Self-Adjuvanting Calcium-Phosphate-Coated Microcrystal-Based Vaccines Induce Pyroptosis in Human and Livestock Immune Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Human Donors
2.2. Formulation of CaP-PCMCs
2.3. Isolation of Bovine and Ovine Peripheral Blood Mononuclear Cells (PBMC)
2.4. Monocyte and Lymphocyte Purification from Cattle PBMCs
2.5. CaP-PCMCs Induced Apoptosis in Cattle Immune Cells
2.5.1. Detection of CaP-PCMCs Induced Apoptosis
2.5.2. Influence of CaCl2 and CaP-PCMC Composition on Apoptosis
2.5.3. Cell–CaP-PCMC Interaction Studies
2.6. Generation of Monocyte-Derived Dendritic Cells (MoDCs)
2.7. Caspase-1 Activation Assay
2.8. Quantification of Cytokines by ELISA
2.9. Statistical Analysis
3. Results
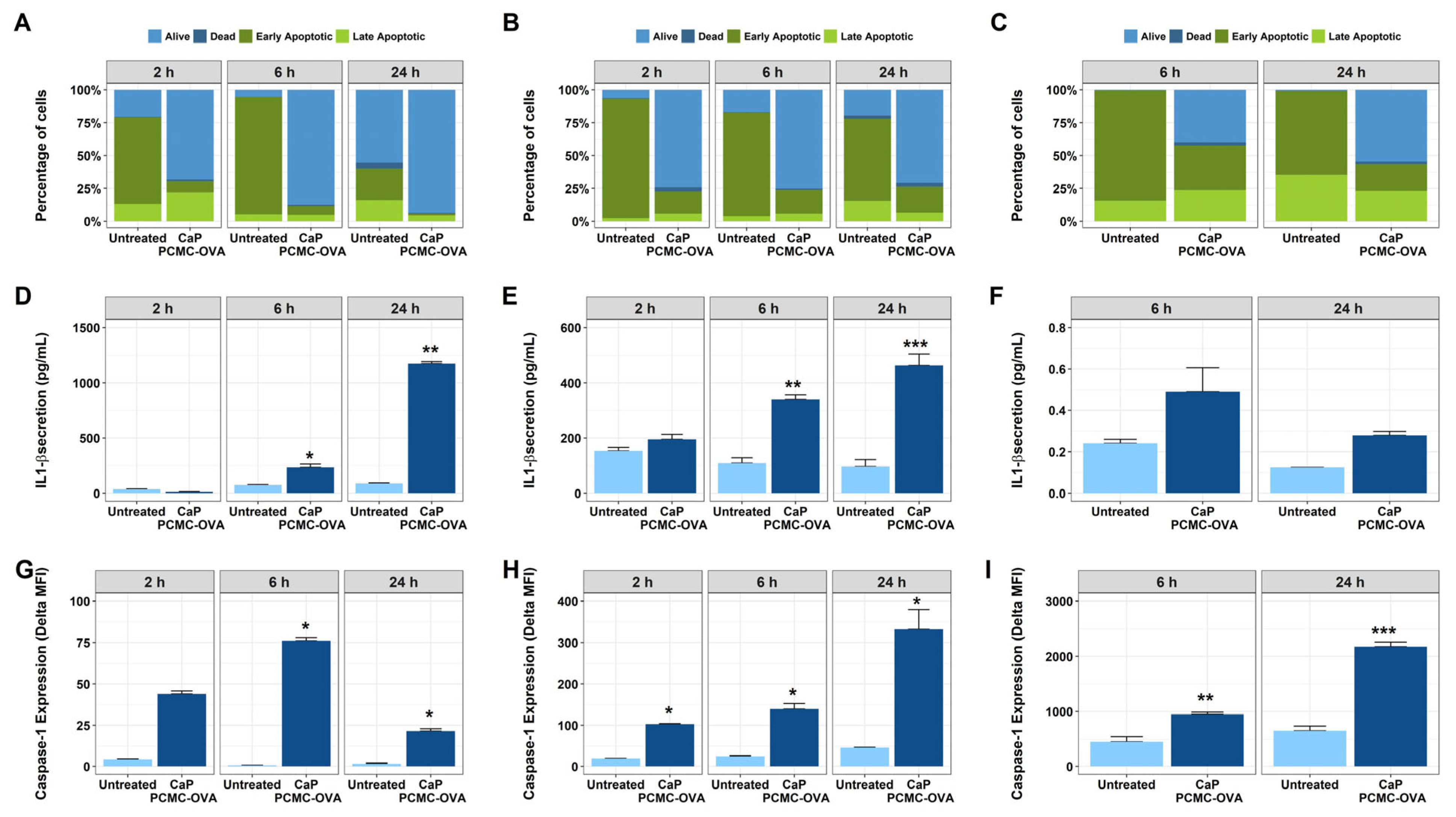
3.1. In Vitro Effect of CaP-PCMCs on Cattle PBMC-Apoptosis Studies
3.1.1. Induction of Cell Death by CaP-PCMCs
3.1.2. Dose Dependence Studies
3.1.3. Levels of CaP-PCMCs Induced Cell Death in White Blood Cell Populations
3.2. Investigating Factors That Influence Cell Death: Effects of Composition and Contact with CaP-PCMCs on Apoptosis
3.2.1. Effects of the Composition of CaP-PCMCs on Cell Death
3.2.2. Effect of Cell-to-CaP-PCMC Contact on Cell Death
3.3. CaP-PCMCs Induced Apoptosis in Model Antigen-Presenting Cells in Three Mammalian Species
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bloom, D.E.; Black, S.; Salisbury, D.; Rappuoli, R. Antimicrobial resistance and the role of vaccines. Proc. Natl. Acad. Sci. USA 2018, 115, 12868–12871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lahariya, C. A brief history of vaccines & vaccination in India. Indian J. Med. Res. 2014, 139, 491. [Google Scholar] [PubMed]
- Hajj Hussein, I.; Chams, N.; Chams, S.; El Sayegh, S.; Badran, R.; Raad, M.; Gerges-Geagea, A.; Leone, A.; Jurjus, A. Vaccines Through Centuries: Major Cornerstones of Global Health. Front. Public Health 2015, 3, 269. [Google Scholar] [CrossRef] [PubMed]
- Liniger, M.; Zuniga, A.; Naim, H.Y. Use of viral vectors for the development of vaccines. Expert Rev. Vaccines 2007, 6, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Leroux-Roels, G. Unmet needs in modern vaccinology: Adjuvants to improve the immune response. Vaccine 2010, 28, C25–C36. [Google Scholar] [CrossRef]
- Soler, E.; Houdebine, L.-M. Preparation of recombinant vaccines. In Biotechnology Annual Review; El-Gewely, M.R., Ed.; Elsevier: Amsterdam, The Netherlands, 2007; Volume 13, pp. 65–94. [Google Scholar]
- Li, H.; Nookala, S.; Re, F. Aluminum Hydroxide Adjuvants Activate Caspase-1 and Induce IL-1β and IL-18 Release. J. Immunol. 2007, 178, 5271–5276. [Google Scholar] [CrossRef]
- Lindblad, E.B. Aluminium adjuvants—In retrospect and prospect. Vaccine 2004, 22, 3658–3668. [Google Scholar] [CrossRef]
