The Burden of Genital Warts in Finland: Cross-Sectional Analysis of the Prevalence and Direct Medical Costs in 2018
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design, Permits, and Data Sharing
2.2. Eligibility Criteria
2.3. Data Sources, Patient Characteristics, and Healthcare Resource Use
3. Results
3.1. Prevalence of Genital Warts in 2018
3.2. Health Care Resource Use and Direct Medical Care Costs of Genital Warts in 2018
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seksitaudit. Suomalaisen Lääkäriseuran Duodecimin Ja Sukupuolitautien Vastustamisyhdistys Ry:N Asettama Työryhmä. Available online: https://www.kaypahoito.fi/en/ (accessed on 27 June 2020).
- Gilson, R.; Nugent, D.; Werner, R.N.; Ballesteros, J.; Ross, J. 2019 IUSTI-Europe Guideline for the Management of Anogenital Warts. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 1644–1653. [Google Scholar] [CrossRef]
- Burchell, A.N.; Coutlee, F.; Tellier, P.-P.; Hanley, J.; Franco, E.L. Genital Transmission of Human Papillomavirus in Recently Formed Heterosexual Couples. J. Infect. Dis. 2011, 204, 1723–1729. [Google Scholar] [CrossRef] [Green Version]
- Patel, H.; Wagner, M.; Singhal, P.; Kothari, S. Systematic Review of the Incidence and Prevalence of Genital Warts. BMC Infect. Dis. 2013, 13, 39. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization Global Health Sector Strategy on Sexually Transmitted Infections 2016–2021. Towards Ending STIS. Available online: https://www.who.int/publications/i/item/WHO-RHR-16.09 (accessed on 29 March 2023).
- Papilloomavirustautien Torjuntatyöryhmä. Terveyden ja Hyvinvoinnin Laitoksen Asettaman Papilloomavirustautien Torjuntaryhmän Selvitys; Raportti 28/2011; Terveyden ja Hyvinvoinnin Laitos (THL): Helsinki, Finland, 2011; pp. 1–121.
- Työryhmä ja Terveyden ja Hyvinvoinnin Laitos. Tulisiko Poikien HPV-Rokotusten Olla Osa Kansallista Rokotusohjelmaa? Työryhmän Loppuraportti; Raportti 2/2019; Terveyden ja Hyvinvoinnin Laitos (THL): Helsinki, Finland, 2019; pp. 1–101.
- Adeli, M.; Moghaddam-Banaem, L.; Shahali, S. Sexual Dysfunction in Women with Genital Warts: A Systematic Review. BMC Women’s Health 2022, 22, 516. [Google Scholar] [CrossRef]
- Gissmann, L.; Wolnik, L.; Ikenberg, H.; Koldovsky, U.; Schnürch, H.G.; zur Hausen, H. Human Papillomavirus Types 6 and 11 DNA Sequences in Genital and Laryngeal Papillomas and in Some Cervical Cancers. Proc. Natl. Acad. Sci. USA 1983, 80, 560–563. [Google Scholar] [CrossRef] [PubMed]
- Ball, S.L.R.; Winder, D.M.; Vaughan, K.; Hanna, N.; Levy, J.; Sterling, J.C.; Stanley, M.A.; Goon, P.K.C. Analyses of Human Papillomavirus Genotypes and Viral Loads in Anogenital Warts. J. Med. Virol. 2011, 83, 1345–1350. [Google Scholar] [CrossRef] [Green Version]
- European Centre for Disease Prevention and Control Vaccine Scheduler. Human Papillomavirus Infection: Recommended Vaccinations. Available online: https://www.ecdc.europa.eu/en/publications-data/ecdc-vaccine-scheduler (accessed on 29 March 2023).
