COVID-19 Vaccination Rate under Different Political Incentive: A Counterfactual Trend Approach Using Nationwide Data
Abstract
:1. Introduction
2. Methods
2.1. Geographical Units
2.2. Vaccination and Its Evolution
2.3. Outcomes
2.4. Covariates
2.4.1. COVID-19 Measures
- The details of this calculation, based on the public list of all deaths in France provided by the national institute of statistics (INSEE) [41], is available in Supplementary Material.
2.4.2. Socio-Economic Conditions
- The rate of poverty. In France, poverty is defined as having an income below 60% that of the national median income.
- The rate of the population above 25 years old without secondary education.
- The income inequality, defined as the ratio between the higher and the lower decile of the income distribution.
2.4.3. Access to COVID-19 Healthcare
- Distance from the centre of each EPCI to the closest vaccination centre;
- Number of pharmacy and family physician per person in each EPCI, since both were allowed to vaccinate (although only family physicians could prescribe the vaccination);
- Cumulated number of vaccine doses per person at the departmental level delivered up to 12 July 2021, and cumulated number of doses per person remaining to be vaccinated during the month after 12 July.
2.4.4. Territory
2.4.5. Political Leanings
2.5. Statistical Analysis
3. Results
3.1. Vaccination before Health Pass
3.2. Vaccination after Health Pass
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, H.; Paulson, K.R.; Pease, S.A.; Watson, S.; Comfort, H.; Zheng, P.; Aravkin, A.Y.; Bisignano, B.; Barber, R.M.; Alam, T.; et al. Estimating excess mortality due to the COVID-19 pandemic: A systematic analysis of COVID-19-related mortality, 20–2021. Lancet 2022, 399, 1513–1536. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Xu, E.; Bowe, B.; Al-Aly, Z. Long-term cardiovascular outcomes of COVID-19. Nat. Med. 2022, 28, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Park, D.W.; Yang, Y. Delay, Death, and Heterogeneity of Primary PCI During the COVID-19 Pandemic. J. Am. Coll. Cardiol. 2020, 76, 2331–2333. [Google Scholar] [CrossRef] [PubMed]
- Larson, D.W.; Aziz, M.A.A.E.; Mandrekar, J.N. How Many Lives Will Delay of Colon Cancer Surgery Cost During the COVID-19 Pandemic? An Analysis Based on the US National Cancer Database. Mayo Clin. Proc. 2020, 95, 1805–1807. [Google Scholar] [CrossRef] [PubMed]
- Thorne, L.G.; Bouhaddou, M.; Reuschl, A.K.; Zuliani-Alvarez, L.; Polacco, B.; Pelin, A.; Batra, J.; Whelan, M.V.X.; Hosmillo, M.; Fossati, A.; et al. Evolution of enhanced innate immune evasion by SARS-CoV-2. Nature 2022, 602, 487–495. [Google Scholar] [CrossRef]
- Miller, J.; Hachmann, N.P.; Collier, A.Y.; Lasrado, N.; Mazurek, C.R.; Patio, R.C.; Powers, O.; Surve, N.; Beth Israel Deaconess Medical Center; Theiler, J.; et al. Substantial Neutralization Escape by SARS-CoV-2 Omicron Variants BQ.1.1 and XBB.1. N. Engl. J. Med. 2023, 388, 662–664. [Google Scholar] [CrossRef]
- Ge, Y.; Zhang, W.B.; Wu, X.; Ruktanonchai, C.W.; Liu, H.; Wang, J.; Song, Y.; Liu, M.; Yan, W.; Yang, J.; et al. Untangling the changing impact of non-pharmaceutical interventions and vaccination on European COVID-19 trajectories. Nat. Commun. 2022, 13, 3106. [Google Scholar] [CrossRef]
- Watson, O.J.; Barnsley, G.; Toor, J.; Hogan, A.B.; Winskill, P.; Ghani, A.C. Global impact of the first year of COVID-19 vaccination: A mathematical modelling study. Lancet Infect Dis. 2022, 22, 1293–1302. [Google Scholar] [CrossRef]
- Feikin, D.R.; Higdon, M.M.; Abu-Raddad, L.J.; Andrews, N.; Araos, R.; Goldberg, Y.; Groome, M.J.; Huppert, A.; O’Brien, K.L.; Smith, P.G.; et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: Results of a systematic review and meta-regression. Lancet 2022, 399, 924–944. [Google Scholar] [CrossRef]
- Marani, M.; Katul, G.G.; Pan, W.K.; Parolari, A.J. Intensity and frequency of extreme novel epidemics. Proc. Natl. Acad. Sci. USA 2021, 118, e2105482118. [Google Scholar] [CrossRef]
- Caiazzo, V.; Witkoski Stimpfel, A. Vaccine hesitancy in American healthcare workers during the COVID-19 vaccine roll out: An integrative review. Public Health 2022, 207, 94–104. [Google Scholar] [CrossRef]
