The Development of Methods for the Production of New Molecular Vaccines and Appropriate RNA Fragments to Counteract Unwanted Genes: A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cellular Cultures
2.2. Previously Designed Recombinant DNA Viral Vectors, Based on Adeno-Associated Virus DNA Genome, Containing Inserted Nucleotide Genes/DNA Fragments
2.3. Development of Methods for the Application of Vaccine Avipoxviral Strains as Vectors for the Design of Recombinant DNA Constructs, as Well as for the Exchange of Nucleotide (DNA and/or RNA Fragments) between Viral Particles and Cells
2.3.1. Virus Inoculation of In Vitro-Incubated Cells
2.3.2. Separation of Intra- and Extra-Cellular Forms of Each Vaccine Avipoxviral Strain on Each Cellular Type
2.3.3. Statistical Analysis
3. Results
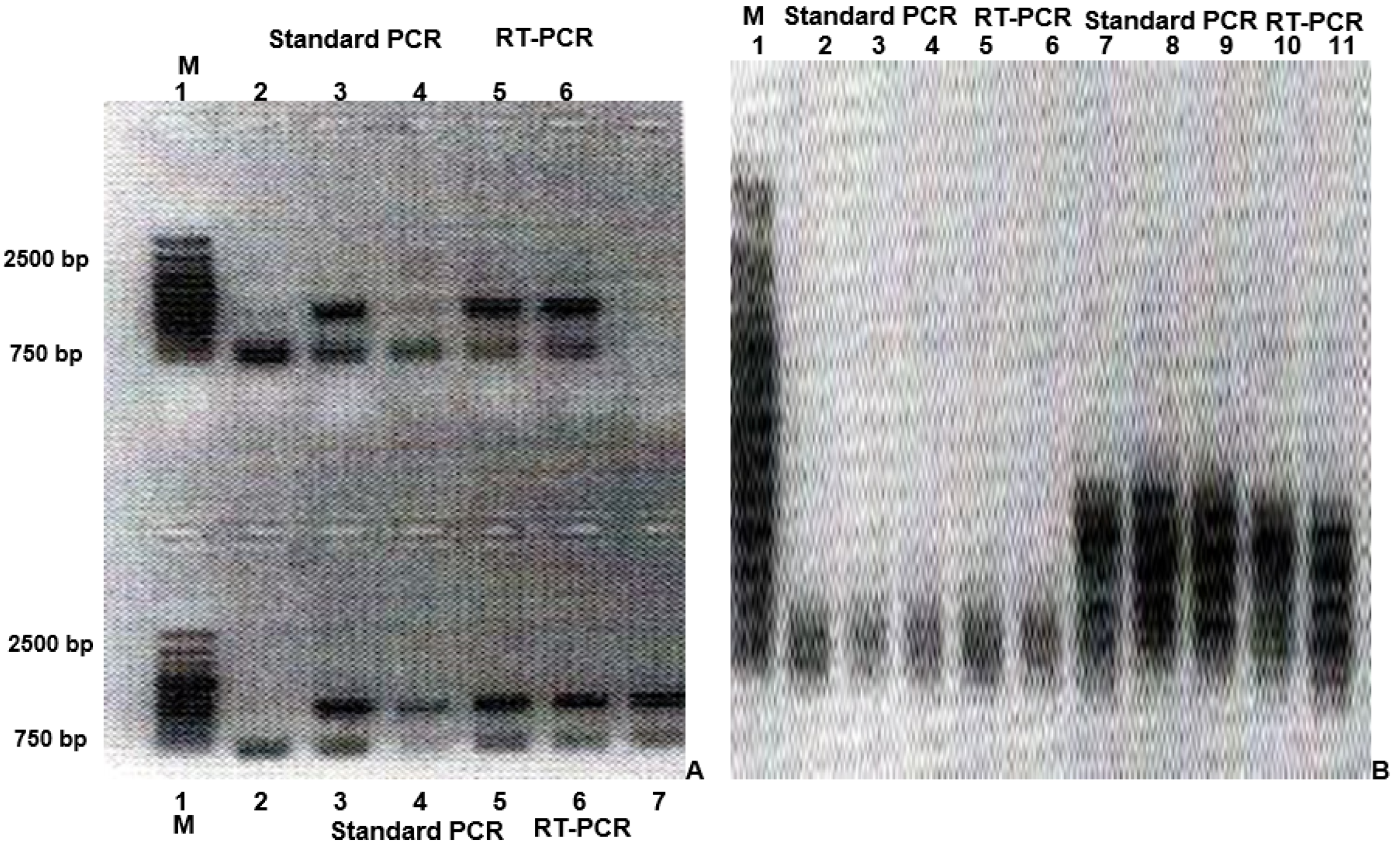
3.1. Investigation on the Presence and Expression of Inserted Copies of One or More Specific Genes of Interest in Previously Designed Recombinant DNA Constructs, Based on the AAV DNA Genome
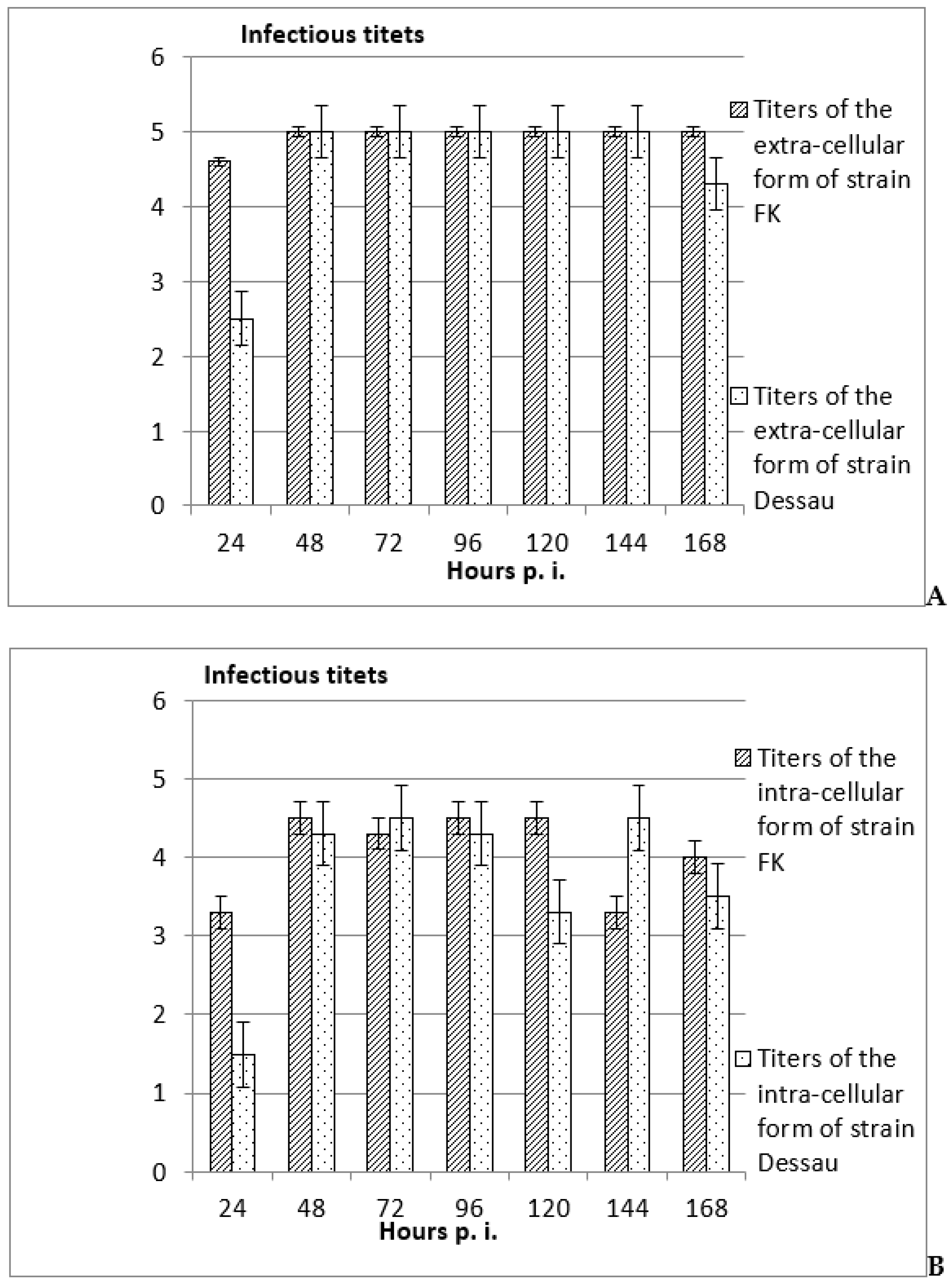
3.2. Investigation of the Possibility for Productive and Non-Productive Replication of Vaccine Avipoxvirus Strains and Isolation of Intra- and Extra-Cellular Forms in Non-Permissive Mammalian Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Palese, P.; Roizman, B. Genetic engineering of viruses and of virus vectors: A preface. Proc. Natl. Acad. Sci. USA 1996, 93, 11287. [Google Scholar] [CrossRef] [PubMed]
- Burt, R.K.; Drobisky, W.R.; Seregina, T.; Traynor, A.; Oyama, Y.; Keever-Taylor, C.; Stefka, J.; Kuzel, T.M.; Brush, M.; Rodriquez, J.; et al. Herpes simplex thymidine kinase gene-transduced donor lymphocyte infusions. Exp. Hematol. 2003, 31, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Barrette, S.; Douglas, J.L.; Seidel, N.E.; Bodine, D.M. Lentivirus-based vectors transducer mouse hematopoietic stem cells with similar efficiency to moloney murine leukemia virus-based vectors. Blood 2000, 96, 3385–3391. [Google Scholar] [CrossRef] [PubMed]
- Brachmann, C.; Davies, A.; Cost, G.J.; Caputo, E.; Li, J.; Hieter, P.; Boeke, J.D. Designer deletion strains derived from Sacharomyces cerevisiae S288C: A useful set of strains and plasmids for PCR-mediated gene disruption and other applications. Yeast 1998, 14, 115–132. [Google Scholar] [CrossRef]
- Domi, A.; Moss, B. Engineering of a vaccinia virus bacterial artificial chromosome in Escherichia coli by bacteriophage λ-based recombination. Nat. Methods 2005, 2, 95–97. [Google Scholar] [CrossRef]
