Application of Baculovirus Expression Vector System (BEVS) in Vaccine Development
Abstract
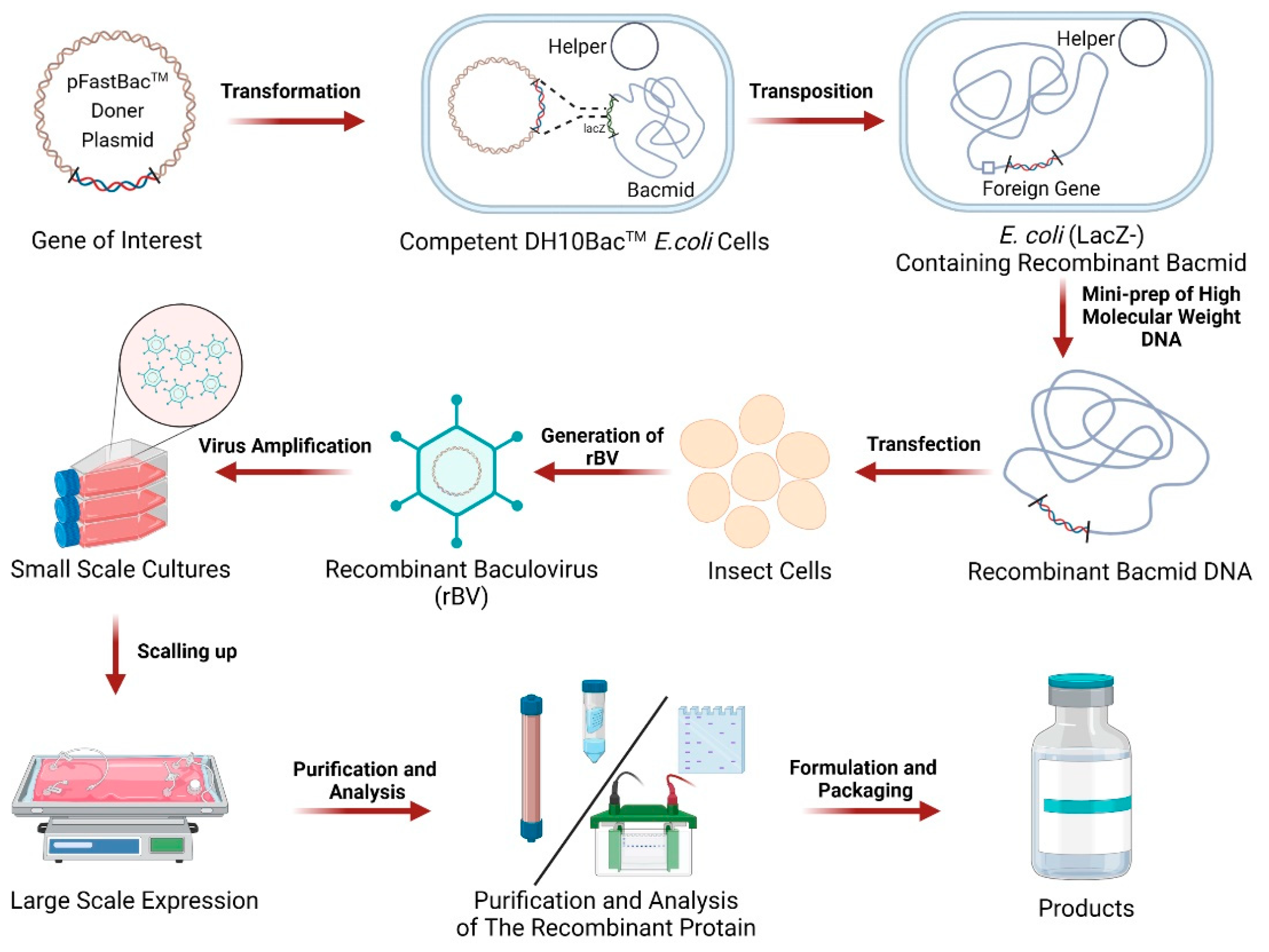
:1. Introduction
2. Composition and Workflow of BEVS
3. Advantages and Limitations of BEVS
4. Strategies for Optimizing BEVS
4.1. Engineering of the Baculovirus Vector
4.2. Engineering of the Cellular Host
5. BEVS-Derived Vaccines
5.1. BEVS-Derived Commercial Vaccines
5.2. BEVS-Derived Clinical Vaccines
5.2.1. SARS-CoV-2 Vaccines in Clinical Trials
5.2.2. Other Vaccines in Clinical Trials
5.3. BEVS-Derived Preclinical Vaccines
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BEVS | Baculovirus expression vector system |
| HPV | Human papillomavirus |
| AcMNPV | Autographa californica multicapsid nucleopolyhedrovirus |
| BmNPV | Bombyx mori nucleopolyhedrovirus |
| VLPs | Virus-like particles |
| RNAi | RNA interference |
| BSS | Burst sequences |
| hr1 | Homologous region 1 |
| sgMultiple | Multiple editing anti-BmNPV therapeutic complex CRISPR-Cas9 system, PSL1180-Cas9-sgIE1-sgLEF11-sgGP64 |
| rBV | Recombinant baculovirus |
| RNAi | RNA interference |
| FDL | Fused lobed |
| rHA | Recombinant trivalent hemagglutinin |
| HA | Hemagglutinin |
| RIV4 | Quadrivalent recombinant influenza vaccine |
| IIV4 | Quadrivalent-inactivated influenza vaccines |
| hACE2 | Human angiotensin-converting enzyme 2 |
| S | spike |
| preS dTM | Prefusion transmembrane-deleted spike |
| M1 | Matrix protein 1 |
| EBOV | Ebola virus |
| RSV | Respiratory syncytial virus |
| F | Fusion |
| AAV | Adeno-associated virus |
| ZIKV | Zika virus |
| E | Envelope |
| rWNV-E | Recombinant West Nile virus truncated envelope protein antigen |
| DENV | Dengue virus |
References
- Hong, M.; Li, T.; Xue, W.; Zhang, S.; Cui, L.; Wang, H.; Zhang, Y.; Zhou, L.; Gu, Y.; Xia, N.; et al. Genetic engineering of baculovirus-insect cell system to improve protein production. Front. Bioeng. Biotechnol. 2022, 10, 994743. [Google Scholar] [CrossRef] [PubMed]
- Summers, M.D. Milestones leading to the genetic engineering of baculoviruses as expression vector systems and viral pesticides. Adv. Virus Res. 2006, 68, 3–73. [Google Scholar] [CrossRef]
- Smith, G.E.; Summers, M.D.; Fraser, M.J. Production of human beta interferon in insect cells infected with a baculovirus expression vector. Mol. Cell. Biol. 1983, 3, 2156–2165. [Google Scholar] [CrossRef]
- Szarewski, A. HPV vaccine: Cervarix. Expert Opin. Biol. Ther. 2010, 10, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.P. Recombinant trivalent influenza vaccine (flublok(®)): A review of its use in the prevention of seasonal influenza in adults. Drugs 2013, 73, 1357–1366. [Google Scholar] [CrossRef]
- Trombetta, C.M.; Marchi, S.; Montomoli, E. The baculovirus expression vector system: A modern technology for the future of influenza vaccine manufacturing. Expert Rev. Vaccines 2022, 21, 1233–1242. [Google Scholar] [CrossRef]
- Sokolenko, S.; George, S.; Wagner, A.; Tuladhar, A.; Andrich, J.M.; Aucoin, M.G. Co-expression vs. co-infection using baculovirus expression vectors in insect cell culture: Benefits and drawbacks. Biotechnol. Adv. 2012, 30, 766–781. [Google Scholar] [CrossRef]
- Felberbaum, R.S. The baculovirus expression vector system: A commercial manufacturing platform for viral vaccines and gene therapy vectors. Biotechnol. J. 2015, 10, 702–714. [Google Scholar] [CrossRef]
- Harrison, R.L.; Jarvis, D.L. Protein N-glycosylation in the baculovirus-insect cell expression system and engineering of insect cells to produce “mammalianized” recombinant glycoproteins. Adv. Virus Res. 2006, 68, 159–191. [Google Scholar] [CrossRef]