- Dhanani, L.Y.; Franz, B. A meta-analysis of COVID-19 vaccine attitudes and demographic characteristics in the United States. Public Health 2022, 207, 31–38. [Google Scholar] [CrossRef]
- Schwarzinger, M.; Watson, V.; Arwidson, P.; Alla, F.; Luchini, S. COVID-19 vaccine hesitancy in a representative working-age population in France: A survey experiment based on vaccine characteristics. Lancet Public Health 2021, 6, e210–e221. [Google Scholar] [CrossRef]
- Lazarus, J.V.; Wyka, K.; White, T.M.; Picchio, C.A.; Rabin, K.; Ratzan, S.C.; Leigh, J.P.; Hu, J.; El-Mohandes, A. Revisiting COVID-19 vaccine hesitancy around the world using data from 23 countries in 2021. Nat. Commun. 2022, 13, 3801. [Google Scholar] [CrossRef]
- Sharif, A.; Botlero, R.; Hoque, N.; Alif, S.M.; Karim, M.N.; Islam, S.M.S. A pragmatic approach to COVID-19 vaccine passport. BMJ Glob. Health 2021, 6, e006956. [Google Scholar] [CrossRef]
- Bardosh, K.; de Figueiredo, A.; Gur-Arie, R.; Jamrozik, E.; Doidge, J.; Lemmens, T.; Keshavjee, S.; Graham, J.E.; Baral, S. The unintended consequences of COVID-19 vaccine policy: Why mandates, passports and restrictions may cause more harm than good. BMJ Glob. Health 2022, 7, e008684. [Google Scholar] [CrossRef]
- Smith, M.J. Evaluating potential unintended consequences of COVID-19 vaccine mandates and passports. BMJ Glob. Health 2022, 7, e009759. [Google Scholar] [CrossRef]
- Bullock, J.; Lane, J.E.; Shults, F.L. What causes COVID-19 vaccine hesitancy? Ignorance and the lack of bliss in the United Kingdom. Humanit. Soc. Sci. Commun. 2022, 9, 87. [Google Scholar] [CrossRef]
- Murphy, J.; Vallières, F.; Bentall, R.P.; Shevlin, M.; McBride, O.; Hartman, T.K.; McKay, R.; Bennett, K.; Mason, L.; Gibson-Miller, J.; et al. Psychological characteristics associated with COVID-19 vaccine hesitancy and resistance in Ireland and the United Kingdom. Nat. Commun. 2021, 12, 29. [Google Scholar] [CrossRef]
- Weitzer, J.; Birmann, B.M.; Steffelbauer, I.; Bertau, M.; Zenk, L.; Caniglia, G.; Laubichler, M.D.; Steiner, G.; Schernhammer, E.S. Willingness to receive an annual COVID-19 booster vaccine in the German-speaking D-A-CH region in Europe: A cross-sectional study. Lancet Reg. Health—Eur. 2022, 18, 100414. [Google Scholar] [CrossRef]
- Soares, P.; Rocha, J.V.; Moniz, M.; Gama, A.; Laires, P.A.; Pedro, A.R.; Pedro, A.R.; Dias, S.; Leite, A.; Nunes, C. Factors Associated with COVID-19 Vaccine Hesitancy. Vaccines 2021, 9, 300. [Google Scholar] [CrossRef] [PubMed]
- Palamenghi, L.; Barello, S.; Boccia, S.; Graffigna, G. Mistrust in biomedical research and vaccine hesitancy: The forefront challenge in the battle against COVID-19 in Italy. Eur. J. Epidemiol. 2020, 35, 785–788. [Google Scholar] [CrossRef] [PubMed]
- Stamm, T.A.; Partheymüller, J.; Mosor, E.; Ritschl, V.; Kritzinger, S.; Eberl, J.M. Coronavirus vaccine hesitancy among unvaccinated Austrians: Assessing underlying motivations and the effectiveness of interventions based on a cross-sectional survey with two embedded conjoint experiments. Lancet Reg. Health—Eur. 2022, 17, 100389. [Google Scholar] [CrossRef] [PubMed]
- Yarmol-Matusiak, E.A.; Cipriano, L.E.; Stranges, S. A comparison of COVID-19 epidemiological indicators in Sweden, Norway, Denmark, and Finland. Scand. J. Public Health 2021, 49, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Campos-Mercade, P.; Meier, A.N.; Schneider, F.H.; Meier, S.; Pope, D.; Wengström, E. Monetary incentives increase COVID-19 vaccinations. Science 2021, 374, 879–882. [Google Scholar] [CrossRef]
- Chang, T.; Jacobson, M.; Shah, M.; Pramanik, R.; Shah, S.B. Financial Incentives and Other Nudges Do Not Increase COVID-19 Vaccinations among the Vaccine Hesitant. National Bureau of Economic Research; 2021 (Working Paper Series). Available online: https://www.nber.org/papers/w29403 (accessed on 30 January 2023).
- COVID-19 Vaccine Incentives. National Governors Association. 2021. Available online: https://www.nga.org/publications/covid-19-vaccine-incentives/ (accessed on 28 February 2023).