- Leval, A.; Herweijer, E.; Arnheim-Dahlstrom, L.; Walum, H.; Frans, E.; Sparen, P.; Simard, J.F. Incidence of Genital Warts in Sweden Before and After Quadrivalent Human Papillomavirus Vaccine Availability. J. Infect. Dis. 2012, 206, 860–866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herweijer, E.; Ploner, A.; Sparén, P. Substantially Reduced Incidence of Genital Warts in Women and Men Six Years after HPV Vaccine Availability in Sweden. Vaccine 2018, 36, 1917–1920. [Google Scholar] [CrossRef]
- Baandrup, L.; Blomberg, M.; Dehlendorff, C.; Sand, C.; Andersen, K.K.; Kjaer, S.K. Significant Decrease in the Incidence of Genital Warts in Young Danish Women After Implementation of a National Human Papillomavirus Vaccination Program. Sex. Transm. Dis. 2013, 40, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Yakely, A.E.; Avni-Singer, L.; Oliveira, C.R.; Niccolai, L.M. Human Papillomavirus Vaccination and Anogenital Warts: A Systematic Review of Impact and Effectiveness in the United States. Sex. Trans. Dis. 2019, 46, 213–220. [Google Scholar] [CrossRef]
- Righolt, C.H.; Willows, K.; Kliewer, E.V.; Mahmud, S.M. Incidence of Anogenital Warts after the Introduction of the Quadrivalent HPV Vaccine Program in Manitoba, Canada. PLoS ONE 2022, 17, e0267646. [Google Scholar] [CrossRef] [PubMed]
- Guerra, F.M.; Rosella, L.C.; Dunn, S.; Wilson, S.E.; Chen, C.; Deeks, S.L. Health Service Utilisation for Anogenital Warts in Ontario, Canada Prior to the Human Papillomavirus (HPV) Vaccine Programme Introduction: A Retrospective Longitudinal Population-Based Study. BMJ Open 2016, 6, e009914. [Google Scholar] [CrossRef] [Green Version]
- Guerra, F.M.; Rosella, L.C.; Dunn, S.; Wilson, S.E.; Chen, C.; Deeks, S.L. Early Impact of Ontario’s Human Papillomavirus (HPV) Vaccination Program on Anogenital Warts (AGWs): A Population-Based Assessment. Vaccine 2016, 34, 4678–4683. [Google Scholar] [CrossRef] [Green Version]
- Kansallinen Rokotusohjelma. Terveyden Ja Hyvinvoinnin Laitos. Available online: https://thl.fi/fi/web/infektiotaudit-ja-rokotukset/rokotteet-a-o/hpv-eli-papilloomavirusrokote (accessed on 29 June 2022).
- Salo, H.; Leino, T.; Kilpi, T.; Auranen, K.; Tiihonen, P.; Lehtinen, M.; Vänskä, S.; Linna, M.; Nieminen, P. The Burden and Costs of Prevention and Management of Genital Disease Caused by HPV in Women: A Population-Based Registry Study in Finland: The Burden and Costs of HPV in Finland. Int. J. Cancer 2013, 133, 1459–1469. [Google Scholar] [CrossRef]
- Mäklin, S.; Kokko, P. Terveyden-ja Sosiaalihuollon Yksikkökustannukset Suomessa Vuonna 2017; Raportti 21/2020; Terveyden ja Hyvinvoinnin Laitos (THL): Helsinki, Finland, 2021; pp. 1–55.
- Suomen Virallinen Tilasto (SVT): Julkisten Menojen Hintaindeksi. Available online: http://www.stat.fi/til/jmhi/index.html (accessed on 28 June 2022).
- Kapiainen, S.; Väisänen, A.; Haula, T. Terveyden-ja Sosiaalihuollon Yksikkökustannukset Suomessa Vuonna 2011; Raportti 3/2014; Terveyden ja Hyvinvoinnin Laitos (THL): Helsinki, Finland, 2014; pp. 1–104.
