Oral Vaccines: A Better Future of Immunization
Abstract
:1. Introduction
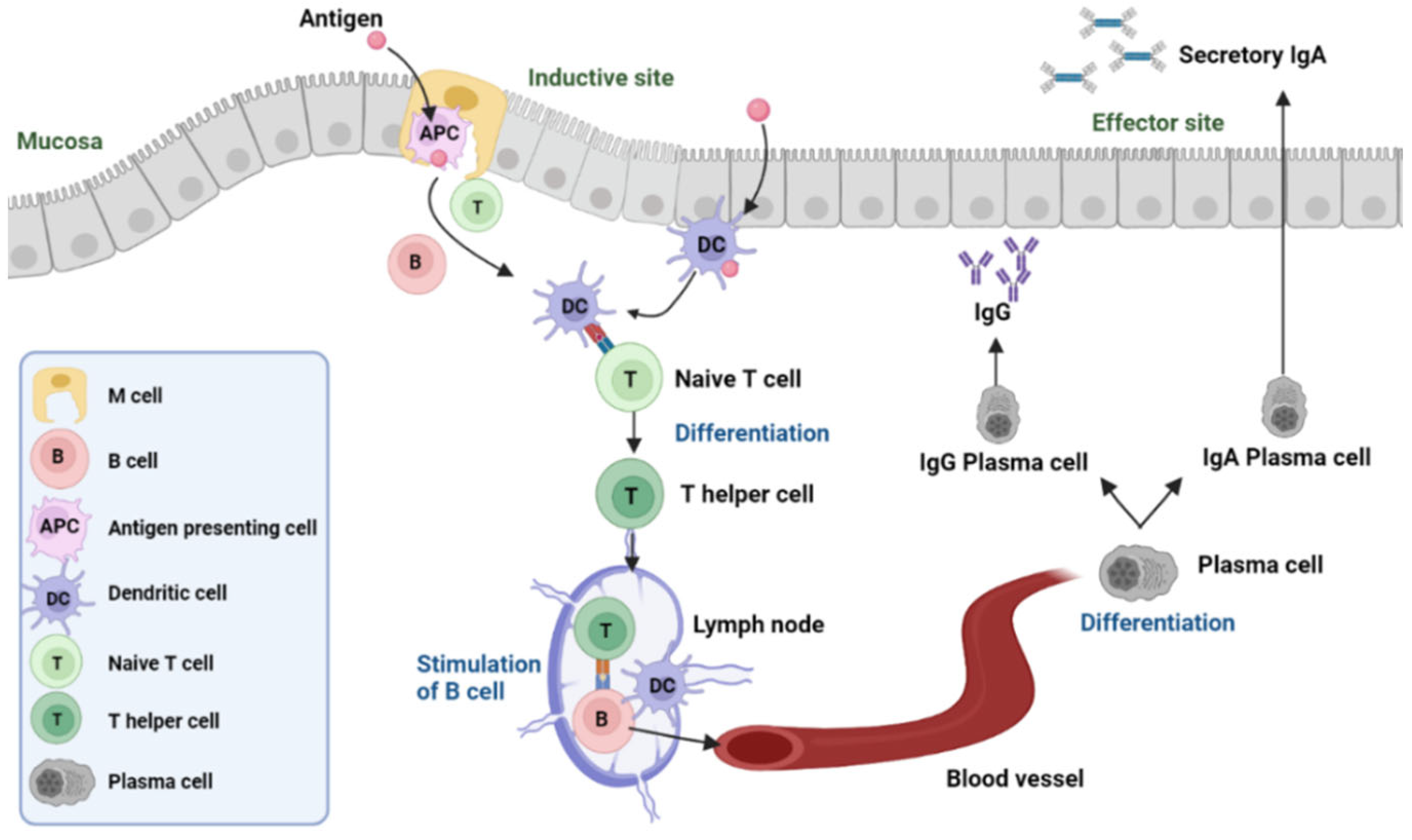
2. The Mechanism of Inactivated Virus/Bacteria Oral Vaccines
The Dominance of Secretory IgA in the Oral Vaccine-Induced Immune Response
3. Current Developed and Certificated Oral Vaccines
3.1. Inactivated Virus/Bacteria Oral Vaccine
3.2. Adenovirus Oral Vaccines
3.3. Recombinant Protein-Based Oral Vaccines
3.4. Transgenic Plant-Based Oral Vaccines
4. Regulatory Procedures and Clinical Trials for Injectable Vaccines vs. Oral Vaccines
Cost of Clinical Trials: Injectable Vaccines vs. Oral Vaccines
5. Challenges of Oral Vaccines
6. Recent Development of Oral Vaccines
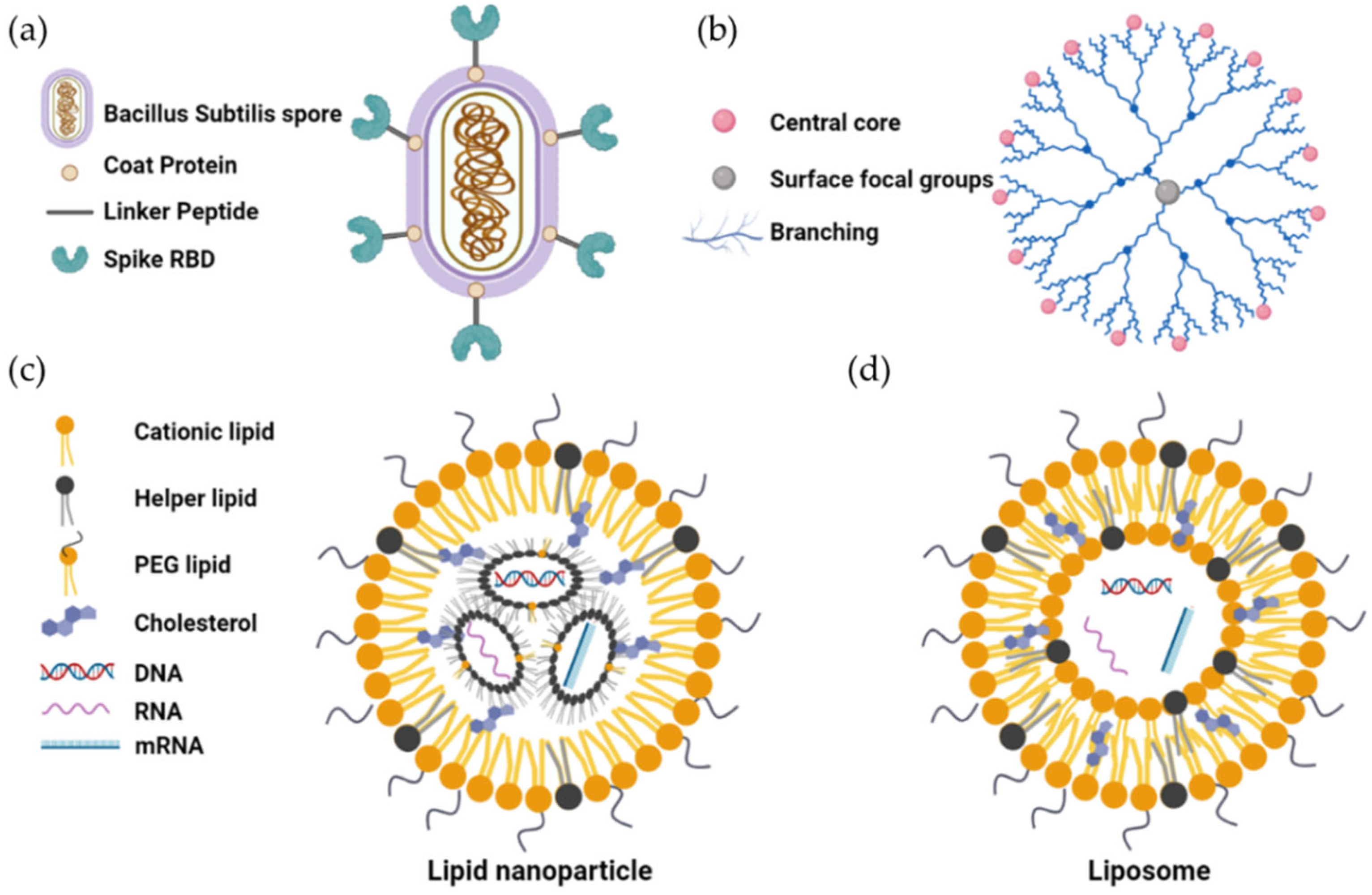
6.1. Bacillus-Subtilis-Based Oral Vaccines
6.2. Nanoparticle-Based Delivery System for Oral Vaccines
7. Conclusions
8. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bazin, H. Introduction. In The Eradication of Smallpox; Bazin, H., Ed.; Academic Press: London, UK, 2000; pp. xix–xx. [Google Scholar]
- Bandyopadhyay, A.S.; Zipursky, S. A novel tool to eradicate an ancient scourge: The novel oral polio vaccine type 2 story. Lancet Infect. Dis. 2023, 23, e67–e71. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.C.; Efstratiou, A.; Mokrousov, I.; Mutreja, A.; Das, B.; Ramamurthy, T. Diphtheria. Nat. Rev. Dis. Prim. 2019, 5, 81. [Google Scholar] [CrossRef] [PubMed]
- Brisse, M.; Vrba, S.M.; Kirk, N.; Liang, Y.; Ly, H. Emerging Concepts and Technologies in Vaccine Development. Front. Immunol. 2020, 11, 583077. [Google Scholar] [CrossRef] [PubMed]
- Zaffran, M. Vaccine transport and storage: Environmental challenges. Dev. Biol. Stand. 1996, 87, 9–17. [Google Scholar]
- Li, M.; Wang, H.; Tian, L.; Pang, Z.; Yang, Q.; Huang, T.; Fan, J.; Song, L.; Tong, Y.; Fan, H. COVID-19 vaccine development: Milestones, lessons and prospects. Signal Transduct. Target. Ther. 2022, 7, 146. [Google Scholar] [CrossRef]
- Mason, H.S.; Lam, O.Y.-Y.; Froberg, C.A.; Lam, D.M.-K. The Expression of hepatitis B surface antigen in transgenic tomato fruit. Adv. Sci. Res. 1994, 1, 1–5. [Google Scholar]
- Lam, D.M.-K.; Shi, J. Edible Vaccines. In Agro-Food-Industry Hi-Tech 7-12; TKS: Milano, Italy, 1996. [Google Scholar]
- Lei, H.; Sheng, Z.; Ding, Q.; Chen, J.; Wei, X.; Lam, D.M.-K.; Xu, Y. Evaluation of Oral Immunization with Recombinant Avian Influenza Virus HA1 Displayed on the Lactococcus lactis Surface and Combined with the Mucosal Adjuvant Cholera Toxin Subunit B. Clin. Vaccine Immunol. 2011, 18, 1046–1051. [Google Scholar] [CrossRef] [Green Version]