- He, Q.; Mitchell, A.R.; Johnson, S.L.; Wagner-Bartak, C.; Morcol, T.; Bell, S.J.D. Calcium Phosphate Nanoparticle Adjuvant. Clin. Diagn. Lab. Immunol. 2000, 7, 899–903. [Google Scholar] [CrossRef] [Green Version]
- McKee, A.S.; Marrack, P. Old and new adjuvants. Curr Opin Immunol 2017, 47, 44–51. [Google Scholar] [CrossRef]
- Karch, C.P.; Burkhard, P. Vaccine technologies: From whole organisms to rationally designed protein assemblies. Biochem. Pharmacol. 2016, 120, 1–14. [Google Scholar] [CrossRef]
- O’Hagan, D.T.; Fox, C.B. New generation adjuvants—From empiricism to rational design. Vaccine 2015, 33, B14–B20. [Google Scholar] [CrossRef] [PubMed]
- Foged, C. Subunit vaccines of the future: The need for safe, customized and optimized particulate delivery systems. Ther. Deliv. 2011, 2, 1057–1077. [Google Scholar] [CrossRef] [PubMed]
- Bramwell, V.W.; Perrie, Y. Particulate Delivery Systems for Vaccines. Crit Rev Drug Carr. Syst 2005, 22, 151–214. [Google Scholar] [CrossRef] [PubMed]
- Pashine, A.; Valiante, N.M.; Ulmer, J.B. Targeting the innate immune response with improved vaccine adjuvants. Nat. Med. 2005, 11, S63–S68. [Google Scholar] [CrossRef] [PubMed]
- Bergmann-Leitner, E.S.; Leitner, W.W. Adjuvants in the Driver’s Seat: How Magnitude, Type, Fine Specificity and Longevity of Immune Responses Are Driven by Distinct Classes of Immune Potentiators. Vaccines 2014, 2, 252–296. [Google Scholar] [CrossRef] [Green Version]
- McSorley, S.J.; Ehst, B.D.; Yu, Y.; Gewirtz, A.T. Bacterial Flagellin Is an Effective Adjuvant for CD4+ T Cells In Vivo. J. Immunol. 2002, 169, 3914–3919. [Google Scholar] [CrossRef]
- Bali, P. Modulating vaccine responses with dendritic cells and Toll-like receptors. Immunol. Rev. 2004, 199, 227–250. [Google Scholar] [CrossRef]
- Ishii, K.J.; Akira, S. Toll or Toll-Free Adjuvant Path Toward the Optimal Vaccine Development. J. Clin. Immunol. 2007, 27, 363–371. [Google Scholar] [CrossRef]
- Weeratna, R.D.; Makinen, S.R.; McCluskie, M.J.; Davis, H.L. TLR agonists as vaccine adjuvants: Comparison of CpG ODN and Resiquimod (R-848). Vaccine 2005, 23, 5263–5270. [Google Scholar] [CrossRef]
- Aderem, A.; Ulevitch, R.J. Toll-like receptors in the induction of the innate immune response. Nature 2000, 406, 782–787. [Google Scholar] [CrossRef]
- Knuschke, T.; Epple, M.; Westendorf, A.M. The type of adjuvant strongly influences the T-cell response during nanoparticle-based immunization. Hum. Vaccines Immunother. 2014, 10, 164–169. [Google Scholar] [CrossRef] [Green Version]
- Apostólico, J.d.S.; Boscardin, S.B.; Yamamoto, M.M.; de Oliveira-Filho, J.N.; Kalil, J.; Cunha-Neto, E.; Rosa, D.S. HIV Envelope Trimer Specific Immune Response Is Influenced by Different Adjuvant Formulations and Heterologous Prime-Boost. PLoS ONE 2016, 11, e0145637. [Google Scholar] [CrossRef] [Green Version]