- Fath-Goodin, A.; Kroemer, J.; Martin, S.; Reeves, K.; Webb, B.A. Polydnavirus genes that enhance the baculovirus expression vector system. Adv. Virus Res. 2006, 68, 75–90. [Google Scholar] [CrossRef]
- Hitchman, R.B.; Locanto, E.; Possee, R.D.; King, L.A. Optimizing the baculovirus expression vector system. Methods 2011, 55, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Possee, R.D.; Griffiths, C.M.; Hitchman, R.B.; Chambers, A.C.; Murguía-Meca, F.; Danquah, J.O.; Jeshtadi, A.; King, L.A. Baculoviruses: Biology, replication and exploitation. In Insect Virology; Asgari, S., Johnson, K.N., Eds.; Caister Academic Press: Wymondham, UK, 2010; pp. 35–58. [Google Scholar]
- Ayres, M.D.; Howard, S.C.; Kuzio, J.; Lopez-Ferber, M.; Possee, R.D. The complete DNA sequence of Autographa californica nuclear polyhedrosis virus. Virology 1994, 202, 586–605. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Liang, A.; Fu, Y. Ac154 carried out anti-apoptotic role during AcMNPV infection process in the host insect cells. Mol. Cell. Biochem. 2020, 463, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.S.; Dojima, T.; Park, E.Y. Comparative characterization of growth and recombinant protein production among three insect cell lines with four kinds of serum free media. Biotechnol. Bioprocess Eng. 2003, 8, 142–146. [Google Scholar] [CrossRef]
- Mishra, V. A Comprehensive Guide to the Commercial Baculovirus Expression Vector Systems for Recombinant Protein Production. Protein Pept. Lett. 2020, 27, 529–537. [Google Scholar] [CrossRef]
- Grohskopf, L.A.; Alyanak, E.; Ferdinands, J.M.; Broder, K.R.; Blanton, L.H.; Talbot, H.K.; Fry, A.M. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices, United States, 2021–2022 Influenza Season. MMWR Recomm. Rep. 2021, 70, 1–28. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Zhang, X.; Liu, L. Influenza and Universal Vaccine Research in China. Viruses 2022, 15, 116. [Google Scholar] [CrossRef]
- Cox, M.M.; Hashimoto, Y. A fast track influenza virus vaccine produced in insect cells. J. Invertebr. Pathol. 2011, 107, S31–S41. [Google Scholar] [CrossRef]
- Cox, M.M.; Hollister, J.R. FluBlok, a next generation influenza vaccine manufactured in insect cells. Biologicals 2009, 37, 182–189. [Google Scholar] [CrossRef]
- Buckland, B.; Boulanger, R.; Fino, M.; Srivastava, I.; Holtz, K.; Khramtsov, N.; McPherson, C.; Meghrous, J.; Kubera, P.; Cox, M.M. Technology transfer and scale-up of the Flublok recombinant hemagglutinin (HA) influenza vaccine manufacturing process. Vaccine 2014, 32, 5496–5502. [Google Scholar] [CrossRef]
- Bieniossek, C.; Imasaki, T.; Takagi, Y.; Berger, I. MultiBac: Expanding the research toolbox for multiprotein complexes. Trends Biochem. Sci. 2012, 37, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Kolesnikova, O.; Zachayus, A.; Pichard, S.; Osz, J.; Rochel, N.; Rossolillo, P.; Kolb-Cheynel, I.; Troffer-Charlier, N.; Compe, E.; Bensaude, O.; et al. Hr-Bac, a Toolbox Based on Homologous Recombination for Expression, Screening and Production of Multiprotein Complexes Using the Baculovirus Expression System. Sci Rep. 2022, 12, 2030. [Google Scholar] [CrossRef]
- Chen, N.; Kong, X.; Zhao, S.; Xiaofeng, W. Post-translational modification of baculovirus-encoded proteins. Virus Res. 2020, 279, 197865. [Google Scholar] [CrossRef] [PubMed]
- Sari, D.; Gupta, K.; Thimiri Govinda Raj, D.B.; Aubert, A.; Drncová, P.; Garzoni, F.; Fitzgerald, D.; Berger, I. The MultiBac Baculovirus/Insect Cell Expression Vector System for Producing Complex Protein Biologics. Adv. Exp. Med. Biol. 2016, 896, 199–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shokrollahi, N.; Habibi-Anbouhi, M.; Jahanian-Najafabadi, A.; Alirahimi, E.; Behdani, M. Expressing of Recombinant VEGFR2-specific Nanobody in Baculovirus Expression System. Iran. J. Biotechnol. 2021, 19, e2783. [Google Scholar] [CrossRef]