- Lei, H.; Gao, T.; Hamicd, Y.; Howard, J.; Liu, J.; Davidson, R.; Tong, R.; Lam, O.Y.; Lam, F.W.; Lam, D.M.-K. The development of a yeast-derived oral vaccine against Hepatitis B. Biotechnol. Hong Kong 2020, 4, 123–134. [Google Scholar]
- Blume, S.; Geesink, I. A Brief History of Polio Vaccines. Science 2000, 288, 1593–1594. [Google Scholar] [CrossRef]
- Howard, B.D. A Prototype Live Oral Cholera Vaccine. Nature 1971, 230, 97–99. [Google Scholar] [CrossRef]
- Parker, E.P.K.; Bronowski, C.; Sindhu, K.N.C.; Babji, S.; Benny, B.; Carmona-Vicente, N.; Chasweka, N.; Chinyama, E.; Cunliffe, N.A.; Dube, Q.; et al. Impact of maternal antibodies and microbiota development on the immunogenicity of oral rotavirus vaccine in African, Indian, and European infants. Nat. Commun. 2021, 12, 7288. [Google Scholar] [CrossRef] [PubMed]
- Wahid, R.; Fresnay, S.; Levine, M.M.; Sztein, M.B. Immunization with Ty21a live oral typhoid vaccine elicits crossreactive multifunctional CD8+ T-cell responses against Salmonella enterica serovar Typhi, S. Paratyphi A, and S. Paratyphi B in humans. Mucosal Immunol. 2015, 8, 1349–1359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lei, H.; Xu, Y.; Chen, J.; Wei, X.; Lam, D.M.-K. Immunoprotection against influenza H5N1 virus by oral administration of enteric-coated recombinant Lactococcus lactis mini-capsules. Virology 2010, 407, 319–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mörbe, U.M.; Jørgensen, P.B.; Fenton, T.M.; von Burg, N.; Riis, L.B.; Spencer, J.; Agace, W.W. Human gut-associated lymphoid tissues (GALT); diversity, structure, and function. Mucosal Immunol. 2021, 14, 793–802. [Google Scholar] [CrossRef]
- Flitter, B.A.; Braun, M.R.; Tucker, S.N. Drop the Needle; A Temperature Stable Oral Tablet Vaccine Is Protective against Respiratory Viral Pathogens. Vaccines 2022, 10, 593. [Google Scholar] [CrossRef]
- Wang, S.; Liu, H.; Zhang, X.; Qian, F. Intranasal and oral vaccination with protein-based antigens: Advantages, challenges and formulation strategies. Protein Cell 2015, 6, 480–503. [Google Scholar] [CrossRef] [Green Version]
- Holmgren, J.; Czerkinsky, C. Mucosal immunity and vaccines. Nat. Med. 2005, 11, S45–S53. [Google Scholar] [CrossRef]
- Skwarczynski, M.; Toth, I. Non-invasive mucosal vaccine delivery: Advantages, challenges and the future. Expert Opin. Drug Deliv. 2020, 17, 435–437. [Google Scholar] [CrossRef] [Green Version]
- Tam, J.; Lam, F.; Lam, Y.; Lam, D. Oral immunization and edible vaccines: A viable option or mirage. In Biotechonology Hong Kong; USCIPI: Whitestone, NY, USA, 2015; Volume 2, pp. 201–211. [Google Scholar]
- Brandtzaeg, P. Function of Mucosa-Associated Lymphoid Tissue in Antibody Formation. Immunol. Investig. 2010, 39, 303–355. [Google Scholar] [CrossRef]