- Östensson, E.; Fröberg, M.; Leval, A.; Hellström, A.-C.; Bäcklund, M.; Zethraeus, N.; Andersson, S. Cost of Preventing, Managing, and Treating Human Papillomavirus (HPV)-Related Diseases in Sweden before the Introduction of Quadrivalent HPV Vaccination. PLoS ONE 2015, 10, e0139062. [Google Scholar] [CrossRef] [Green Version]
- Burger, E.A.; Sy, S.; Nygård, M.; Kristiansen, I.S.; Kim, J.J. Prevention of HPV-Related Cancers in Norway: Cost-Effectiveness of Expanding the HPV Vaccination Program to Include Pre-Adolescent Boys. PLoS ONE 2014, 9, e89974. [Google Scholar] [CrossRef] [Green Version]
- Olsen, J.; Jepsen, M.R. Human Papillomavirus Transmission and Cost-Effectiveness of Introducing Quadrivalent HPV Vaccination in Denmark. Int. J. Technol. Assess Health Care 2010, 26, 183–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orumaa, M.; Kjaer, S.K.; Dehlendorff, C.; Munk, C.; Olsen, A.O.; Hansen, B.T.; Campbell, S.; Nygård, M. The Impact of HPV Multi-Cohort Vaccination: Real-World Evidence of Faster Control of HPV-Related Morbidity. Vaccine 2020, 38, 1345–1351. [Google Scholar] [CrossRef]
- Feiring, B.; Laake, I.; Christiansen, I.K.; Hansen, M.; Stålcrantz, J.; Ambur, O.H.; Magnus, P.; Jonassen, C.M.; Trogstad, L. Substantial Decline in Prevalence of Vaccine-Type and Nonvaccine-Type Human Papillomavirus (HPV) in Vaccinated and Unvaccinated Girls 5 Years After Implementing HPV Vaccine in Norway. J. Infect. Dis. 2018, 218, 1900–1910. [Google Scholar] [CrossRef]
- Drolet, M.; Bénard, É.; Pérez, N.; Brisson, M.; Ali, H.; Boily, M.-C.; Baldo, V.; Brassard, P.; Brotherton, J.M.L.; Callander, D.; et al. Population-Level Impact and Herd Effects Following the Introduction of Human Papillomavirus Vaccination Programmes: Updated Systematic Review and Meta-Analysis. Lancet 2019, 394, 497–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khawar, L.; McManus, H.; Vickers, T.; Chow, E.P.F.; Fairley, C.K.; Donovan, B.; Machalek, D.A.; Regan, D.G.; Grulich, A.E.; Guy, R.J.; et al. Genital Warts Trends in Australian and Overseas-Born People in Australia: A Cross-Sectional Trend Analysis to Measure Progress towards Control and Elimination. Lancet Reg. Health-West. Pac. 2021, 16, 100251. [Google Scholar] [CrossRef] [PubMed]