- Pavot, V.; Rochereau, N.; Rességuier, J.; Gutjahr, A.; Genin, C.; Tiraby, G.; Perouzel, E.; Lioux, T.; Vernejoul, F.; Verrier, B.; et al. Cutting Edge: New Chimeric NOD2/TLR2 Adjuvant Drastically Increases Vaccine Immunogenicity. J. Immunol. 2014, 193, 5781–5785. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, T.; Momota, M.; Kuroda, E.; Kusakabe, T.; Kobari, S.; Makisaka, K.; Ohno, Y.; Suzuki, Y.; Nakagawa, F.; Lee, M.S.J.; et al. DAMP-Inducing Adjuvant and PAMP Adjuvants Parallelly Enhance Protective Type-2 and Type-1 Immune Responses to Influenza Split Vaccination. Front. Immunol. 2018, 9, 2619. [Google Scholar] [CrossRef]
- Bergsbaken, T.; Fink, S.L.; Cookson, B.T. Pyroptosis: Host cell death and inflammation. Nat. Rev. Microbiol. 2009, 7, 99–109. [Google Scholar] [CrossRef] [Green Version]
- Fink, S.L.; Cookson, B.T. Apoptosis, Pyroptosis, and Necrosis: Mechanistic Description of Dead and Dying Eukaryotic Cells. Infect. Immun. 2005, 73, 1907–1916. [Google Scholar] [CrossRef] [Green Version]
- Frank, D.; Vince, J.E. Pyroptosis versus necroptosis: Similarities, differences, and crosstalk. Cell Death Differ. 2019, 26, 99–114. [Google Scholar] [CrossRef] [Green Version]
- Aimanianda, V.; Haensler, J.; Lacroix-Desmazes, S.; Kaveri, S.V.; Bayry, J. Novel cellular and molecular mechanisms of induction of immune responses by aluminum adjuvants. Trends Pharmacol. Sci. 2009, 30, 287–295. [Google Scholar] [CrossRef]
- Marichal, T.; Ohata, K.; Bedoret, D.; Mesnil, C.; Sabatel, C.; Kobiyama, K.; Lekeux, P.; Coban, C.; Akira, S.; Ishii, K.J.; et al. DNA released from dying host cells mediates aluminum adjuvant activity. Nat. Med. 2011, 17, 996–1002. [Google Scholar] [CrossRef]
- Arakelian, T.; Oosterhuis, K.; Tondini, E.; Los, M.; Vree, J.; van Geldorp, M.; Camps, M.; Teunisse, B.; Zoutendijk, I.; Arens, R.; et al. Pyroptosis-inducing active caspase-1 as a genetic adjuvant in anti-cancer DNA vaccination. Vaccine 2022, 40, 2087–2098. [Google Scholar] [CrossRef]
- Murdan, S.; Somavarapu, S.; Ross, A.C.; Alpar, H.O.; Parker, M.C. Immobilisation of vaccines onto micro-crystals for enhanced thermal stability. Int. J. Pharm. 2005, 296, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.; Asokanathan, C.; Kmiec, D.; Irvine, J.; Fleck, R.; Xing, D.; Moore, B.; Parton, R.; Coote, J. Protein coated microcrystals formulated with model antigens and modified with calcium phosphate exhibit enhanced phagocytosis and immunogenicity. Vaccine 2014, 32, 4234–4242. [Google Scholar] [CrossRef] [Green Version]
- Brandau, D.T.; Jones, L.S.; Wiethoff, C.M.; Rexroad, J.; Middaugh, C.R. Thermal Stability of Vaccines. J. Pharm. Sciences 2003, 92, 218–231. [Google Scholar] [CrossRef] [PubMed]