- Lycke, N. Recent progress in mucosal vaccine development: Potential and limitations. Nat. Rev. Immunol. 2012, 12, 592–605. [Google Scholar] [CrossRef]
- Oh, S.-H.; Kim, S.-H.; Jeon, J.-H.; Kim, E.B.; Lee, N.-K.; Beck, S.; Choi, Y.-J.; Kang, S.-K. Cytoplasmic expression of a model antigen with M Cell-Targeting moiety in lactic acid bacteria and implication of the mechanism as a mucosal vaccine via oral route. Vaccine 2021, 39, 4072–4081. [Google Scholar] [CrossRef]
- Lund, F.E.; Randall, T.D. Effector and regulatory B cells: Modulators of CD4+ T cell immunity. Nat. Rev. Immunol. 2010, 10, 236–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiu, C.; Openshaw, P.J. Antiviral B cell and T cell immunity in the lungs. Nat. Immunol. 2015, 16, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, M.; Du, G.; Chen, X.; Sun, X. Advancedoral vaccine delivery strategies for improving the immunity. Adv. Drug Deliv. Rev. 2021, 177, 113928. [Google Scholar] [CrossRef] [PubMed]
- Kunisawa, J.; Kurashima, Y.; Kiyono, H. Gut-associated lymphoid tissues for the development of oral vaccines. Adv. Drug Deliv. Rev. 2012, 64, 523–530. [Google Scholar] [CrossRef]
- Robbins, J.B.; Schneerson, R.; Szu, S.C. Perspective: Hypothesis: Serum IgG Antibody Is Sufficient to Confer Protection against Infectious Diseases by Inactivating the Inoculum. J. Infect. Dis. 1995, 171, 1387–1398. [Google Scholar] [CrossRef]
- Bauer, G. The potential significance of high avidity immunoglobulin G (IgG) for protective immunity towards SARS-CoV-2. Int. J. Infect. Dis. 2021, 106, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Sabin, A.B.; Boulger, L.R. History of Sabin attenuated poliovirus oral live vaccine strains. J. Biol. Stand. 1973, 1, 115–118. [Google Scholar] [CrossRef]
- Jelinek, T.; Kollaritsch, H. Vaccination with Dukoral® against travelers’ diarrhea (ETEC) and cholera. Expert Rev. Vaccines 2008, 7, 561–567. [Google Scholar] [CrossRef]
- Pezzoli, L. Global oral cholera vaccine use, 2013–2018. Vaccine 2020, 38, A132–A140. [Google Scholar] [CrossRef]
- Mosley, J.F., 2nd; Smith, L.L.; Brantley, P.; Locke, D.; Como, M. Vaxchora: The First FDA-Approved Cholera Vaccination in the United States. Pharm. Ther. 2017, 42, 638–640. [Google Scholar]
- Saluja, T.; Mogasale, V.V.; Excler, J.-L.; Kim, J.H.; Mogasale, V. An overview of VaxchoraTM, a live attenuated oral cholera vaccine. Hum. Vaccines Immunother. 2020, 16, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Ciarlet, M.; Schödel, F. Development of a rotavirus vaccine: Clinical safety, immunogenicity, and efficacy of the pentavalent rotavirus vaccine, RotaTeq®. Vaccine 2009, 27, G72–G81. [Google Scholar] [CrossRef]