- Chow, E.P.F.; Read, T.R.H.; Wigan, R.; Donovan, B.; Chen, M.Y.; Bradshaw, C.S.; Fairley, C.K. Ongoing Decline in Genital Warts among Young Heterosexuals 7 Years after the Australian Human Papillomavirus (HPV) Vaccination Programme. Sex. Transm. Infect. 2015, 91, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Donovan, B.; Wand, H.; Read, T.R.H.; Regan, D.G.; Grulich, A.E.; Fairley, C.K.; Guy, R.J. Genital Warts in Young Australians Five Years into National Human Papillomavirus Vaccination Programme: National Surveillance Data. BMJ 2013, 346, f2032. [Google Scholar] [CrossRef] [Green Version]
- Patel, C.; Brotherton, J.M.; Pillsbury, A.; Jayasinghe, S.; Donovan, B.; Macartney, K.; Marshall, H. The Impact of 10 Years of Human Papillomavirus (HPV) Vaccination in Australia: What Additional Disease Burden Will a Nonavalent Vaccine Prevent? Eurosurveillance 2018, 23, 1700737. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| ICPC-2 | ICD-10 | ATC Code (Effective Substance) |
|---|---|---|
| Inclusion criteria: | ||
| Y76 (Condylomata acuminata male) | A63.0 [Anogenital (venereal) warts] | ATC D06BB10 (imiquimod) |
| X91 (Condylomata acuminata female) | ATC D06BB04 (podophyllotoxin) | |
| Exclusion criteria: | ||
| S77 (Malignant neoplasm of skin) | C44 (Other and unspecified malignant neoplasm of skin) | |
| X77 (Malignant neoplasm genital female other) | C51 (Malignant neoplasm of vulva) | |
| S80 (Solar keratosis/sunburn) | L57.0 (Actinic keratosis) | |
| S79 (Neoplasm skin benign) | D04 (Carcinoma in situ of skin) | |
| NCSP Code | Description |
|---|---|
| KGD10 | Destruction of lesion of penis |
| LFB10 | Excision of lesion of vulva or perineum |
| LFB20 | Destruction of lesion of vulva or perineum |
| KDD32 | Urethroscopic destruction of tumor of urethra |
| LDB00 | Excision of lesion of cervix uteri |
| LDB20 | Electrocoagulation or laser therapy of cervix uteri |
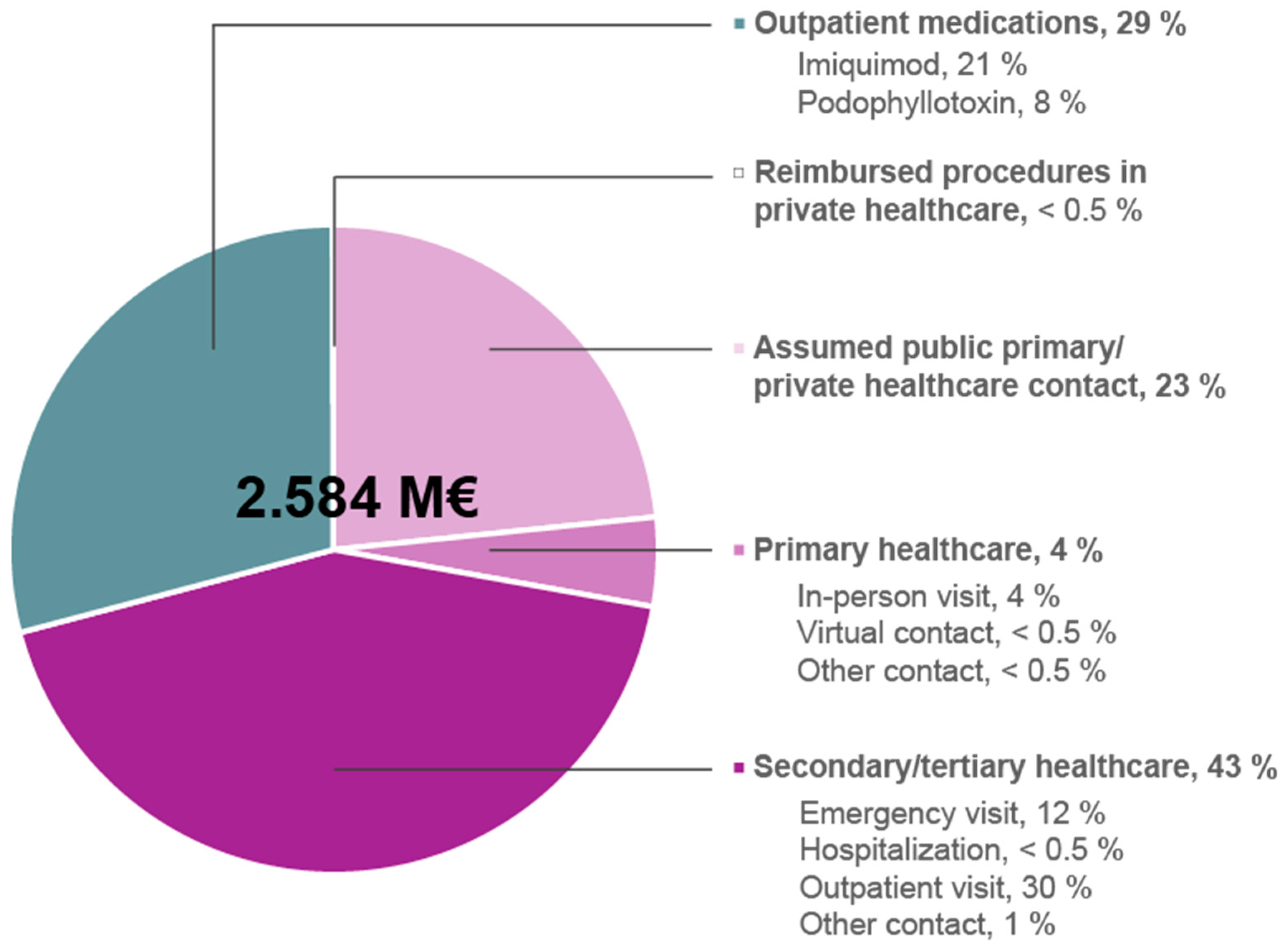
| Number of Subjects 1 | Subcategory | Number of Subjects Per Event 1 | Number of Events 1,2 | Total Costs (€) 1 | |