- Matthias, D.M.; Robertson, J.; Garrison, M.M.; Newland, S.; Nelson, C. Freezing temperatures in the vaccine cold chain: A systematic literature review. Vaccine 2007, 25, 3980–3986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feinmann, J. How the world is (not) handling surplus doses and expiring vaccines. BMJ 2021, 374, n2062. [Google Scholar] [CrossRef]
- Kreiner, M.; Amorim Fernandes, J.F.; O’Farrell, N.; Halling, P.J.; Parker, M.-C. Stability of protein-coated microcrystals in organic solvents. J. Mol. Catal. B Enzym. 2005, 33, 65–72. [Google Scholar] [CrossRef]
- Khosravani, A.; Parker, M.-C.; Parton, R.; Coote, J. Formulation of the adenylate cyclase toxin of Bordetella pertussis as protein-coated microcrystals. Vaccine 2007, 25, 4361–4367. [Google Scholar] [CrossRef]
- Corripio-Miyar, Y.; Hope, J.; McInnes, C.J.; Wattegedera, S.R.; Jensen, K.; Pang, Y.; Entrican, G.; Glass, E.J. Phenotypic and functional analysis of monocyte populations in cattle peripheral blood identifies a subset with high endocytic and allogeneic T-cell stimulatory capacity. Vet. Res. 2015, 46, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Sokolovska, A.; Becker, C.E.; Ip, W.K.E.; Rathinam, V.A.K.; Brudner, M.; Paquette, N.; Tanne, A.; Vanaja, S.K.; Moore, K.J.; Fitzgerald, K.A.; et al. Activation of caspase-1 by the NLRP3 inflammasome regulates the NADPH oxidase NOX2 to control phagosome function. Nat. Immunol. 2013, 14, 543–553. [Google Scholar] [CrossRef] [Green Version]
- Mattson, M.P.; Chan, S.L. Calcium orchestrates apoptosis. Nat. Cell Biol. 2003, 5, 1041–1043. [Google Scholar] [CrossRef] [Green Version]
- Moore, B.D. (University of Strathclyde, Glasgow, UK). Personal communication, 2019. [Google Scholar]
- Knudsen, N.P.H.; Olsen, A.; Buonsanti, C.; Follmann, F.; Zhang, Y.; Coler, R.N.; Fox, C.B.; Meinke, A.; D’Oro, U.; Casini, D.; et al. Different human vaccine adjuvants promote distinct antigen-independent immunological signatures tailored to different pathogens. Sci. Rep. 2016, 6, 19570. [Google Scholar] [CrossRef] [Green Version]
- Pifferi, C.; Fuentes, R.; Fernández-Tejada, A. Natural and synthetic carbohydrate-based vaccine adjuvants and their mechanisms of action. Nat. Rev. Chem. 2021, 5, 197–216. [Google Scholar] [CrossRef]
- Lindblad, E.B. Aluminium compounds for use in vaccines. Immunol. Cell Biol. 2004, 82, 497–505. [Google Scholar] [CrossRef]
- Eisenbarth, S.C.; Colegio, O.R.; O’Connor, W.; Sutterwala, F.S.; Flavell, R.A. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature 2008, 453, 1122–1126. [Google Scholar] [CrossRef] [Green Version]
- Kool, M.; Fierens, K.; Lambrecht, B.N. Alum adjuvant: Some of the tricks of the oldest adjuvant. J. Med. Microbiol. 2012, 61, 927–934. [Google Scholar] [CrossRef]