- Lu, H.Y.; Chen, Y.H.; Liu, H.J. Baculovirus as a vaccine vector. Bioengineered 2012, 3, 271–274. [Google Scholar] [CrossRef]
- Organisation forEconomic Co-Operation and Development (OECD) Environment. Consensus Document on Information Ulsed in the Assessment of Enuironmental Applications InvoloingBaculovirus. In Proceedings of the Series on Harmonization of Regulatory Oversight in Biotechnology, Paris, France, 8 January 2002; Number 20. p. 79. [Google Scholar]
- Caubet, J.C.; Ponvert, C. Vaccine allergy. Immunol. Allergy Clin. N. Am. 2014, 34, 597–613, ix. [Google Scholar] [CrossRef]
- Jarvis, D.L. Developing baculovirus-insect cell expression systems for humanized recombinant glycoprotein production. Virology 2003, 310, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Altmann, F. More than silk and honey--or, can insect cells serve in the production of therapeutic glycoproteins? Glycoconj. J. 1997, 14, 643–646. [Google Scholar] [CrossRef]
- Rosano, G.L.; Morales, E.S.; Ceccarelli, E.A. New tools for recombinant protein production in Escherichia coli: A 5-year update. Protein Sci. 2019, 28, 1412–1422. [Google Scholar] [CrossRef]
- Martínez-Solís, M.; Gómez-Sebastián, S.; Escribano, J.M.; Jakubowska, A.K.; Herrero, S. A novel baculovirus-derived promoter with high activity in the baculovirus expression system. PeerJ 2016, 4, e2183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Solís, M.; Herrero, S.; Targovnik, A.M. Engineering of the baculovirus expression system for optimized protein production. Appl. Microbiol. Biotechnol. 2019, 103, 113–123. [Google Scholar] [CrossRef]
- Futatsumori-Sugai, M.; Tsumoto, K. Signal peptide design for improving recombinant protein secretion in the baculovirus expression vector system. Biochem. Biophys. Res. Commun. 2010, 391, 931–935. [Google Scholar] [CrossRef] [PubMed]
- Bleckmann, M.; Schürig, M.; Chen, F.F.; Yen, Z.Z.; Lindemann, N.; Meyer, S.; Spehr, J.; van den Heuvel, J. Identification of Essential Genetic Baculoviral Elements for Recombinant Protein Expression by Transactivation in Sf21 Insect Cells. PLoS ONE 2016, 11, e0149424. [Google Scholar] [CrossRef] [Green Version]
- Masumoto, M.; Ohde, T.; Shiomi, K.; Yaginuma, T.; Niimi, T. A Baculovirus immediate-early gene, ie1, promoter drives efficient expression of a transgene in both Drosophila melanogaster and Bombyx mori. PLoS ONE 2012, 7, e49323. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.-H.; Gwak, W.-S.; Bae, S.-M.; Choi, J.-B.; Han, B.-K.; Woo, S.-D. Increased productivity of the baculovirus expression vector system by combining enhancing factors. J. Asia-Pac. Entomol. 2018, 21, 1079–1084. [Google Scholar] [CrossRef]
- Tiwari, P.; Saini, S.; Upmanyu, S.; Benjamin, B.; Tandon, R.; Saini, K.S.; Sahdev, S. Enhanced expression of recombinant proteins utilizing a modified baculovirus expression vector. Mol. Biotechnol. 2010, 46, 80–89. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, L.; Zhang, Y.; Liu, D.; Du, E.; Yang, Z. Functional analysis of RNAi suppressor P19 on improving baculovirus yield and transgene expression in Sf9 cells. Biotechnol. Lett. 2015, 37, 2159–2166. [Google Scholar] [CrossRef]
- Rao, R.; Fiandra, L.; Giordana, B.; de Eguileor, M.; Congiu, T.; Burlini, N.; Arciello, S.; Corrado, G.; Pennacchio, F. AcMNPV ChiA protein disrupts the peritrophic membrane and alters midgut physiology of Bombyx mori larvae. Insect Biochem. Mol. Biol. 2004, 34, 1205–1213. [Google Scholar] [CrossRef]