- Munzert, S.; Ramirez-Ruiz, S.; Çalı, B.; Stoetzer, L.F.; Gohdes, A.; Lowe, W. Prioritization preferences for COVID-19 vaccination are consistent across five countries. Humanit. Soc. Sci. Commun. 2022, 9, 439. [Google Scholar] [CrossRef]
- Oliu-Barton, M.; Pradelski, B.S.R.; Woloszko, N.; Guetta-Jeanrenaud, L.; Aghion, P.; Artus, P.; Fontanet, A.; Martin, P.; Wolff, G.B. The effect of COVID certificates on vaccine uptake, health outcomes, and the economy. Nat. Commun. 2022, 13, 3942. [Google Scholar] [CrossRef]
- Mills, M.C.; Rüttenauer, T. The effect of mandatory COVID-19 certificates on vaccine uptake: Synthetic-control modelling of six countries. Lancet Public Health 2022, 7, e15–e22. [Google Scholar] [CrossRef]
- Karaivanov, A.; Kim, D.; Lu, S.E.; Shigeoka, H. COVID-19 vaccination mandates and vaccine uptake. Nat. Hum. Behav. 2022, 6, 1615–1624. [Google Scholar] [CrossRef]
- Débarre, F.; Lecoeur, E.; Guimier, L.; Jauffret-Roustide, M.; Jannot, A.S. The French Covid-19 vaccination policy did not solve vaccination inequities: A nationwide study on 64.5 million people. Eur. J. Public Health 2022, 32, 825–830. [Google Scholar] [CrossRef]
- Ward, J.K.; Gauna, F.; Gagneux-Brunon, A.; Botelho-Nevers, E.; Cracowski, J.L.; Khouri, C.; Launay, O.; Verger, P.; Peretti-Watel, P. The French health pass holds lessons for mandatory COVID-19 vaccination. Nat. Med. 2022, 28, 232–235. [Google Scholar] [CrossRef]
- Données Relatives Aux Stocks des Doses de Vaccins Contre la COVID-19-Data.gouv.fr. Available online: https://www.data.gouv.fr/fr/datasets/donnees-relatives-aux-stocks-des-doses-de-vaccins-contre-la-covid-19/ (accessed on 26 October 2022).
- French National Health Insurance Données Vaccination par, E.P.C.I. Available online: https://datavaccin-covid.ameli.fr/explore/dataset/donnees-de-vaccination-par-epci/ (accessed on 30 January 2023).
- Rogers, E.M. Diffusion of Innovations; Free Press of Glencoe: New York, NY, USA, 1962; 396p. [Google Scholar]
- Bass, F.M. A New Product Growth for Model Consumer Durables. Manag. Sci. 1969, 15, 215–227. [Google Scholar] [CrossRef]
- Berwick, D.M. Disseminating Innovations in Health Care. JAMA 2003, 289, 1969–1975. [Google Scholar] [CrossRef]
- Santé Publique France. Données Hospitalières Relatives à L’épidémie de COVID-19 (SIVIC)—Data.gouv.fr. Available online: https://www.data.gouv.fr/fr/datasets/donnees-hospitalieres-relatives-a-lepidemie-de-covid-19/ (accessed on 30 January 2023).
- Aron, J.; Muellbauer, J.; Giattino, C.; Ritchie, H. A Pandemic Primer on Excess Mortality Statistics and Their Comparability across Countries. Our World in Data. Available online: https://ourworldindata.org/covid-excess-mortality (accessed on 30 January 2023).
- National Institute of Statistics and Economical Studies. Fichiers des Personnes Décédées Depuis 1970|Insee. Available online: https://www.insee.fr/fr/information/4190491 (accessed on 30 January 2023).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017; Available online: https://www.R-project.org/ (accessed on 20 June 2023).
- Dormann, F.C.; McPherson, J.M.; Araújo, M.B.; Bivand, R.; Bolliger, J.; Carl, G.; Davies, R.G.; Hirzel, A.; Jetzet, W.; Kisslingal, W.D.; et al. Methods to account for spatial autocorrelation in the analysis of species distributional data: A review. Ecography 2007, 30, 609–628. [Google Scholar] [CrossRef] [Green Version]
- Bivand, R.; Piras, G. Comparing Implementations of Estimation Methods for Spatial Econometrics. J. Stat. Softw. 2015, 63, 1–36. [Google Scholar] [CrossRef] [Green Version]
- Plummer, M. Rjags: Bayesian Graphical Models Using MCMC. 2021. Available online: https://CRAN.R-project.org/package=rjags (accessed on 20 June 2023).
- van Buuren, S.; Groothuis-Oudshoorn, K. mice: Multivariate Imputation by Chained Equations in R. J. Stat. Softw. 2011, 45, 1–67. [Google Scholar] [CrossRef] [Green Version]
- Schafer, J.L. Multiple imputation: A primer. Stat. Methods Med. Res. 1999, 8, 3–15. [Google Scholar] [CrossRef]
- Wilson, S.L.; Wiysonge, C. Social media and vaccine hesitancy. BMJ Glob. Health 2020, 5, e004206. [Google Scholar] [CrossRef]
- Biswas, M.R.; Alzubaidi, M.S.; Shah, U.; Abd-Alrazaq, A.A.; Shah, Z. A Scoping Review to Find out Worldwide COVID-19 Vaccine Hesitancy and Its Underlying Determinants. Vaccines 2021, 9, 1243. [Google Scholar] [CrossRef]
- Treyens, P.E.; Catherine, A. Dans les DOM, L’activité et L’emploi Stagnent Depuis dix Ans-Insee Analyses Martinique-04. INSEE Anal Martin. Available online: https://www.insee.fr/fr/statistiques/1285790 (accessed on 31 January 2023).