|---|---|---|---|---|---|
| Secondary/ tertiary public healthcare | ≈2220 | Emergency care visit 3 | NA 4 | 997.5 | 315,179 |
| Hospitalization days | 5.0 | 3851 | |||
| Outpatient visit | 3111.5 | 763,927 | |||
| Other speciality care contact | 100.5 | 30,060 | |||
| Total costs | 1,113,017 | ||||
| Primary public healthcare | 2773 | In-person visit | NA 4 | 2225.0 | 102,784 |
| Virtual contact | 190.0 | 5742 | |||
| Other | 188.5 | 5892 | |||
| Total costs | 114,418 | ||||
| Assumed public primary/ private healthcare contact 5 | ≈7190 | - | 7190 | 7190 | 604,532 |
| Reimbursed procedures at private sector | ≈10–16 | KGD10 | X | X | X |
| LFB10 | 8 | 8 | 1155 | ||
| LFB20 | X | X | X | ||
| KDD32 | 0 | 0 | 0 | ||
| LDB00 | 0 | 0 | 0 | ||
| LDB20 | 0 | 0 | 0 | ||
| LEB30 | 0 | 0 | 0 | ||
| Total costs 6 | 1155 | ||||
| Outpatient prescription medications | 9721 | Imiquimod | 4703 | 6545 | 547,953 |
| Podo- phyllotoxin | 5612 | 7063 | 203,391 | ||
| Total costs | 751,344 | ||||
| Total costs | 2,584,465 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gylling, A.; Uusi-Rauva, K.; Toppila, I.; Hiltunen-Back, E. The Burden of Genital Warts in Finland: Cross-Sectional Analysis of the Prevalence and Direct Medical Costs in 2018. Vaccines 2023, 11, 1202. https://doi.org/10.3390/vaccines11071202
Gylling A, Uusi-Rauva K, Toppila I, Hiltunen-Back E. The Burden of Genital Warts in Finland: Cross-Sectional Analysis of the Prevalence and Direct Medical Costs in 2018. Vaccines. 2023; 11(7):1202. https://doi.org/10.3390/vaccines11071202
Chicago/Turabian StyleGylling, Annette, Kristiina Uusi-Rauva, Iiro Toppila, and Eija Hiltunen-Back. 2023. "The Burden of Genital Warts in Finland: Cross-Sectional Analysis of the Prevalence and Direct Medical Costs in 2018" Vaccines 11, no. 7: 1202. https://doi.org/10.3390/vaccines11071202
APA StyleGylling, A., Uusi-Rauva, K., Toppila, I., & Hiltunen-Back, E. (2023). The Burden of Genital Warts in Finland: Cross-Sectional Analysis of the Prevalence and Direct Medical Costs in 2018. Vaccines, 11(7), 1202. https://doi.org/10.3390/vaccines11071202