- Dong, Z.; Qin, Q.; Hu, Z.; Chen, P.; Huang, L.; Zhang, X.; Tian, T.; Lu, C.; Pan, M. Construction of a One-Vector Multiplex CRISPR/Cas9 Editing System to Inhibit Nucleopolyhedrovirus Replication in Silkworms. Virol. Sin. 2019, 34, 444–453. [Google Scholar] [CrossRef]
- Hebert, C.G.; Valdes, J.J.; Bentley, W.E. In vitro and in vivo RNA interference mediated suppression of Tn-caspase-1 for improved recombinant protein production in High Five cell culture with the baculovirus expression vector system. Biotechnol. Bioeng. 2009, 104, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Hebert, C.G.; Valdes, J.J.; Bentley, W.E. Investigating apoptosis: Characterization and analysis of Trichoplusia ni-caspase-1 through overexpression and RNAi mediated silencing. Insect Biochem. Mol. Biol. 2009, 39, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, K.; Lan, L.; Shi, N.; Nan, H.; Shi, Y.; Xu, X.; Chen, H. Improvement of protein production by engineering a novel antiapoptotic baculovirus vector to suppress the expression of Sf-caspase-1 and Tn-caspase-1. Biotechnol. Bioeng. 2021, 118, 2977–2989. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, V.; Hackl, M.; Druz, A.; Shridhar, S.; Chung, C.Y.; Heffner, K.M.; Kreil, D.P.; Betenbaugh, M.; Shiloach, J.; Barron, N.; et al. CHO microRNA engineering is growing up: Recent successes and future challenges. Biotechnol. Adv. 2013, 31, 1501–1513. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Zhou, Y.; Chen, K.; Ju, X. Suppression of Bm-Caspase-1 Expression in BmN Cells Enhances Recombinant Protein Production in a Baculovirus Expression Vector System. Mol. Biotechnol. 2016, 58, 319–327. [Google Scholar] [CrossRef]
- Lai, Y.K.; Hsu, J.T.; Chu, C.C.; Chang, T.Y.; Pan, K.L.; Lin, C.C. Enhanced recombinant protein production and differential expression of molecular chaperones in sf-caspase-1-repressed stable cells after baculovirus infection. BMC Biotechnol. 2012, 12, 83. [Google Scholar] [CrossRef] [Green Version]
- Legardinier, S.; Klett, D.; Poirier, J.C.; Combarnous, Y.; Cahoreau, C. Mammalian-like nonsialyl complex-type N-glycosylation of equine gonadotropins in Mimic insect cells. Glycobiology 2005, 15, 776–790. [Google Scholar] [CrossRef] [Green Version]
- Toth, A.M.; Kuo, C.W.; Khoo, K.H.; Jarvis, D.L. A new insect cell glycoengineering approach provides baculovirus-inducible glycogene expression and increases human-type glycosylation efficiency. J. Biotechnol. 2014, 182–183, 19–29. [Google Scholar] [CrossRef] [Green Version]
- Aumiller, J.J.; Mabashi-Asazuma, H.; Hillar, A.; Shi, X.; Jarvis, D.L. A new glycoengineered insect cell line with an inducibly mammalianized protein N-glycosylation pathway. Glycobiology 2012, 22, 417–428. [Google Scholar] [CrossRef] [Green Version]
- Mabashi-Asazuma, H.; Jarvis, D.L. CRISPR-Cas9 vectors for genome editing and host engineering in the baculovirus-insect cell system. Proc. Natl. Acad. Sci. USA 2017, 114, 9068–9073. [Google Scholar] [CrossRef]
- Cox, M.M.; Patriarca, P.A.; Treanor, J. FluBlok, a recombinant hemagglutinin influenza vaccine. Influenza Other Respir. Viruses 2008, 2, 211–219. [Google Scholar] [CrossRef] [Green Version]
- Dunkle, L.M.; Izikson, R.; Patriarca, P.A.; Goldenthal, K.L.; Muse, D.; Cox, M.M.J. Randomized Comparison of Immunogenicity and Safety of Quadrivalent Recombinant Versus Inactivated Influenza Vaccine in Healthy Adults 18-49 Years of Age. J. Infect. Dis. 2017, 216, 1219–1226. [Google Scholar] [CrossRef] [Green Version]
- Schwarz, T.F. Clinical update of the AS04-adjuvanted human papillomavirus-16/18 cervical cancer vaccine, Cervarix. Adv. Ther. 2009, 26, 983–998. [Google Scholar] [CrossRef]