- Galindo, I.; Lorenso, M.; Blasco, R. Set of vectors for the expression of histidine-tagged proteins in vaccinia virus recombinants. Biotechniques 2001, 30, 524–526. [Google Scholar] [CrossRef] [Green Version]
- Flynn, R.A.; Belk, J.A.; Qi, Y.; Yasumoto, Y.; Schmitz, C.O.; Mumbach, M.R.; Limaye, A.; Wei, J.; Alfajaro, M.M.; Parker, K.R.; et al. Systematic discovery and functional interrogation of SARS-CoV-2 RNA-host protein interactions during infection. bioRxiv 2020, 1862, 253. [Google Scholar]
- Chen, Y.; Liu, Q.; Guo, D. Emerging coronaviruses: Genome structure, replication, and pathogenesis. J. Med. Virol. 2020, 92, 418–423. [Google Scholar] [CrossRef] [Green Version]
- Naqvi, A.B.T.; Fatima, K.; Mohammad, T.; Fatima, U.; Singh, I.K.; Singh, A.; Atif, S.M.; Hariprasad, G.; Hasan, G.M.; Hassan, I. Insights into SARS-CoV-2 genome, structure, evolution, pathogenesis and therapies: Structural genomics approach. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165878. [Google Scholar] [CrossRef]
- Perrotta, F.; Matera, M.G.; Cazzola, M.; Bianco, A. Severe respiratory SARS-CoV-2 infection: Does ACE2 receptor matter? Respir. Med. 2020, 168, 105996. [Google Scholar] [CrossRef]
- Manfredonia, I.; Incamato, D. Structure and regulation of coronavirus genomes: State-of-the-art and novel insights from SARS-CoV-2 studies. Biochem. Soc. Transcations 2020, 49, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Mousavisadeh, L.; Ghasemi, S. Genotype and phenotype of COVID-19: Their roles and pathogenesis. J. Microbiol. Immunol. Infect. 2020, 54, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.M.; McFall, E.R.; Burns, J.K.; Parks, R.J. The role of chromatin in adenoviral vector function. Viruses 2013, 5, 1500–1515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonçalves, M.A.F.V. Adeno-associated virus: From defective virus to effective vector. Virol. J. 2005, 2, 43. [Google Scholar] [CrossRef] [Green Version]
- McLaughlin, S.K.; Collis, P.; Hermonat, P.L.; Muzyczka, N. Adeno-associated virus general transduction vectors: Analysis of proviral structures. J. Virol. 1988, 62, 1963–1973. [Google Scholar] [CrossRef] [Green Version]
- Vincent-Lacaze, N.; Snyder, R.O.; Gluzman, R.; Bohl, D.; Lagarde, C.; Danos, O. Structure of adeno associated virus vector following transduction of the skeletal muscle. J. Virol. 1999, 73, 1949–1955. [Google Scholar] [CrossRef] [Green Version]
