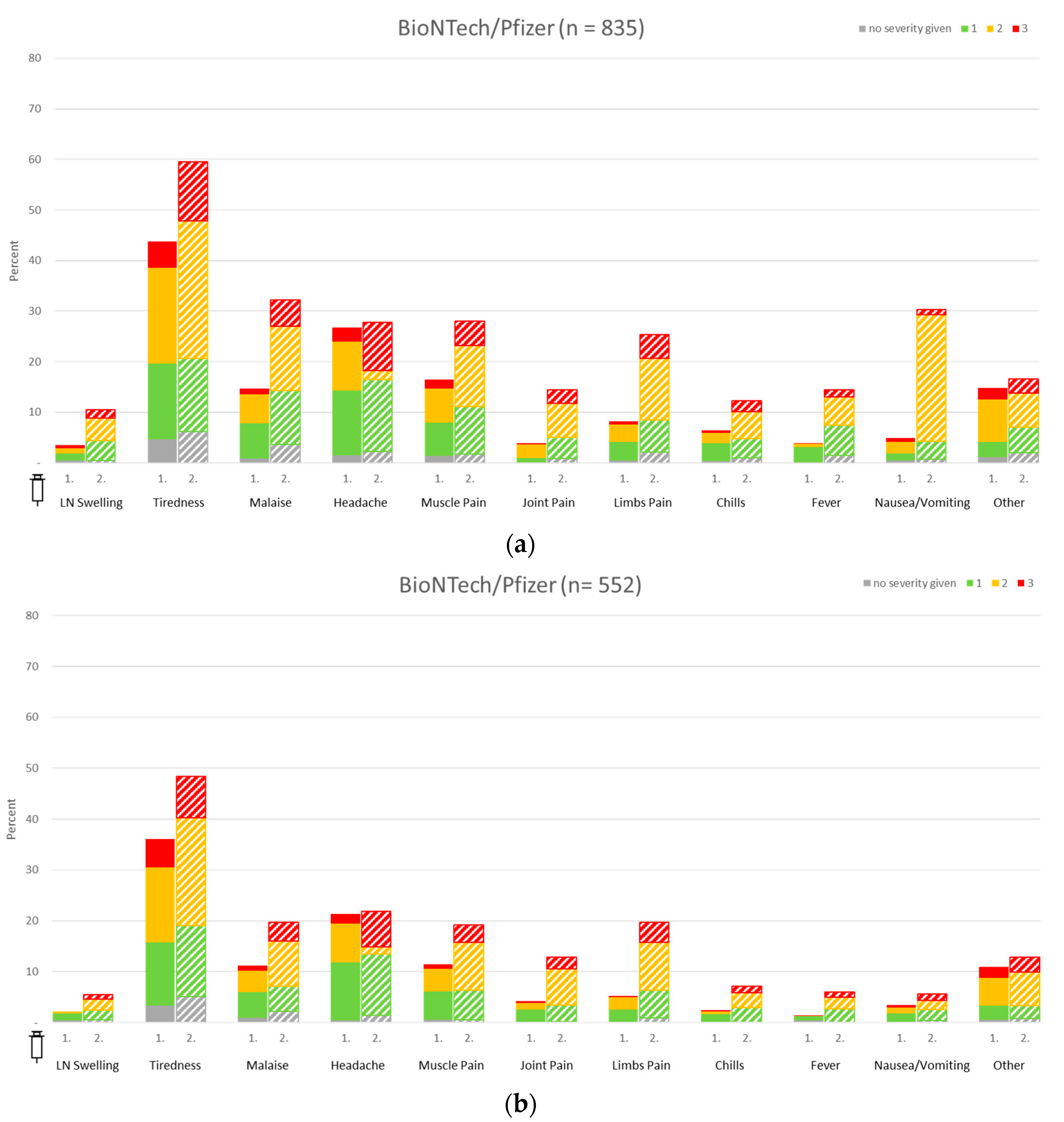
3.2. Local Vaccine Reactions in HCW and Police Staff
3.3. Systemic Vaccine Reactions in Healthcare Workers and Police Staff
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Rothe, C.; Schunk, M.; Sothmann, P.; Bretzel, G.; Froeschl, G.; Wallrauch, C.; Zimmer, T.; Thiel, V.; Janke, C.; Guggemos, W.; et al. Transmission of 2019-nCoV Infection from an Asymptomatic Contact in Germany. N. Engl. J. Med. 2020, 382, 970–971. [Google Scholar] [CrossRef]
- The Lancet. Global governance for COVID-19 vaccines. Lancet 2020, 395, 1883. [Google Scholar] [CrossRef] [PubMed]
- The Recovery Collaborative Group. Dexamethasone in Hospitalized Patients with COVID-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Feldt, T.; Guggemos, W.; Heim, K.; Jensen, B.; Kellner, N.; Kobbe, R.; Koch, T.; Lübbert, C.; Mikolajewska, A.; Niebank, M.; et al. Hinweise zu Erkennung, Diagnostik und Therapie von Patienten mit COVID-19. In Ständiger Arbeitskr der Kompetenz-und Behandlungszentren für Krankheiten Durch Hochpathogene Erreger am Robert Koch-Institut; Robert Koch Institute: Berlin, Germany, 2021; pp. 1–25. [Google Scholar] [CrossRef]
- Shah, S.; Chawla, R. Cancer in corona times. Saudi J. Anaesth. 2020, 14, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Anderson, E.J.; Rouphael, N.G.; Widge, A.T.; Jackson, L.A.; Roberts, P.C.; Makhene, M.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; Pruijssers, A.J.; et al. Safety and Immunogenicity of SARS-CoV-2 mRNA-1273 Vaccine in Older Adults. N. Engl. J. Med. 2020, 383, 2427–2438. [Google Scholar] [CrossRef]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- Sun, Y.; Lin, W.; Dong, W.; Xu, J. Origin and evolutionary analysis of the SARS-CoV-2 Omicron variant. J. Biosaf. Biosecurity 2022, 4, 33–37. [Google Scholar] [CrossRef]
- Goutam Mukherjee, A.; Ramesh Wanjari, U.; Murali, R.; Chaudhary, U.; Renu, K.; Madhyastha, H.; Iyer, M.; Vellingiri, B.; Gopalakrishnan, A.V. Omicron variant infection and the associated immunological scenario. Immunobiology 2022, 227, 152222. [Google Scholar] [CrossRef]