- Pollet, J.; Chen, W.H.; Strych, U. Recombinant protein vaccines, a proven approach against coronavirus pandemics. Adv. Drug Deliv. Rev. 2021, 170, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Heath, P.T.; Galiza, E.P.; Baxter, D.N.; Boffito, M.; Browne, D.; Burns, F.; Chadwick, D.R.; Clark, R.; Cosgrove, C.; Galloway, J.; et al. Safety and Efficacy of NVX-CoV2373 Covid-19 Vaccine. N. Engl. J. Med. 2021, 385, 1172–1183. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.H.; Patel, N.; Haupt, R.; Zhou, H.; Weston, S.; Hammond, H.; Logue, J.; Portnoff, A.D.; Norton, J.; Guebre-Xabier, M.; et al. SARS-CoV-2 spike glycoprotein vaccine candidate NVX-CoV2373 immunogenicity in baboons and protection in mice. Nat. Commun. 2021, 12, 372. [Google Scholar] [CrossRef]
- Yang, J.; Wang, W.; Chen, Z.; Lu, S.; Yang, F.; Bi, Z.; Bao, L.; Mo, F.; Li, X.; Huang, Y.; et al. A vaccine targeting the RBD of the S protein of SARS-CoV-2 induces protective immunity. Nature 2020, 586, 572–577. [Google Scholar] [CrossRef]
- Pavot, V.; Berry, C.; Kishko, M.; Anosova, N.G.; Huang, D.; Tibbitts, T.; Raillard, A.; Gautheron, S.; Gutzeit, C.; Koutsoukos, M.; et al. Protein-based SARS-CoV-2 spike vaccine booster increases cross-neutralization against SARS-CoV-2 variants of concern in non-human primates. Nat. Commun. 2022, 13, 1699. [Google Scholar] [CrossRef]
- Honda-Okubo, Y.; Antipov, A.; Andre, G.; Barati, S.; Kafi, H.; Petrovsky, N. Ability of SpikoGen®, an Advax-CpG adjuvanted recombinant spike protein vaccine, to induce cross-neutralising antibodies against SARS-CoV-2 variants. Immunology 2023. [Google Scholar] [CrossRef]
- Uttenthal, A.; Le Potier, M.F.; Romero, L.; De Mia, G.M.; Floegel-Niesmann, G. Classical swine fever (CSF) marker vaccine. Trial I. Challenge studies in weaner pigs. Vet. Microbiol. 2001, 83, 85–106. [Google Scholar] [CrossRef] [PubMed]
- Fachinger, V.; Bischoff, R.; Jedidia, S.B.; Saalmüller, A.; Elbers, K. The effect of vaccination against porcine circovirus type 2 in pigs suffering from porcine respiratory disease complex. Vaccine 2008, 26, 1488–1499. [Google Scholar] [CrossRef] [PubMed]
- Azali, M.A.; Mohamed, S.; Harun, A.; Hussain, F.A.; Shamsuddin, S.; Johan, M.F. Application of Baculovirus Expression Vector system (BEV) for COVID-19 diagnostics and therapeutics: A review. J. Genet. Eng. Biotechnol. 2022, 20, 98. [Google Scholar] [CrossRef] [PubMed]
- Amanat, F.; Strohmeier, S.; Rathnasinghe, R.; Schotsaert, M.; Coughlan, L.; García-Sastre, A.; Krammer, F. Introduction of Two Prolines and Removal of the Polybasic Cleavage Site Lead to Higher Efficacy of a Recombinant Spike-Based Sars-Cov-2 Vaccine in the Mouse Model. mBio 2021, 12, 10-1128. [Google Scholar] [CrossRef]
- Li, T.; Zheng, Q.; Yu, H.; Wu, D.; Xue, W.; Xiong, H.; Huang, X.; Nie, M.; Yue, M.; Rong, R.; et al. SARS-CoV-2 spike produced in insect cells elicits high neutralization titres in non-human primates. Emerg. Microbes Infect. 2020, 9, 2076–2090. [Google Scholar] [CrossRef] [PubMed]
- Francica, J.R.; Flynn, B.J.; Foulds, K.E.; Noe, A.T.; Werner, A.P.; Moore, I.N.; Gagne, M.; Johnston, T.S.; Tucker, C.; Davis, R.L.; et al. Protective antibodies elicited by SARS-CoV-2 spike protein vaccination are boosted in the lung after challenge in nonhuman primates. Sci. Transl. Med. 2021, 13, eabi4547. [Google Scholar] [CrossRef]
- Mi, Y.; Xie, T.; Zhu, B.; Tan, J.; Li, X.; Luo, Y.; Li, F.; Niu, H.; Han, J.; Lv, W.; et al. Production of SARS-CoV-2 Virus-Like Particles in Insect Cells. Vaccines 2021, 9, 554. [Google Scholar] [CrossRef]
- Lu, X.; Chen, Y.; Bai, B.; Hu, H.; Tao, L.; Yang, J.; Chen, J.; Chen, Z.; Hu, Z.; Wang, H. Immune responses against severe acute respiratory syndrome coronavirus induced by virus-like particles in mice. Immunology 2007, 122, 496–502. [Google Scholar] [CrossRef] [Green Version]