- Xie, J.; Mao, Q.; Tai, P.W.; He, R.; Ai, J.; Su, Q.; Zhu, Y.; Ma, H.; Li, J.; Gong, S.; et al. Short DNA hairpins compromise recombinant adeno-associated virus genome homogeneity. Mol. Ther. 2017, 25, 1363–1374. [Google Scholar] [CrossRef] [Green Version]
- Mackett, M.; Smith, G.; Moss, B. Vaccinia virus: A selectable eukaryotic cloning and expression vector. Proc. Natl. Acad. Sci. USA 1982, 79, 7415–7419. [Google Scholar] [CrossRef]
- Schnitzlein, W.; Ghildial, N.; Tripathy, D. A rapid method for identifying the thymidine kinase genes of avipoxviruses. J. Virol. Methods 1988, 20, 341–352. [Google Scholar] [CrossRef]
- Spehner, D.; Drillen, R.; Lecocq, J.P. Construction of fowlpox virus vectors with intergenic insertions: Expression of the β-galactosidase gene and the measles fusion protein. J. Virol. 1990, 64, 527–533. [Google Scholar] [CrossRef] [Green Version]
- Simeonov, K.; Ivanov, I.; Popova, T. Propagation of avian pox vaccine strains in mammalian MDBK cell line. Mecedonian Veterninary Res. 2000, 29, 45–50. [Google Scholar]
- Somogyi, P.; Fraizer, J.; Skinner, M.A. Fowlpox virus host range restriction: Gene expression, DNA replication, and morphogenesis in non-permissive mammalian cells. Virology 1993, 197, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Cadoz, M.; Strady, A.; Meignier, B.; Taylor, J.; Tartaglia, J.; Paoletti, E.; Plotkin, S. Immunization of man with canarypox virus expressing rabies glycoprotein. Lancet 1992, 339, 1429–1432. [Google Scholar] [CrossRef] [PubMed]
- Sainova, I.; Kril, A.; Simeonov, K.; Popova, T.; Ivanov, I. Investigation of the morphology of cell clones, derived from the mammalian EBTr cell line and their susceptibility to the vaccine avian poxvirus strains FK and Dessau. J. Virol. Methods 2005, 127, 37–40. [Google Scholar] [CrossRef]
- Ivanov, I.; Kril, A.; Simeonov, K.; Popova, T.; Sainova, I.; Tzvetkov, Y. Propagation of avian pox virus vaccine strains in duck embryo cell cultures. Maced. Vet. Rev. 2000, 29, 33–38. [Google Scholar]
- Kent, S.; Zhao, A.; Best, S.; Chandler, J.; Boyle, D.; Ramshaw, I. Enchanced T-cell immunogenecity and protective efficacy of a human immunodeficiency virus type 1 vaccine regimen consisting of consequtive priming with DNA and boosting with recombinant fowlpox virus. J. Virol. 1998, 72, 10180–10188. [Google Scholar] [CrossRef] [Green Version]
- Lantermann, M.; Schwantes, A.; Sliva, K.; Sutter, G.; Schnierle, B.S. Vaccinia virus double-stranded RNA-binding protein E3 does not interfere with siRNA-mediated gene silencing in mammalian cells. Virus Res. 2007, 126, 1–8. [Google Scholar] [CrossRef]