- Vesikari, T.; Itzler, R.; Karvonen, A.; Korhonen, T.; Van Damme, P.; Behre, U.; Bona, G.; Gothefors, L.; Heaton, P.M.; Dallas, M.; et al. RotaTeq®, a pentavalent rotavirus vaccine: Efficacy and safety among infants in Europe. Vaccine 2009, 28, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Ward, R.L.; Bernstein, D.I. Rotarix: A Rotavirus Vaccine for the World. Clin. Infect. Dis. 2009, 48, 222–228. [Google Scholar] [CrossRef]
- O’Ryan, M.; Linhares, A.C. Update on Rotarix™: An oral human rotavirus vaccine. Exp. Rev. Vaccines 2009, 8, 1627–1641. [Google Scholar] [CrossRef]
- Ferreccio, C.; Levine, M.M.; Rodriguez, H.; Contreras, R. Comparative efficacy of two, three, or four doses of TY21a live oral typhoid vaccine in enteric-coated capsules: A field trial in an endemic area. J. Infect. Dis. 1989, 159, 766–769. [Google Scholar] [CrossRef]
- Amicizia, D.; Arata, L.; Zangrillo, F.; Panatto, D.; Gasparini, R. Overview of the impact of Typhoid and Paratyphoid fever. Utility of Ty21a vaccine (Vivotif®). J. Prev. Med. Hyg. 2017, 58, E1–E8. [Google Scholar]
- Collins, N.D.; Adhikari, A.; Yang, Y.; Kuschner, R.A.; Karasavvas, N.; Binn, L.N.; Walls, S.D.; Graf, P.C.F.; Myers, C.A.; Jarman, R.G.; et al. Live Oral Adenovirus Type 4 and Type 7 Vaccine Induces Durable Antibody Response. Vaccines 2020, 8, 411. [Google Scholar] [CrossRef]
- Wang, M.; Fu, T.; Hao, J.; Li, L.; Tian, M.; Jin, N.; Ren, L.; Li, C. A recombinant Lactobacillus plantarum strain expressing the spike protein of SARS-CoV-2. Int. J. Biol. Macromol. 2020, 160, 736–740. [Google Scholar] [CrossRef]
- Chan, H.T.; Daniell, H. Plant-made oral vaccines against human infectious diseases-Are we there yet? Plant Biotechnol. J. 2015, 13, 1056–1070. [Google Scholar] [CrossRef] [Green Version]
- Platt, L.R.; Estívariz, C.F.; Sutter, R.W. Vaccine-Associated Paralytic Poliomyelitis: A Review of the Epidemiology and Estimation of the Global Burden. J. Infect. Dis. 2014, 210, S380–S389. [Google Scholar] [CrossRef]
- Snider, C.J.; Zaman, K.; Estivariz, C.F.; Yunus, M.; Weldon, W.C.; Wannemuehler, K.A.; Oberste, M.S.; Pallansch, M.A.; Wassilak, S.G.; Bari, T.I.A.; et al. Inactivated poliovirus vaccine: PastImmunogenicity of full and present experiencefractional dose of inactivated poliovirus vaccine for use in routine immunisation and outbreak response: An open-label, randomised controlled trial. Lancet 2019, 393, 2624–7462634. [Google Scholar] [CrossRef]
- Cryz, S.J., Jr.; Levine, M.M.; Kaper, J.B.; Fürer, E.; Althaus, B. Randomized double-blind placebo controlled trial to evaluate the safety and immunogenicity of the live oral cholera vaccine strain CVD 103-HgR in Swiss adults. Vaccine 1990, 8, 577–580. [Google Scholar] [CrossRef]