- Chu, K.B.; Kang, H.J.; Yoon, K.W.; Lee, H.A.; Moon, E.K.; Han, B.K.; Quan, F.S. Influenza Virus-like Particle (VLP) Vaccines Expressing the SARS-CoV-2 S Glycoprotein, S1, or S2 Domains. Vaccines 2021, 9, 920. [Google Scholar] [CrossRef]
- van Oosten, L.; Altenburg, J.J.; Fougeroux, C.; Geertsema, C.; van den End, F.; Evers, W.A.C.; Westphal, A.H.; Lindhoud, S.; van den Berg, W.; Swarts, D.C.; et al. Two-Component Nanoparticle Vaccine Displaying Glycosylated Spike S1 Domain Induces Neutralizing Antibody Response against SARS-CoV-2 Variants. mBio 2021, 12, e0181321. [Google Scholar] [CrossRef]
- Glass, R.I.; Parashar, U.D.; Estes, M.K. Norovirus gastroenteritis. N. Engl. J. Med. 2009, 361, 1776–1785. [Google Scholar] [CrossRef] [Green Version]
- Adler, J.L.; Zickl, R. Winter vomiting disease. J. Infect. Dis. 1969, 119, 668–673. [Google Scholar] [CrossRef]
- El-Kamary, S.S.; Pasetti, M.F.; Mendelman, P.M.; Frey, S.E.; Bernstein, D.I.; Treanor, J.J.; Ferreira, J.; Chen, W.H.; Sublett, R.; Richardson, C.; et al. Adjuvanted intranasal Norwalk virus-like particle vaccine elicits antibodies and antibody-secreting cells that express homing receptors for mucosal and peripheral lymphoid tissues. J. Infect. Dis. 2010, 202, 1649–1658. [Google Scholar] [CrossRef]
- Atmar, R.L.; Bernstein, D.I.; Harro, C.D.; Al-Ibrahim, M.S.; Chen, W.H.; Ferreira, J.; Estes, M.K.; Graham, D.Y.; Opekun, A.R.; Richardson, C.; et al. Norovirus vaccine against experimental human Norwalk Virus illness. N. Engl. J. Med. 2011, 365, 2178–2187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernstein, D.I.; El Sahly, H.M.; Keitel, W.A.; Wolff, M.; Simone, G.; Segawa, C.; Wong, S.; Shelly, D.; Young, N.S.; Dempsey, W. Safety and immunogenicity of a candidate parvovirus B19 vaccine. Vaccine 2011, 29, 7357–7363. [Google Scholar] [CrossRef] [Green Version]
- Nicastri, E.; Kobinger, G.; Vairo, F.; Montaldo, C.; Mboera, L.E.G.; Ansunama, R.; Zumla, A.; Ippolito, G. Ebola Virus Disease: Epidemiology, Clinical Features, Management, and Prevention. Infect. Dis. Clin. N. Am. 2019, 33, 953–976. [Google Scholar] [CrossRef]
- Bengtsson, K.L.; Song, H.; Stertman, L.; Liu, Y.; Flyer, D.C.; Massare, M.J.; Xu, R.H.; Zhou, B.; Lu, H.; Kwilas, S.A.; et al. Matrix-M adjuvant enhances antibody, cellular and protective immune responses of a Zaire Ebola/Makona virus glycoprotein (GP) nanoparticle vaccine in mice. Vaccine 2016, 34, 1927–1935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, C.B.; Walsh, E.E.; Long, C.E.; Schnabel, K.C. Immunity to and frequency of reinfection with respiratory syncytial virus. J. Infect. Dis. 1991, 163, 693–698. [Google Scholar] [CrossRef]
- Falsey, A.R.; Hennessey, P.A.; Formica, M.A.; Cox, C.; Walsh, E.E. Respiratory syncytial virus infection in elderly and high-risk adults. N. Engl. J. Med. 2005, 352, 1749–1759. [Google Scholar] [CrossRef] [PubMed]
- Raghunandan, R.; Lu, H.; Zhou, B.; Xabier, M.G.; Massare, M.J.; Flyer, D.C.; Fries, L.F.; Smith, G.E.; Glenn, G.M. An insect cell derived respiratory syncytial virus (RSV) F nanoparticle vaccine induces antigenic site II antibodies and protects against RSV challenge in cotton rats by active and passive immunization. Vaccine 2014, 32, 6485–6492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fries, L.; Shinde, V.; Stoddard, J.J.; Thomas, D.N.; Kpamegan, E.; Lu, H.; Smith, G.; Hickman, S.P.; Piedra, P.; Glenn, G.M. Immunogenicity and safety of a respiratory syncytial virus fusion protein (RSV F) nanoparticle vaccine in older adults. Immun. Ageing 2017, 14, 8. [Google Scholar] [CrossRef]
- Murphy, G.S.; Oldfield, E.C., 3rd. Falciparum malaria. Infect. Dis. Clin. N. Am. 1996, 10, 747–775. [Google Scholar] [CrossRef]