- Jin, J.; Arias, E.E.; Chen, J.; Harper, J.W.; Walter, J.C. A family of diverse Cul4-Dbd1-interacting proteins includes Cdt2, which is required for S phase destruction of the replication factor Cdt1. Mol. Cell 2006, 23, 709–721. [Google Scholar] [CrossRef]
- Kurz, T.; Ozlu, N.; Rudolf, F.; O’Rourke, S.M.; Luke, B.; Hofmann, K.; Hyman, A.A.; Bowerman, B.; Peter, M. The conversed protein DCN-1/Dcn1p is required for cullin neddylation in C. elegans and S. cerevisiae. Nature 2005, 435, 1257–1261. [Google Scholar] [CrossRef]
- Kurz, T.; Chou, Y.-C.; Willems, A.R.; Meyer-Schaller, N.; Hecht, M.-L.; Tyers, M.; Peter, M.; Sicheri, F. Dcn1 functions as a scaffold-type E3 ligase for cullin neddylation. Mol. Cell 2008, 29, 23–35. [Google Scholar] [CrossRef]
- Meyer-Schaller, N.; Chou, Y.-C.; Sumara, I.; Martin, D.D.O.; Kurz, T.; Katheder, N.; Hofmann, K.; Berthiaume, L.G.; Sicheri, F.; Peter, M. The human Dcn1-like protein DCNL3 promotes Cul3 neddylation at membranes. Proc. Natl. Acad. Sci. USA 2009, 106, 12365–12370. [Google Scholar] [CrossRef]
- O’Charoenrat, P.; Sarkaria, I.; Talbot, S.G.; Reddy, P.; Dao, S.; Ngai, I.; Shaha, A.; Kraus, D.; Shah, J.; Rusch, V.; et al. SCCRO (DCUN1D1) induces extracellular matrix invasion by activating matrix metalloproteinase 2. Clin. Cancer Res. 2008, 14, 6780–6789. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Anglesio, M.S.; O’Sullivan, M.; Zhang, F.; Yang, G.; Sarao, R.; Nghiem, M.P.; Cronin, S.; Hara, H.; Melnyk, N.; et al. The E3 ligase HACE1 is a critical chromosome 6q21 tumor suppressor involved in multiple cancers. Nat. Med. 2007, 13, 1060–1069. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Zhu, H. Single-stranded conformational polymorphism analysis: Basic principles and routine practice. Meth. Mol. Med. 2005, 108, 149–157. [Google Scholar]
- Kakavas, K.V. Sensitivity and applications of the PCR single-strand conformation polymorphism method. Mol. Biol. Rep. 2021, 48, 3629–3635. [Google Scholar] [CrossRef]
- Sainova, I.; Vavrek, I.; Pavlova, V.; Daneva, T.; Manchev, S.; Nikolova, E. Experimental model for safe gene transfer by recombinant gene constructs. Biotechnol. Biotechnol. Equip. 2010, 24, 134–138. [Google Scholar] [CrossRef]
- Sainova, I.; Vavrek, I.; Pavlova, V.; Iliev, I.; Yossifova, L.; Gardeva, E.; Nikolova, E. Experimental model for safe in vitro- and in vivo-influence of internal and external factors on cell differentiation. Afr. J. Pharm. Pharmacol. 2011, 5, 786–791. [Google Scholar] [CrossRef]