- Burke, R.M.; Tate, J.E.; Kirkwood, C.D.; Steele, A.D.; Parashar, U.D. Current and new rotavirus vaccines. Curr. Opin. Infect. Dis. 2019, 32, 435–444. [Google Scholar] [CrossRef]
- Isanaka, S.; Guindo, O.; Langendorf, C.; Matar Seck, A.; Plikaytis, B.D.; Sayinzoga-Makombe, N.; McNeal, M.M.; Meyer, N.; Adehossi, E.; Djibo, A.; et al. Efficacy of a Low-Cost, Heat-Stable Oral Rotavirus Vaccine in Niger. N. Engl. J. Med. 2017, 376, 1121–1130. [Google Scholar] [CrossRef]
- Carvalho, M.F.; Gill, D. Rotavirus vaccine efficacy: Current status and areas for improvement. Hum. Vaccines Immunother. 2019, 15, 1237–1250. [Google Scholar] [CrossRef] [Green Version]
- Syed, K.A.; Saluja, T.; Cho, H.; Hsiao, A.; Shaikh, H.; Wartel, T.A.; Mogasale, V.; Lynch, J.; Kim, J.H.; Excler, J.-L.; et al. Review on the Recent Advances on Typhoid Vaccine Development and Challenges Ahead. Clin. Infect. Dis. 2020, 71, S141–S150. [Google Scholar] [CrossRef] [PubMed]
- Chang, J. Adenovirus Vectors: Excellent Tools for Vaccine Development. Immune Netw. 2021, 21, e6. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, F.; Tachibana, M.; Mizuguchi, H. Adenovirus vector-based vaccine for infectious diseases. Drug Metab. Pharmacokinet. 2022, 42, 100432. [Google Scholar] [CrossRef] [PubMed]
- Tatsis, N.; Ertl, H.C.J. Adenoviruses as vaccine vectors. Mol. Ther. 2004, 10, 616–629. [Google Scholar] [CrossRef]
- Simon-Loriere, E.; Schwartz, O. Towards SARS-CoV-2 serotypes? Nat. Rev. Microbiol. 2022, 20, 187–188. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.Q.; Gao, G.P.; Reyes-Sandoval, A.; Li, Y.; Wilson, J.M.; Ertl, H.C. Oral vaccination of mice with adenoviral vectors is not impaired by preexisting immunity to the vaccine carrier. J. Virol. 2003, 77, 10780–10789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langel, S.N.; Johnson, S.; Martinez, C.I.; Tedjakusuma, S.N.; Peinovich, N.; Dora, E.G.; Kuehl, P.J.; Irshad, H.; Barrett, E.G.; Werts, A.D.; et al. Adenovirus type 5 SARS-CoV-2 vaccines delivered orally or intranasally reduced disease severity and transmission in a hamster model. Sci. Transl. Med. 2022, 14, eabn6868. [Google Scholar] [CrossRef]
- Serradell, M.C.; Rupil, L.L.; Martino, R.A.; Prucca, C.G.; Carranza, P.G.; Saura, A.; Fernández, E.A.; Gargantini, P.R.; Tenaglia, A.H.; Petiti, J.P.; et al. Efficient oral vaccination by bioengineering virus-like particles with protozoan surface proteins. Nat. Commun. 2019, 10, 361. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.S.; Seo, J.Y.; Park, S.I.; Kim, T.G.; Kim, T.J. Oral immunization with recombinant protein antigen expressed in tobacco against fish nervous necrosis virus. J. Vet. Med. Sci. 2018, 80, 272–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sung, J.C.; Lai, N.C.; Wu, K.C.; Choi, M.C.; Ma, C.H.; Lin, J.; Kuok, C.N.; Leong, W.L.; Lam, W.K.; Hamied, Y.K.; et al. Safety and Immunogenicity of Inactivated Bacillus subtilis Spores as a Heterologous Antibody Booster for COVID-19 Vaccines. Vaccines 2022, 10, 1014. [Google Scholar] [CrossRef]
- Nascimento, I.P.; Leite, L.C.C. Recombinant vaccines and the development of new vaccine strategies. Braz. J. Med. Biol. Res. 2012, 45, 1102–1111. [Google Scholar] [CrossRef] [Green Version]
- De Smet, R.; Allais, L.; Cuvelier, C.A. Recent advances in oral vaccine development. Hum. Vaccines Immunother. 2014, 10, 1309–1318. [Google Scholar] [CrossRef] [Green Version]
- Schreuder, M.P.; Deen, C.; Boersma, W.J.A.; Pouwels, P.H.; Klis, F.M. Yeast expressing hepatitis B virus surface antigen determinants on its surface: Implications for a possible oral vaccine. Vaccine 1996, 14, 383–388. [Google Scholar] [CrossRef]
- Harper, D.M.; DeMars, L.R. HPV vaccines—A review of the first decade. Gynecol. Oncol. 2017, 146, 196–204. [Google Scholar] [CrossRef] [Green Version]
- Keating, G.M.; Noble, S. Recombinant Hepatitis B Vaccine (Engerix-B®). Drugs 2003, 63, 1021–1051. [Google Scholar] [CrossRef] [PubMed]
- Mason, H.S.; Lam, D.M.; Arntzen, C.J. Expression of hepatitis B surface antigen in transgenic plants. Proc. Natl. Acad. Sci. USA 1992, 89, 11745–11749. [Google Scholar] [CrossRef] [PubMed]
- Mac, S.; Sumner, A.; Duchesne-Belanger, S.; Stirling, R.; Tunis, M.; Sander, B. Cost-effectiveness of Palivizumab for Respiratory Syncytial Virus: A Systematic Review. Pediatrics 2019, 143, e20184064. [Google Scholar] [CrossRef] [PubMed]
- Pollet, J.; Chen, W.-H.; Strych, U. Recombinant protein vaccines, a proven approach against coronavirus pandemics. Adv. Drug Deliv. Rev. 2021, 170, 71–82. [Google Scholar] [CrossRef]
- Pulendran, B.; Arunachalam, P.S.; O’Hagan, D.T. Emerging concepts in the science of vaccine adjuvants. Nat. Rev. Drug Discov. 2021, 20, 454–475. [Google Scholar] [CrossRef] [PubMed]
- Nanishi, E.; Borriello, F.; O’Meara, T.R.; McGrath, M.E.; Saito, Y.; Haupt, R.E.; Seo, H.S.; van Haren, S.D.; Cavazzoni, C.B.; Brook, B.; et al. An aluminum hydroxide:CpG adjuvant enhances protection elicited by a SARS-CoV-2 receptor binding domain vaccine in aged mice. Sci. Transl. Med. 2022, 14, eabj5305. [Google Scholar] [CrossRef]
- Jin, P.; Sun, F.; Liu, Q.; Wang, Q.; Zhang, Y.; Liu, X. An oral vaccine based on chitosan/aluminum adjuvant induces both local and systemic immune responses in turbot (Scophthalmus maximus). Vaccine 2021, 39, 7477–7484. [Google Scholar] [CrossRef]