- Lauring, A.S.; Tenforde, M.W.; Chappell, J.D.; Gaglani, M.; A Ginde, A.; McNeal, T.; Ghamande, S.; Douin, D.J.; Talbot, H.K.; Casey, J.D.; et al. Clinical severity of, and effectiveness of mRNA vaccines against, COVID-19 from omicron, delta, and alpha SARS-CoV-2 variants in the United States: Prospective observational study. BMJ 2022, 376, e069761. [Google Scholar] [CrossRef] [PubMed]
- Barda, N.; Dagan, N.; Cohen, C.; A Hernán, M.; Lipsitch, M.; Kohane, I.S.; Reis, B.Y.; Balicer, R.D. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: An observational study. Lancet 2021, 398, 2093–2100. [Google Scholar] [CrossRef] [PubMed]
- Chenchula, S.; Karunakaran, P.; Sharma, S.; Chavan, M. Current evidence on efficacy of COVID-19 booster dose vaccination against the Omicron variant: A systematic review. J. Med. Virol. 2022, 94, 2969–2976. [Google Scholar] [CrossRef] [PubMed]
- Sánchez Van Kammen, M.; Aguiar De Sousa, D.; Poli, S.; Cordonnier, C.; Heldner, M.R.; van de Munckhof, A.; Krzywicka, K.; van Haaps, T.; Ciccone, A.; Middeldorp, S.; et al. Characteristics and Outcomes of Patients with Cerebral Venous Sinus Thrombosis in SARS-CoV-2 Vaccine-Induced Immune Thrombotic Thrombocytopenia. JAMA Neurol. 2021, 78, 1314–1323. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.; Klemis, V.; Schub, D.; Mihm, J.; Hielscher, F.; Marx, S.; Abu-Omar, A.; Ziegler, L.; Guckelmus, C.; Urschel, R.; et al. Immunogenicity and reactogenicity of heterologous ChAdOx1 nCoV-19/mRNA vaccination. Nat. Med. 2021, 27, 1530–1535. [Google Scholar] [CrossRef]
- Rashedi, R.; Samieefar, N.; Masoumi, N.; Mohseni, S.; Rezaei, N. COVID-19 vaccines mix-and-match: The concept, the efficacy and the doubts. J. Med. Virol. 2022, 94, 1294–1299. [Google Scholar] [CrossRef]
- Noushad, M.; Rastam, S.; Nassani, M.Z.; Al-Saqqaf, I.S.; Hussain, M.; Yaroko, A.A.; Arshad, M.; Kirfi, A.M.; Koppolu, P.; Niazi, F.H.; et al. A Global Survey of COVID-19 Vaccine Acceptance among Healthcare Workers. Front. Public Health 2022, 9, 2437. [Google Scholar] [CrossRef]
- Hajure, M.; Tariku, M.; Bekele, F.; Abdu, Z.; Dule, A.; Mohammedhussein, M.; Tsegaye, T. Attitude towards COVID-19 vaccination among healthcare workers: A systematic review. Infect. Drug Resist. 2021, 14, 3883–3897. [Google Scholar] [CrossRef]
- Lindner-Pawłowicz, K.; Mydlikowska-śmigórska, A.; Łampika, K.; Sobieszczańska, M. COVID-19 Vaccination Acceptance among Healthcare Workers and General Population at the Very Beginning of the National Vaccination Program in Poland: A Cross-Sectional, Exploratory Study. Vaccines 2022, 10, 66. [Google Scholar] [CrossRef]