- Water Coalition and Working Group on the Rights to Water and Sanitation in France. Human Rights to Water and Sanitation in Overseas Departments and Regions. 2022. Available online: https://www.coalition-eau.org/wp-content/uploads/written-contribution-from-french-ngos-overseas-1.pdf (accessed on 31 January 2023).
- Multigner, L.; Kadhel, P.; Rouget, F.; Blanchet, P.; Cordier, S. Chlordecone exposure and adverse effects in French West Indies populations. Environ. Sci. Pollut. Res. Int. 2016, 23, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Bergmann, M.; Bethmann, A.; Hannemann, T.V.; Schumacher, A.T. Who are the Unvaccinated? Determinants of SARS-CoV-2 Vaccinations Among Older Adults Across Europe. Easy Soc. Sci. 2022, 1, 1–11. [Google Scholar]
- Gleizes, F.; Nougaret, A.; Pla, A.; Viard-Guillot, L. Un Tiers des Adultes ont Renoncé à Effectuer une Démarche Administrative en Ligne en 2021. Insee Focus. Available online: https://www.insee.fr/fr/statistiques/6438420 (accessed on 31 January 2023).
- Vandentorren, S.; Smaïli, S.; Chatignoux, E.; Maurel, M.; Alleaume, C.; Neufcourt, L.; Kelly-Irving, M.; Delpierre, C. The Effect of Social Deprivation on the Dynamic of SARS-CoV-2 Infection in France: A Population-Based Analysis. Lancet Public Health. Available online: https://www.thelancet.com/journals/lanpub/article/PIIS2468-2667(22)00007-X/fulltext (accessed on 21 February 2022).
- Mongin, D.; Cullati, S.; Kelly-Irving, M.; Rosselet, M.; Regard, S.; Courvoisier, D.S. Neighbourhood Socio-Economic Vulnerability and Access to COVID-19 Healthcare during the First Two Waves of the Pandemic in Geneva, Switzerland: A Gender Perspective. eClinicalMedicine. Available online: https://www.thelancet.com/journals/eclinm/article/PIIS2589-5370(22)00082-7/fulltext (accessed on 27 September 2022).
- Riou, J.; Panczak, R.; Althaus, C.L.; Junker, C.; Perisa, D.; Schneider, K.; Criscuolo, N.G.; Low, N.; Egger, M. Socioeconomic Position and the COVID-19 Care Cascade fromTesting to Mortality in Switzerland: A Population-Based Analysis. Lancet Public Health. Available online: https://www.thelancet.com/journals/lanpub/article/PIIS2468-2667(21)00160-2/abstract (accessed on 12 July 2021).
- Hart, J.T. The Inverse Care Law. Lancet 1971, 297, 405–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viskupič, F.; Wiltse, D.L.; Meyer, B.A. Trust in physicians and trust in government predict COVID-19 vaccine uptake. Soc. Sci. Q. 2022, 103, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Bartoš, V.; Bauer, M.; Cahlíková, J.; Chytilová, J. C ommunicating doctors’ consensus persistently increases COVID-19 vaccinations. Nature 2022, 606, 542–549. [Google Scholar] [CrossRef]