- Venkatraman, N.; Anagnostou, N.; Bliss, C.; Bowyer, G.; Wright, D.; Lövgren-Bengtsson, K.; Roberts, R.; Poulton, I.; Lawrie, A.; Ewer, K.; et al. Safety and immunogenicity of heterologous prime-boost immunization with viral-vectored malaria vaccines adjuvanted with Matrix-M™. Vaccine 2017, 35, 6208–6217. [Google Scholar] [CrossRef] [PubMed]
- Mena, J.A.; Kamen, A.A. Insect cell technology is a versatile and robust vaccine manufacturing platform. Expert Rev. Vaccines 2011, 10, 1063–1081. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Miao, Y.; Ke, X.; Tan, Z.; Hu, C.; Li, P.; Wang, T.; Zhang, Y.; Sun, J.; Liu, Y.; et al. Baculovirus Surface Display of Zika Virus Envelope Protein Protects against Virus Challenge in Mouse Model. Virol. Sin. 2020, 35, 637–650. [Google Scholar] [CrossRef]
- Desprès, P.; Dietrich, J.; Girard, M.; Bouloy, M. Recombinant baculoviruses expressing yellow fever virus E and NS1 proteins elicit protective immunity in mice. J. Gen. Virol. 1991, 72 Pt 11, 2811–2816. [Google Scholar] [CrossRef]
- Bonafé, N.; Rininger, J.A.; Chubet, R.G.; Foellmer, H.G.; Fader, S.; Anderson, J.F.; Bushmich, S.L.; Anthony, K.; Ledizet, M.; Fikrig, E.; et al. A recombinant West Nile virus envelope protein vaccine candidate produced in Spodoptera frugiperda expresSF+ cells. Vaccine 2009, 27, 213–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ong, S.H.; Yip, J.T.; Chen, Y.L.; Liu, W.; Harun, S.; Lystiyaningsih, E.; Heriyanto, B.; Beckett, C.G.; Mitchell, W.P.; Hibberd, M.L.; et al. Periodic re-emergence of endemic strains with strong epidemic potential-a proposed explanation for the 2004 Indonesian dengue epidemic. Infect. Genet. Evol. 2008, 8, 191–204. [Google Scholar] [CrossRef]
- Rantam, F.A.; Purwati; Soegijanto, S.; Susilowati, H.; Sudiana, K.; Hendrianto, E.; Soetjipto. Analysis of recombinant, multivalent dengue virus containing envelope (E) proteins from serotypes-1, -3 and -4 and expressed in baculovirus. Trials Vaccinol. 2015, 4, e75–e79. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Li, M.; Wang, Y.; Hao, P.; Jin, X. Elaboration of tetravalent antibody responses against dengue viruses using a subunit vaccine comprised of a single consensus dengue envelope sequence. Vaccine 2017, 35, 6308–6320. [Google Scholar] [CrossRef]

| Strategies | Designation | Optimization Purpose | |
|---|---|---|---|
| Engineering of the baculovirus vector | Promoters: | orf46, pH, p10 | Additive effect when combined |
| Promoters: | 39k, gp64 | Contribute to protein expression | |
| Promoters: | OpIE2, IE1 | As effective initiators of foreign gene expression | |
| Enhancers: | hr5, BSS, vp39 | Enhance the activation ability of the pH promoter | |
| Enhancers: | hr1 | Promote the protein expression | |
| Enhancers: | p19 | Act as an RNAi inhibitor | |
| Remove non-essential genes | p10, p24 | Design baculovirus vectors lacking chitinase and cathepsin | |
| Add beneficial genes to the viral genome | p35 | Produce higher levels of recombinant proteins | |
| Engineering of the cellular host | RNA interference apoptosis-related gene | p35, p49 | Extend the expression of recombinant protein |
| Establish cell lines | derived from Hi5, BmN, Sf9 | Extend the expression of recombinant protein | |
| Classical insect cell lines | SF21, Sf9, Hi5, Tn-368 | Produce less complex N-glycans and baculovirus infection leads to cell death or lysis | |
| Commercial insect cell lines | SfSWT-1, SfSWT-5 | Produce highly processed recombinant proteins with terminal sialic acids complex type N-glycans | |
| Applicable Categories | Target | Name | Antigen | Product Type | Manufacture | Recommended Administration Schedule |
|---|---|---|---|---|---|---|