- Vetvicka, V.; Vannucci, L.; Sima, P. β-glucan as a new tool in vaccine development. Scand. J. Immunol. 2020, 91, e12833. [Google Scholar] [CrossRef]
- Daniell, H.; Rai, V.; Xiao, Y. Cold chain and virus-free oral polio booster vaccine made in lettuce chloroplasts confers protection against all three poliovirus serotypes. Plant Biotechnol. J. 2019, 17, 1357–1368. [Google Scholar] [CrossRef] [Green Version]
- Burnett, M.J.; Burnett, A.C. Therapeutic recombinant protein production in plants: Challenges and opportunities. Plants People Planet 2020, 2, 121–132. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, T.; Hoshikawa, K.; Ezura, K.; Okazawa, R.; Fujita, S.; Takaoka, M.; Mason, H.S.; Ezura, H.; Miura, K. Improvement of the transient expression system for production of recombinant proteins in plants. Sci. Rep. 2018, 8, 4755. [Google Scholar] [CrossRef] [Green Version]
- Giddings, G. Transgenic plants as protein factories. Curr. Opin. Biotechnol. 2001, 12, 450–454. [Google Scholar] [CrossRef] [PubMed]
- Lam, D.M.-K.; Arntzen, C.J. Anti-Viral Vaccines Expressed in Plants. U.S. Patent No. 5,612,487, 18 March 1997. [Google Scholar]
- Laere, E.; Ling, A.P.K.; Wong, Y.P.; Koh, R.Y.; Mohd Lila, M.A.; Hussein, S. Plant-Based Vaccines: Production and Challenges. J. Bot. 2016, 2016, 4928637. [Google Scholar] [CrossRef] [Green Version]
- Floss, D.M.; Falkenburg, D.; Conrad, U. Production of vaccines and therapeutic antibodies for veterinary applications in transgenic plants: An overview. Transgenic Res. 2007, 16, 315–332. [Google Scholar] [CrossRef]
- Dixon, T.A.; Williams, T.C.; Pretorius, I.S. Sensing the future of bio-informational engineering. Nat. Commun. 2021, 12, 388. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, D.A.; Thomas, J.A. Biopharmaceuticals derived from genetically modified plants. QJM: Int. J. Med. 2004, 97, 705–716. [Google Scholar] [CrossRef] [Green Version]
- Giddings, G.; Allison, G.; Brooks, D.; Carter, A. Transgenic plants as factories for biopharmaceuticalsCurrent state-of-the-art in plant-based antibody production systems. Nat. Biotechnol. Lett. 2000, 18, 1151–1155. [Google Scholar] [CrossRef]
- Richter, L.J.; Thanavala, Y.; Arntzen, C.J.; Mason, H.S. Production of hepatitis B surface antigen in transgenic plants for oral immunization. Nat. Biotechnol. 2000, 18, 1167–1171. [Google Scholar] [CrossRef]
- Wagner, R.; Meißner, J.; Grabski, E.; Sun, Y.; Vieths, S.; Hildt, E. Regulatory concepts to guide and promote the accelerated but safe clinical development and licensure of COVID-19 vaccines in Europe. Allergy 2022, 77, 72–82. [Google Scholar] [CrossRef]