- Macy, D.W. Vaccine adjuvants. Semin. Vet. Med. Surg. Small Anim. 1997, 12, 206–211. [Google Scholar] [CrossRef]
- Spickler, A.R.; Roth, J.A. Adjuvants in Veterinary Vaccines: Modes of Action and Adverse Effects. J. Vet. Intern. Med. 2003, 17, 273–281. [Google Scholar] [CrossRef]
- Epple, M.; Ganesan, K.; Heumann, R.; Klesing, J.; Kovtun, A.; Neumann, S.; Sokolova, V. Application of calcium phosphate nanoparticles in biomedicine. J. Mater. Chem. 2010, 20, 18–23. [Google Scholar] [CrossRef]
- Moore, B.D.; New, R.R.C.; Butcher, W.; Mahood, R.; Steward, J.; Bayliss, M.; MacLeod, C.; Bogus, M.; Williamson, E.D. Dual route vaccination for plague with emergency use applications. Vaccine 2018, 36, 5210–5217. [Google Scholar] [CrossRef]
- New, R.R.C.; Moore, B.D.; Butcher, W.; Mahood, R.; Lever, M.S.; Smither, S.; O’Brien, L.; Weller, S.A.; Bayliss, M.; Gibson, L.C.D.; et al. Antibody-mediated protection against MERS-CoV in the murine model. Vaccine 2019, 37, 4094–4102. [Google Scholar] [CrossRef]
- Orrenius, S.; Zhivotovsky, B.; Nicotera, P. Regulation of cell death: The calcium–apoptosis link. Nat. Rev. Mol. Cell Biol. 2003, 4, 552. [Google Scholar] [CrossRef]
- Bagur, R.; Hajnóczky, G. Intracellular Ca(2+) Sensing: Its Role in Calcium Homeostasis and Signaling. Mol Cell 2017, 66, 780–788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dupuis, M.; Denis-Mize, K.; LaBarbara, A.; Peters, W.; Charo, I.F.; McDonald, D.M.; Ott, G. Immunization with the adjuvant MF59 induces macrophage trafficking and apoptosis. Eur. J. Immunol. 2001, 31, 2910–2918. [Google Scholar] [CrossRef] [PubMed]
- Howard, C.J.; Charleston, B.; Stephens, S.A.; Sopp, P.; Hope, J.C. The role of dendritic cells in shaping the immune response. Anim. Health Res. Rev. 2004, 5, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Cypryk, W.; Nyman, T.A.; Matikainen, S. From Inflammasome to Exosome—Does Extracellular Vesicle Secretion Constitute an Inflammasome-Dependent Immune Response? Front. Immunol. 2018, 9, 2188. [Google Scholar] [CrossRef] [Green Version]
- Miao, E.A.; Rajan, J.V.; Aderem, A. Caspase-1-induced pyroptotic cell death. Immunol Rev. 2011, 243, 206–214. [Google Scholar] [CrossRef] [Green Version]
- Bergsbaken, T.; Fink, S.L.; den Hartigh, A.B.; Loomis, W.P.; Cookson, B.T. Coordinated Host Responses during Pyroptosis: Caspase-1–Dependent Lysosome Exocytosis and Inflammatory Cytokine Maturation. J. Immunol. 2011, 187, 2748–2754. [Google Scholar] [CrossRef] [Green Version]
- Boise, L.H.; Collins, C.M. Salmonella-induced cell death: Apoptosis, necrosis or programmed cell death? Trends Microbiol. 2001, 9, 64–67. [Google Scholar] [CrossRef]