- Detoc, M.; Bruel, S.; Frappe, P.; Tardy, B.; Botelho-Nevers, E.; Gagneux-Brunon, A. Intention to participate in a COVID-19 vaccine clinical trial and to get vaccinated against COVID-19 in France during the pandemic. Vaccine 2020, 38, 7002–7006. [Google Scholar] [CrossRef]
- Kreps, S.; Prasad, S.; Brownstein, J.S.; Hswen, Y.; Garibaldi, B.T.; Zhang, B.; Kriner, D.L. Factors Associated with US Adults’ Likelihood of Accepting COVID-19 Vaccination. JAMA Netw. Open 2020, 3, e2025594. [Google Scholar] [CrossRef]
- Gagneux-Brunon, A.; Detoc, M.; Bruel, S.; Tardy, B.; Rozaire, O.; Frappe, P.; Botelho-Nevers, E. Intention to get vaccinations against COVID-19 in French healthcare workers during the first pandemic wave: A cross-sectional survey. J. Hosp. Infect. 2021, 108, 168–173. [Google Scholar] [CrossRef]
- Reilev, M.; Olesen, M.; Kildegaard, H.; Støvring, H.; Andersen, J.H.; Hallas, J.; Lund, L.C.; Ladebo, L.; Ernst, M.T.; Damkier, P. Changing characteristics over time of individuals receiving COVID-19 vaccines in Denmark: A population-based descriptive study of vaccine uptake. Scand. J. Public Health 2022, 50, 686–692. [Google Scholar] [CrossRef]
- Perrotta, D.; Grow, A.; Rampazzo, F.; Cimentada, J.; Del Fava, E.; Gil-Clavel, S.; Zagheni, E. Behaviours and attitudes in response to the COVID-19 pandemic: Insights from a cross-national Facebook survey. EPJ Data Sci. 2021, 10, 17. [Google Scholar] [CrossRef]
- Brouard, S.; Vasilopoulos, P.; Becher, M. Sociodemographic and Psychological Correlates of Compliance with the COVID-19 Public Health Measures in France. Can. J. Polit. Sci. Can. Sci. Polit. 2020, 53, 253–258. [Google Scholar] [CrossRef] [Green Version]
- Viswanath, K.; Bekalu, M.; Dhawan, D.; Pinnamaneni, R.; Lang, J.; McLoud, R. Individual and social determinants of COVID-19 vaccine uptake. BMC Public Health 2021, 21, 818. [Google Scholar] [CrossRef]
- Joshi, A.; Kaur, M.; Kaur, R.; Grover, A.; Nash, D.; El-Mohandes, A. Predictors of COVID-19 Vaccine Acceptance, Intention, and Hesitancy: A Scoping Review. Front. Public Health 2021, 9. Available online: https://www.frontiersin.org/articles/10.3389/fpubh.2021.698111 (accessed on 27 February 2023). [CrossRef]
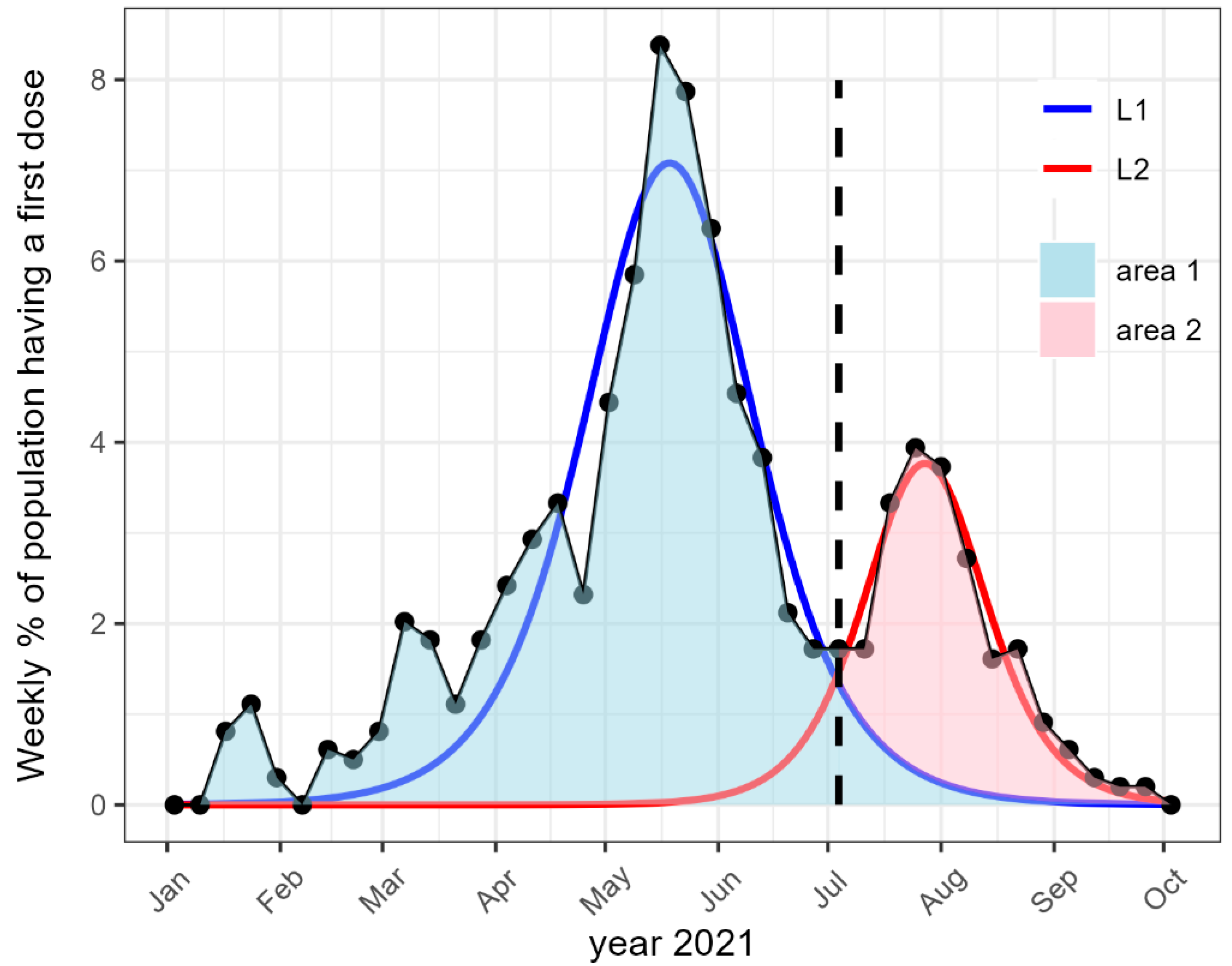