| Human vaccines | Influenza virus | Flublok® | HA protein | Subunit | Sanofi Pasteur (Paris, France) | Each year |
| Influenza virus | Flublok Quadrivalent® | HA protein | Subunit | Sanofi Pasteur | Each year | |
| Papillomavirus | Cervarix™ | HPV16/18 L1 protein | VLP | GSK (London, UK) | Three times in six months | |
| SARS-CoV-2 | NVX-CoV2373 | S protein | Subunit | Novavax (Malvern, PA, USA) | Eight weeks apart, two injections | |
| SARS-CoV-2 | Weikexin | Recombinant RBD monomer | Subunit | Westvac (Chengdu, China) | Six months apart, two booster injections | |
| SARS-CoV-2 | Trivalent Weikexin | Recombinant RBD monomer | Subunit | Westvac (Chengdu, China) | / | |
| SARS-CoV-2 | VidPrevtyn Beta | SARS-CoV-2 preS dTM | Subunit | Sanofi/GSK (Paris, France/London, UK) | Four months | |
| SARS-CoV-2 | SpikoGen® | S protein extracellular domain | Subunit | Vaxine/CinnaGen Co. (Adelaide, Australia/Tehran, Iran) | Three weeks apart | |
| Animal vaccines | Classical swine fever | Porcilis® Pesti | E2 protein | Subunit | MSD Animal Health (Shanghai, China) | Four weeks apart, two injections |
| Classical swine fever | BAYOVAC CSF E2® | E2 protein | Subunit | Bayer AG/Pfizer Animal (Nordrhein-Westfalen, Germany/Groton, CT, USA) | Four to six weeks apart | |
| Porcine circovirus-2 | CircoFLEX® | PCV2 ORF2 protein | VLP | B. Ingelheim (Berlin, Germany) | Piglets once, breeding pigs three times a year | |
| Porcine circovirus-2 | Porcilis® PCV | PCV2 ORF2 protein | VLP | MSD Animal Health | Two to three weeks apart, two injections | |
| Porcine circovirus-2 | Circumvent® PCV G2 | PCV2a Cap protein | VLP | Merck Animal Health (Madison, NJ, USA) | Just one injection |
| Target | Phase | Antigen | Product Type | Manufacture | NCT Number |
|---|---|---|---|---|---|
| Norwalk virus | Phase II | Norwalk virus-VLP | VLP | Baylor College of Medicine (Houston, TX, USA) | NCT00973284 |
| Phase I | Norwalk virus-VLP | VLP | LigoCyte (Bozeman, MT, USA) | NCT00806962 | |
| Parvovirus B19 | Phase I/II | VP1 and VP2 | VLP | Meridian Life Science (Memphis, TN, USA) | NCT00379938 |
| Ebola virus | Phase I | EBOV Glycoprotein | Subunit | Novavax | NCT02370589 |
| RSV | Phase III | Fusion glycoprotein | Nanoparticle | Novavax | NCT02624947 |
| Malaria | Phase I | ChAd63-MVA ME-TRAP | Viral-vectored | Novavax | NCT01669512 |
| Seasonal influenza virus | Phase III | HA, NA and M1 | Nanoparticle | Novavax | NCT04120194 |
| H1N1 influenza | Phase II | H1N1 2009 Influenza Virus-like Particle | VLP | Novavax | NCT01072799 |
| Papillomavirus | Phase II | HPV (6/11/16/18/31/33/35/39/45/51/52/56/58/59) L1 protein | VLP | SinoCellTech (Beijing, China) | NCT05060484 |
| SARS-CoV-2 | Phase II | SARS-CoV-2 preS dTM | Subunit | Sanofi/GSK | NCT04762680 |
| SARS-CoV-2 | Phase I | SARS-CoV-2 S1 protein | VLP | Radboud University Medical Center (Nijmegen, Netherlands) | NCT04839146 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, Q.; Liu, J.; Wei, Y.; Wei, X. Application of Baculovirus Expression Vector System (BEVS) in Vaccine Development. Vaccines 2023, 11, 1218. https://doi.org/10.3390/vaccines11071218
Hong Q, Liu J, Wei Y, Wei X. Application of Baculovirus Expression Vector System (BEVS) in Vaccine Development. Vaccines. 2023; 11(7):1218. https://doi.org/10.3390/vaccines11071218
Chicago/Turabian StyleHong, Qiaonan, Jian Liu, Yuquan Wei, and Xiawei Wei. 2023. "Application of Baculovirus Expression Vector System (BEVS) in Vaccine Development" Vaccines 11, no. 7: 1218. https://doi.org/10.3390/vaccines11071218
APA StyleHong, Q., Liu, J., Wei, Y., & Wei, X. (2023). Application of Baculovirus Expression Vector System (BEVS) in Vaccine Development. Vaccines, 11(7), 1218. https://doi.org/10.3390/vaccines11071218