- Sansonetti, P.J.; Phalipon, A.; Arondel, J.; Thirumalai, K.; Banerjee, S.; Akira, S.; Takeda, K.; Zychlinsky, A. Caspase-1 Activation of IL-1β and IL-18 Are Essential for Shigella flexneri–Induced Inflammation. Immunity 2000, 12, 581–590. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Sun, B. Neutrophil pyroptosis: New perspectives on sepsis. Cell. Mol. Life Sci. 2019, 76, 2031–2042. [Google Scholar] [CrossRef] [Green Version]
- Reinke, S.; Thakur, A.; Gartlan, C.; Bezbradica, J.S.; Milicic, A. Inflammasome-Mediated Immunogenicity of Clinical and Experimental Vaccine Adjuvants. Vaccines 2020, 8, 554. [Google Scholar] [CrossRef]
- Suschak, J.J.; Wang, S.; Fitzgerald, K.A.; Lu, S. Identification of Aim2 as a Sensor for DNA Vaccines. J. Immunol. 2015, 194, 630–636. [Google Scholar] [CrossRef] [Green Version]
- Curtsinger, J.M.; Mescher, M.F. Inflammatory cytokines as a third signal for T cell activation. Curr. Opin. Immunol. 2010, 22, 333–340. [Google Scholar] [CrossRef] [Green Version]
- Duhen, T.; Geiger, R.; Jarrossay, D.; Lanzavecchia, A.; Sallusto, F. Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat. Immunol. 2009, 10, 857–863. [Google Scholar] [CrossRef]
- Pitt, J.M.; Stavropoulos, E.; Redford, P.S.; Beebe, A.M.; Bancroft, G.J.; Young, D.B.; O’Garra, A. Blockade of IL-10 Signaling during Bacillus Calmette-Guérin Vaccination Enhances and Sustains Th1, Th17, and Innate Lymphoid IFN-γ and IL-17 Responses and Increases Protection to Mycobacterium tuberculosis Infection. J. Immunol. 2012, 189, 4079–4087. [Google Scholar] [CrossRef] [Green Version]
- Gopal, R.; Lin, Y.; Obermajer, N.; Slight, S.; Nuthalapati, N.; Ahmed, M.; Kalinski, P.; Khader, S.A. IL-23-dependent IL-17 drives Th1-cell responses following Mycobacterium bovis BCG vaccination. Eur. J. Immunol. 2012, 42, 364–373. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, M.; Jiao, H.; Domingo-Gonzalez, R.; Das, S.; Griffiths, K.L.; Rangel-Moreno, J.; Nagarajan, U.M.; Khader, S.A. Rationalized design of a mucosal vaccine protects against Mycobacterium tuberculosis challenge in mice. J. Leukoc. Biol. 2017, 101, 1373–1381. [Google Scholar] [CrossRef] [Green Version]
- Sharp, F.A.; Ruane, D.; Claass, B.; Creagh, E.; Harris, J.; Malyala, P.; Singh, M.; O’Hagan, D.T.; Pétrilli, V.; Tschopp, J.; et al. Uptake of particulate vaccine adjuvants by dendritic cells activates the NALP3 inflammasome. Proc. Natl. Acad. Sci. USA 2009, 106, 870–875. [Google Scholar] [CrossRef]
- Li, H.; Willingham, S.B.; Ting, J.P.-Y.; Re, F. Cutting Edge: Inflammasome Activation by Alum and Alum’s Adjuvant Effect Are Mediated by NLRP3. J. Immunol. 2008, 181, 17–21. [Google Scholar] [CrossRef]
- McKee, A.S.; Munks, M.W.; MacLeod, M.K.; Fleenor, C.J.; Van Rooijen, N.; Kappler, J.W.; Marrack, P. Alum induces innate immune responses through macrophage and mast cell sensors, but these sensors are not required for alum to act as an adjuvant for specific immunity. J. Immunol. 2009, 183, 4403–4414. [Google Scholar] [CrossRef] [Green Version]