- Rortman, J.J.; Apostolova, M.K.A.; Philip, M. Viral and cellular oncogenes promote immune evasion. Oncogene 2022, 41, 921–929. [Google Scholar] [CrossRef]
- Ali, S.H.; Kasper, S.J.; Arai, T.; DeKaprio, J.A. Cul7/p185/p193 binding to Simian Virus 40 large T antigen has a role in cellular transformation. J. Virol. 2004, 78, 2749–2757. [Google Scholar] [CrossRef] [Green Version]
- Takao, T.; Asanoma, K.; Kato, K.; Fukushima, K.; Tsunematsu, R.; Hirakawa, T.; Matsumura, S.; Seki, H.; Takeda, S.; Wake, N. Isolation and characterization of human trophoblast side population (SP) cells in primary villous cytotrophoblasts and HTR8/SVneo cell line. PLoS ONE 2011, 6, e21990. [Google Scholar] [CrossRef] [Green Version]
- Ni, W.; Yang, X.; Yang, D.; Bao, J.; Li, R.; Xiao, Y.; Hou, C.; Wang, H.; Liu, J.; Yang, D.; et al. Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19. Crit. Care 2020, 24, 422. [Google Scholar] [CrossRef] [PubMed]
- Tikellis, C.; Thomas, M.C. Angiotensin-converting enzyme 2 (ACE2) is a key modulator of the renin angiotensin system in health and disease. Int. J. Pept. 2012, 2012, 256294. [Google Scholar] [CrossRef] [PubMed]
- Clarke, N.E.; Turner, A.J. Angiotensin-converting enzyme 2: The first decade. Int. J. Hypertens. 2012, 2012, 307315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamming, I.; Cooper, M.; Haagmans, B.; Hooper, N.; Korstanje, R.; Osterhaus, A.; Timens, W.; Turner, A.; Navis, G.; van Goor, H. The emerging role of ACE2 in physiology and disease. J. Pathol. 2007, 212, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Davison, A.J.; Benko, M.; Harrach, B. Genetic content and evolution of adenoviruses. J. Gen. Virol. 2003, 84, 2895–2908. [Google Scholar] [CrossRef]
- Kulanayake, S.; Tikoo, S.K. Adenovirus core proteins: Structure and function. Viruses 2021, 13, 388. [Google Scholar] [CrossRef]
- Saha, B.; Wong, C.M.; Parks, R.J. The adenovirus genome contributes to the structural stability. Viruses 2014, 6, 3563–3583. [Google Scholar] [CrossRef] [Green Version]
- Ishii, K.; Ueda, Y.; Matsuo, K.; Matsuura, Y.; Kitamura, T.; Kato, K.; Izumi, Y.; Someya, K.; Ohsu, T.; Honda, M.; et al. Structural analysis of vaccinia virus Dis strain: Application as a new replication-deficient viral vector. Virology 2002, 302, 433–444. [Google Scholar] [CrossRef]
- Larson, G.P.; Tran, V.; Yú, S.; Caì, Y.; Higgins, C.A.; Smith, D.M.; Baker, S.F.; Radoshitzky, S.R.; Kuhn, J.H.; Mehle, A. EPS8 substrate facilitates uncoating of influenza A virus. Cell Rep. 2019, 29, 2175–2183. [Google Scholar] [CrossRef] [Green Version]