- Abukhalil, A.D.; Shatat, S.S.; Abushehadeh, R.R.; Al-Shami, N.; Naseef, H.A.; Rabba, A. Side effects of Pfizer/BioNTech (BNT162b2) COVID-19 vaccine reported by the Birzeit University community. BMC Infect. Dis. 2023, 23, 5. [Google Scholar] [CrossRef]
- Mohammed, R.A.; Yazbik, R.S.; Baajajah, L.H.; Alharthy, S.F.; Alsalahi, H.; Mahjaa, M.A.; Barakat, M.M.; Badawy, M.I.; Sultan, I. Side Effects Associated With Homologous and Heterologous COVID-19 Vaccines: A Cross-Sectional Study in Saudi Arabia. Cureus 2023, 15, e34030. [Google Scholar] [CrossRef]
- Ho, T.C.; Chen, Y.M.A.; Chan, H.P.; Chang, C.-C.; Chuang, K.-P.; Lee, C.-H.; Yuan, C.-H.; Tyan, Y.-C.; Yang, M.-H. The effects of heterologous immunization with prime-boost COVID-19 vaccination against SARS-CoV-2. Vaccines 2021, 9, 1163. [Google Scholar] [CrossRef]
- Der Befragten, D.B. COVID-19 Impfquoten-Monitoring in Deutschland (COVIMO); Robert Koch Institute: Berlin, Germany, 2021; pp. 19–22. [Google Scholar]
- Langer, R.; Thanner, M. Exploratory Research in Clinical and Social Pharmacy Pharmacists’ attitudes toward influenza vaccination: Does the COVID-19 pandemic make a difference? Explor. Res. Clin. Soc. Pharm. 2023, 9, 100235. [Google Scholar] [CrossRef]
- On, K.; Li, K.; In, W.; Tang, A. Since January 2020 Elsevier has created a COVID-19 resource centre with free information in English and Mandarin on the novel coronavirus COVID-19. The COVID-19 resource centre is hosted on Elsevier Connect, the company’ s public news and information. Int. J. Nurs. Stud. 2020, 114, 103854. [Google Scholar]
- Durovic, A.M.; Widmer, A.F.; Dangel, M.; Ulrich, A.; Battegay, M.; Tschudin-Sutter, S. Low rates of influenza vaccination uptake among healthcare workers: Distinguishing barriers between occupational groups. Am. J. Infect. Control 2020, 48, 1139–1143. [Google Scholar] [CrossRef]
- Ibrahim, A.M.; Hamayoun, M.; Farid, M.; Al-Umra, U.; Shube, M.; Sumaili, K.; Shamalla, L.; Malik, S.M.M.R. COVID-19 Vaccine Acceptance and Hesitancy in Health Care Workers in Somalia: Findings from a Fragile Country with No Previous Experience of Mass Adult Immunization. Vaccines 2023, 11, 858. [Google Scholar] [CrossRef]