- Franchi, L.; Núñez, G. The Nlrp3 inflammasome is critical for aluminium hydroxide-mediated IL-1beta secretion but dispensable for adjuvant activity. Eur. J. Immunol. 2008, 38, 2085–2089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Infante-Duarte, C.; Kamradt, T. Th1/Th2 balance in infection. Springer Semin. Immunopathol 1999, 21, 317–338. [Google Scholar] [CrossRef] [PubMed]
- Ciabattini, A.; Pettini, E.; Fiorino, F.; Pastore, G.; Andersen, P.; Pozzi, G.; Medaglini, D. Modulation of Primary Immune Response by Different Vaccine Adjuvants. Front. Immunol. 2016, 7, 427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cayrol, C.; Girard, J.P. The IL-1-like cytokine IL-33 is inactivated after maturation by caspase-1. Proc. Natl. Acad. Sci. USA 2009, 106, 9021–9026. [Google Scholar] [CrossRef]
- Evavold, C.L.; Kagan, J.C. How Inflammasomes Inform Adaptive Immunity. J. Mol. Biol. 2018, 430, 217–237. [Google Scholar] [CrossRef]
- Schmitz, J.; Owyang, A.; Oldham, E.; Song, Y.; Murphy, E.; McClanahan, T.K.; Zurawski, G.; Moshrefi, M.; Qin, J.; Li, X.; et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 2005, 23, 479–490. [Google Scholar] [CrossRef] [Green Version]
- Brewer, J.M.; Conacher, M.; Hunter, C.A.; Mohrs, M.; Brombacher, F.; Alexander, J. Aluminium Hydroxide Adjuvant Initiates Strong Antigen-Specific Th2 Responses in the Absence of IL-4- or IL-13-Mediated Signaling. J. Immunol. 1999, 163, 6448–6454. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corripio-Miyar, Y.; MacLeod, C.L.; Mair, I.; Mellanby, R.J.; Moore, B.D.; McNeilly, T.N. Self-Adjuvanting Calcium-Phosphate-Coated Microcrystal-Based Vaccines Induce Pyroptosis in Human and Livestock Immune Cells. Vaccines 2023, 11, 1229. https://doi.org/10.3390/vaccines11071229
Corripio-Miyar Y, MacLeod CL, Mair I, Mellanby RJ, Moore BD, McNeilly TN. Self-Adjuvanting Calcium-Phosphate-Coated Microcrystal-Based Vaccines Induce Pyroptosis in Human and Livestock Immune Cells. Vaccines. 2023; 11(7):1229. https://doi.org/10.3390/vaccines11071229
Chicago/Turabian StyleCorripio-Miyar, Yolanda, Clair Lyle MacLeod, Iris Mair, Richard J. Mellanby, Barry D. Moore, and Tom N. McNeilly. 2023. "Self-Adjuvanting Calcium-Phosphate-Coated Microcrystal-Based Vaccines Induce Pyroptosis in Human and Livestock Immune Cells" Vaccines 11, no. 7: 1229. https://doi.org/10.3390/vaccines11071229
APA StyleCorripio-Miyar, Y., MacLeod, C. L., Mair, I., Mellanby, R. J., Moore, B. D., & McNeilly, T. N. (2023). Self-Adjuvanting Calcium-Phosphate-Coated Microcrystal-Based Vaccines Induce Pyroptosis in Human and Livestock Immune Cells. Vaccines, 11(7), 1229. https://doi.org/10.3390/vaccines11071229