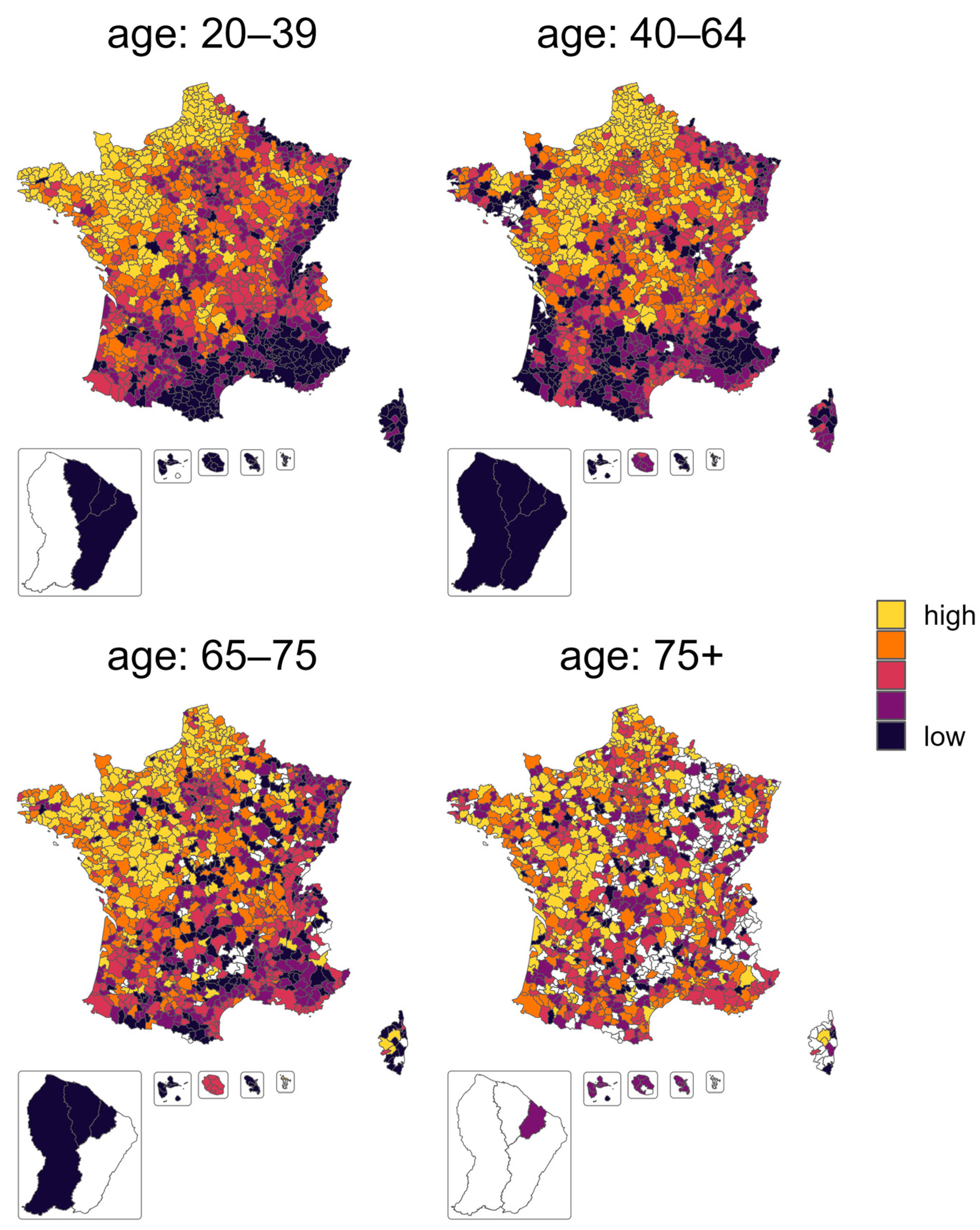
| Age Class | 20–39 | 40–64 | 65–75 | 75+ |
|---|---|---|---|---|
| Outcomes | ||||
| Vaccination rate before health pass | 37.3 [32.3, 41.9] | 62.0 [57.7, 66.0] | 81.6 [78.7, 84.1] | 84.0 [81.4, 86.1] |
| SEHP | 53.6 [48.6, 58.1] | 48.6 [41.5, 54.7] | 41.6 [34.2, 48.0] | 30.1 [16.6, 37.6] |
| Fit L1 | ||||
| Time in day from the health pass | −21.3 [−25.5, −17.8] | −37.9 [−41.9, −34.2] | −70.9 [−74.9, −67.8] | −92.8 [−97.5, −88.3] |
| Amplitude Vmax in weekly % of the population receiving first vaccination dose | 6.1 [5.1, 7.1] | 6.4 [5.7, 7.2] | 9.3 [8.4, 10.4] | 8.7 [7.3, 10.3] |
| Full width at half maximum | 37.9 [33.9, 43.1] | 60.2 [51.4, 70.6] | 53.4 [46.5, 60.4] | 44.1 [33.7, 56.1] |
| R2 of the regression | 0.93 [0.90, 0.96] | 0.84 [0.76, 0.89] | 0.84 [0.77, 0.90] | 0.82 [0.74, 0.88] |
| Fit L2 | ||||
| Time | 32.7 [30.7, 35.0] | 32.5 [30.4, 34.7] | 31.9 [29.3, 34.9] | 34.9 [30.0, 39.1] |
| Amplitude | 6.4 [5.8, 7.1] | 3.6 [3.3, 4.0] | 1.3 [1.1, 1.6] | 0.8 [0.6, 1.0] |
| Width | 35.8 [31.7, 40.6] | 40.1 [35.2, 45.0] | 43.3 [31.2, 51.1] | 47.1 [26.4, 64.0] |
| R2 | 0.92 [0.88, 0.94] | 0.92 [0.88, 0.95] | 0.84 [0.75, 0.90] | 0.71 [0.50, 0.84] |
| Overall | Metropolitan | Overseas | Missing | |
|---|---|---|---|---|
| Territory | ||||
| Number of EPCI | 1252 | 1229 | 23 | |
| Density | 0.4 | |||
| Low (below 50/km2) | 508 (40.7%) | 504 (41.0%) | 4 (22.2%) | |
| Medium (between 50/km2 and 300/km2) | 611 (49.0%) | 603 (49.1%) | 8 (44.4%) | |
| High (above 300/km2) | 128 (10.3%) | 122 (9.9%) | 6 (33.3%) | |
| COVID-19 | ||||
| Death excess (p score) | 8.70 [1.46, 17.26] | 8.62 [1.31, 17.24] | 10.75 [6.73, 22.62] | 0.0 |
| hospitalization (% of the population) | 0.67 [0.45, 0.85] | 0.67 [0.45, 0.85] | 0.52 [0.28, 0.52] | 0.5 |
| Socio-economics | ||||
| population below the poverty threshold (%) | 12.90 [9.70, 16.00] | 12.90 [9.70, 15.90] | 33.55 [30.58, 38.78] | 1.3 |
| population without a secondary education (%) | 17.50 [16.60, 18.60] | 17.60 [16.70, 18.60] | 15.90 [15.15, 17.58] | 0.4 |
| Income inequality | 2.80 [2.70, 3.10] | 2.80 [2.70, 3.10] | 4.15 [3.98, 4.32] | 1.3 |
| Median living (k euros) | 21.10 [20.20, 22.30] | 21.10 [20.28, 22.30] | 16.85 [15.45, 17.65] | 1.3 |
| COVID-19 health access | ||||
| Epci with a vaccination center (%) | 930 (74.3%) | 911 (74.1%) | 19 (82.6%) | 0.0 |
| Distance to the closest vaccination center (km) | 5.85 [3.17, 10.51] | 5.75 [3.15, 10.40] | 10.60 [6.90, 19.17] | 0.0 |