- Vaux, S.; Fonteneau, L.; Péfau, M.; Venier, A.G.; Gautier, A.; Altrach, S.S.; Parneix, P.; Levy-Bruhl, D. Acceptability of mandatory vaccination against influenza, measles, pertussis and varicella by workers in healthcare facilities: A national cross-sectional study, France, 2019. Arch. Public Health 2023, 81, 51. [Google Scholar] [CrossRef] [PubMed]
- Beattie, A.; Palmer, K.; Rees, E.; Riddell, Z.; Roberts, C.; Jordan, R. Factors Affecting the Acceptance of Pandemic Influenza A H1N1 Vaccine amongst Essential Service Providers: A Cross Sectional Study. Vaccines 2012, 1, 17–33. [Google Scholar] [CrossRef] [PubMed]
- Caban-Martinez, A.J.; Gaglani, M.; Olsho, L.E.W.; Grant, L.; Schaefer-Solle, N.; Thompson, M.G.; Burgess, J.L. COVID-19 Vaccination Perspectives and Illnesses Among Law Enforcement Officers, Firefighters, and Other First Responders in the US, January to September 2021. JAMA Netw. Open. 2022, 5, e2222640. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rau, K.; von Heeringen, E.; Bühler, N.; Wagenpfeil, S.; Becker, S.L.; Schneitler, S. Recipient-Reported Reactogenicity of Different SARS-CoV-2 Vaccination Regimens among Healthcare Professionals and Police Staff in Germany. Vaccines 2023, 11, 1147. https://doi.org/10.3390/vaccines11071147
Rau K, von Heeringen E, Bühler N, Wagenpfeil S, Becker SL, Schneitler S. Recipient-Reported Reactogenicity of Different SARS-CoV-2 Vaccination Regimens among Healthcare Professionals and Police Staff in Germany. Vaccines. 2023; 11(7):1147. https://doi.org/10.3390/vaccines11071147
Chicago/Turabian StyleRau, Katharina, Edgar von Heeringen, Nina Bühler, Stefan Wagenpfeil, Sören L. Becker, and Sophie Schneitler. 2023. "Recipient-Reported Reactogenicity of Different SARS-CoV-2 Vaccination Regimens among Healthcare Professionals and Police Staff in Germany" Vaccines 11, no. 7: 1147. https://doi.org/10.3390/vaccines11071147
APA StyleRau, K., von Heeringen, E., Bühler, N., Wagenpfeil, S., Becker, S. L., & Schneitler, S. (2023). Recipient-Reported Reactogenicity of Different SARS-CoV-2 Vaccination Regimens among Healthcare Professionals and Police Staff in Germany. Vaccines, 11(7), 1147. https://doi.org/10.3390/vaccines11071147