- Jacob, S.W.; Herschler, R. Pharmacology of DMSO. Cryobiology 1986, 23, 14–27. [Google Scholar] [CrossRef]
- de Ménorvan, M.A.; Mir, L.M.; Fernández, L.M.; Reigada, R. Effects of dymethyl sulfoxide in cholesterolcontaining lipid membranes: A comparative study of experiments in silico and with cells. PLoS ONE 2012, 7, e41733. [Google Scholar]
- Norwood, T.H.; Zeigler, C.J.; Martin, G.M. Dimethyl sulfoxide enchances polyethylene glycol-mediated somatic cell fusion. Somat. Cell Genet. 1976, 2, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.A.; Fukushima, M.; Davis, R.W. DMSO and betaine greatly improve amplification of GC-rich constructs in de novo synthesis. PLoS ONE 2010, 5, e11024. [Google Scholar] [CrossRef] [Green Version]
- Hart, C.A.; Ahkong, Q.F.; Fisher, D.; Goodall, A.H.; Hallinan, T.; Lucy, J.A. Effects of detergents on virus- and chemically-induced fusion of erythrocytes. Biochem. Soc. Trans. 1975, 3, 733–734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frey, S.; Marsh, M.; Günther, S.; Pelchen-Matthews, A.; Stephens, P.; Ortlepp, S.; Stegmann, T. Temperature dependence of cell-cell fusion induced by the envelope glycoprotein of human immunodeficiency virus type 1. J. Virol. 1995, 69, 1462–1472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manandhar, G.; Onishchenko, G.E. Centriolar cycle of fused cells. J. Cell Sci. 1995, 108, 667–673. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sainova, I.; Kolyovska, V.; Ilieva, I.; Markova, T.; Dimitrova-Dikanarova, D.; Hadjiolova, R. The Development of Methods for the Production of New Molecular Vaccines and Appropriate RNA Fragments to Counteract Unwanted Genes: A Pilot Study. Vaccines 2023, 11, 1226. https://doi.org/10.3390/vaccines11071226
Sainova I, Kolyovska V, Ilieva I, Markova T, Dimitrova-Dikanarova D, Hadjiolova R. The Development of Methods for the Production of New Molecular Vaccines and Appropriate RNA Fragments to Counteract Unwanted Genes: A Pilot Study. Vaccines. 2023; 11(7):1226. https://doi.org/10.3390/vaccines11071226
Chicago/Turabian StyleSainova, Iskra, Vera Kolyovska, Iliana Ilieva, Tzvetanka Markova, Dimitrina Dimitrova-Dikanarova, and Radka Hadjiolova. 2023. "The Development of Methods for the Production of New Molecular Vaccines and Appropriate RNA Fragments to Counteract Unwanted Genes: A Pilot Study" Vaccines 11, no. 7: 1226. https://doi.org/10.3390/vaccines11071226