| Number of family physicians and pharmacies per 10,000 inhabitants | 1.1 [0.9, 1.3] | 1.1 [0.9, 1.3] | 1.2 [0.9, 1.3] | 0.6 |
| Doses delivered before the health pass (% of the population) | 90.0 [85.0, 94.6] | 90.0 [85.0, 94.6] | 82.9 [75.7, 134.2] | 0.1 |
| Doses delivered to vaccination centers after health pass implementation (% of unvaccinated people) | 124.5 [109.4, 140.5] | 125.3 [109.9, 141.4] | 60.1 [12.3, 76.1] | 0.1 |
| Politics | ||||
| Percentage of abstention during first round of 2022 presidential elections | 22.42 [20.34, 24.62] | 22.35 [20.31, 24.42] | 56.54 [52.44, 60.47] | 0.0 |
| Percentage of voting for a pro-pass candidate | 31.25 [28.14, 35.02] | 31.37 [28.36, 35.08] | 21.16 [17.98, 22.33] | 0.0 |
| Term | 20–39 | 40–64 | 65–75 | 75+ |
|---|---|---|---|---|
| R2 | 0.58 | 0.81 | 0.94 | 0.95 |
| Territory | ||||
| Oversea (vs metropolitan) | 4.0 [−5.1,13.1] | −14.2 [−20.2,−8.1] | −32.7 [−36.8,−28.6] | −38.0 [−41.1,−35.0] |
| Medium density (ref low) | 1.3 [0.8,1.7] | 1.2 [0.8,1.7] | 0.8 [0.4,1.2] | 0.5 [0.07,0.9] |
| High density (ref low) | 2.9 [2.1,3.7] | 2.4 [1.6,3.1] | 1.7 [1.1,2.4] | 1.4 [0.8,2.0] |
| Proportion women | 0.2 [0.02,0.4] | 0.2 [−0.002,0.4] | 0.06 [−0.1,0.2] | −0.08 [−0.2,0.09] |
| COVID-19 | ||||
| Death excess | −0.004 [−0.01, 0.004] | −0.005 [−0.01, 0.003] | −0.008 [−0.01, −0.001] | −0.01 [−0.02, −0.004] |
| Hospitalization | 0.9 [−1.1, 2.8] | −0.6 [−2.7, 1.5] | −0.5 [−2.4, 1.5] | −0.3 [−1.2, 0.6] |
| Socio-economic | ||||
| Poverty | −0.5 [−0.53, −0.4] | −0.4 [−0.5, −0.3] | −0.2 [−0.3, −0.16] | −0.07 [−0.1, −0.02] |
| Population without secondary education | −0.1 [−0.2, 0.03] | −0.1 [−0.3, −0.01] | −0.03 [−0.1, 0.08] | −0.01 [−0.1, 0.09] |
| Inequalities | −0.5 [−1.2, 0.3] | −1.7 [−2.5, −0.9] | −1.7 [−2.4, −1.0] | −1.4 [−2.0, −0.8] |
| COVID-19 health access | ||||
| Min distance to vaccination center | −0.04 [−0.06, −0.03] | −0.05 [−0.08, −0.03] | −0.01 [−0.03, 0.007] | −0.006 [−0.02, 0.01] |
| Pharmacy and physician density | −0.3 [−0.7, 0.1] | 0.2 [−0.1, 0.6] | 0.2 [−0.1, 0.5] | 0.1 [−0.2, 0.5] |
| Doses available | −0.005 [−0.02, 0.01] | 0.03 [0.01, 0.05] | 0.02 [0.007, 0.04] | 0.01 [0.002, 0.02] |
| Politics | ||||
| Abstention | −0.2 [−0.3, −0.1] | −0.2 [−0.25, −0.1] | −0.1 [−0.2, −0.06] | −0.2 [−0.24, −0.1] |
| Result of pro-pass candidate | 0.5 [0.4, 0.55] | 0.5 [0.46, 0.6] | 0.3 [0.27, 0.4] | 0.3 [0.31, 0.4] |
| Term | 20–39 | 40–64 | 65–75 | 75+ |
|---|---|---|---|---|
| R2 | 0.32 | 0.18 | 0.14 | 0.23 |
| Territory | ||||
| Oversea (vs metropolitan) | −2.1 [−7.6, 3.3] | −28.6 [−38.5, −18.8] | −21.2 [−30.4, −12.0] | −39.1 [−50.3, −27.9] |
| Medium density (ref low) | 0.4 [−0.2, 0.9] | 0.5 [−0.7, 1.7] | 5.0 [3.2, 6.7] | 10.6 [8.4, 12.7] |
| High density (ref low) | 0.5 [−0.5, 1.4] | 0.8 [−1.2, 2.8] | 5.3 [2.3, 8.2] | 9.9 [6.4, 13.4] |
| Proportion women | 0.2 [−0.07, 0.4] | 1.2 [0.7, 1.7] | 2.4 [1.5, 3.3] | 3.8 [2.7, 4.8] |
| COVID-19 | ||||
| Death excess | −0.01 [−0.02, 0.002] | 0.002 [−0.02, 0.03] | 0.08 [0.02, 0.1] | −0.03 [−0.09, 0.04] |
| Hospitalization | −1.7 [−3.4, 0.002] | 1.0 [−2.7, 4.6] | −5.0 [−8.6, −1.4] | −6.6 [−10.3, −2.8] |
| Socio-economic | ||||
| Poverty | −0.2 [−0.3, −0.1] | −0.3 [−0.5, −0.1] | 0.1 [−0.2, 0.4] | 0.2 [−0.1, 0.5] |
| Pop without secondary education | −0.1 [−0.3, 0.06] | −0.2 [−0.6, 0.1] | −0.9 [−1.4, −0.4] | −1.6 [−2.2, −1.0] |
| Inequalities | −3.2 [−4.1, −2.2] | −3.2 [−5.1, −1.2] | −5.0 [−7.5, −2.5] | −1.7 [−4.4, 1.1] |
| COVID-19 health access | ||||
| Min distance to vaccination center | −0.008 [−0.04, 0.02] | −0.03 [−0.08, 0.03] | −0.08 [−0.2, −0.003] | −0.07 [−0.2, 0.02] |
| Pharmacy and physician density | −0.5 [−1.1, 0.1] | −3.9 [−5.1, −2.6] | −1.6 [−3.8, 0.6] | −2.6 [−5.2, 0.06] |
| Doses available | 0.03 [0.02, 0.05] | 0.002 [−0.03, 0.04] | 0.05 [0.01, 0.09] | 0.005 [−0.04, 0.05] |
| Politics | ||||
| Abstention | −0.1 [−0.2, −0.02] | 0.3 [0.04, 0.5] | 0.4 [0.2, 0.7] | 0.5 [0.2, 0.7] |
| Result of pro-pass candidate | 0.3 [0.28, 0.4] | 0.1 [−0.01, 0.3] | 0.3 [0.1, 0.5] | 0.2 [−0.06, 0.4] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mongin, D.; Buclin, C.P.; Cullati, S.; Courvoisier, D.S. COVID-19 Vaccination Rate under Different Political Incentive: A Counterfactual Trend Approach Using Nationwide Data. Vaccines 2023, 11, 1149. https://doi.org/10.3390/vaccines11071149
Mongin D, Buclin CP, Cullati S, Courvoisier DS. COVID-19 Vaccination Rate under Different Political Incentive: A Counterfactual Trend Approach Using Nationwide Data. Vaccines. 2023; 11(7):1149. https://doi.org/10.3390/vaccines11071149
Chicago/Turabian StyleMongin, Denis, Clement P. Buclin, Stephane Cullati, and Delphine S. Courvoisier. 2023. "COVID-19 Vaccination Rate under Different Political Incentive: A Counterfactual Trend Approach Using Nationwide Data" Vaccines 11, no. 7: 1149. https://doi.org/10.3390/vaccines11071149