- Khehra, N.; Padda, I.; Jaferi, U.; Atwal, H.; Narain, S.; Parmar, M.S. Tozinameran (BNT162b2) Vaccine: The Journey from Preclinical Research to Clinical Trials and Authorization. AAPS PharmSciTech 2021, 22, 172. [Google Scholar] [CrossRef]
- Pöllabauer, E.M.; Fritsch, S.; Pavlova, B.G.; Löw-Baselli, A.; Firth, C.; Koska, M.; Maritsch, F.; Barrett, P.N.; Ehrlich, H.J. Clinical evaluation to determine the appropriate paediatric formulation of a tick-borne encephalitis vaccine. Vaccine 2010, 28, 4558–4565. [Google Scholar] [CrossRef]
- Handel, A.; Li, Y.; McKay, B.; Pawelek, K.A.; Zarnitsyna, V.; Antia, R. Exploring the impact of inoculum dose on host immunity and morbidity to inform model-based vaccine design. PLoS Comput. Biol. 2018, 14, e1006505. [Google Scholar] [CrossRef] [Green Version]
- Lavelle, E.C.; Ward, R.W. Mucosal vaccines—Fortifying the frontiers. Nat. Rev. Immunol. 2022, 22, 236–250. [Google Scholar] [CrossRef] [PubMed]
- Qadri, F.; Akhtar, M.; Bhuiyan, T.R.; Chowdhury, M.I.; Ahmed, T.; Rafique, T.A.; Khan, A.; Rahman, S.I.A.; Khanam, F.; Lundgren, A.; et al. Safety and immunogenicity of the oral, inactivated, enterotoxigenic Escherichia coli vaccine ETVAX in Bangladeshi children and infants: A double-blind, randomised, placebo-controlled phase 1/2 trial. Lancet Infect. Dis. 2020, 20, 208–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, Y.; Nie, L.; Zhu, S.; Zhang, X. Nanovesicles-Mediated Drug Delivery for Oral Bioavailability Enhancement. Int. J. Nanomed. 2022, 17, 4861–4877. [Google Scholar] [CrossRef]
- Zafar, A.; Arshad, R.; Ur Rehman, A.; Ahmed, N.; Akhtar, H. Recent Developments in Oral Delivery of Vaccines Using Nanocarriers. Vaccines 2023, 11, 490. [Google Scholar] [CrossRef]
- McNeil, M.M.; Gee, J.; Weintraub, E.S.; Belongia, E.A.; Lee, G.M.; Glanz, J.M.; Nordin, J.D.; Klein, N.P.; Baxter, R.; Naleway, A.L.; et al. The Vaccine Safety Datalink: Successes and challenges monitoring vaccine safety. Vaccine 2014, 32, 5390–5398. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.E.; Webster, R.K.; Weinman, J.; Amlôt, R.; Yiend, J.; Rubin, G.J. Psychological factors associated with uptake of the childhood influenza vaccine and perception of post-vaccination side-effects: A cross-sectional survey in England. Vaccine 2017, 35, 1936–1945. [Google Scholar] [CrossRef] [Green Version]
- Riad, A.; Pokorná, A.; Klugarová, J.; Antalová, N.; Kantorová, L.; Koščík, M.; Klugar, M. Side Effects of mRNA-Based COVID-19 Vaccines among Young Adults (18–30 Years Old): An Independent Post-Marketing Study. Pharmaceuticals 2021, 14, 1049. [Google Scholar] [CrossRef]
- Cadorna-Carlos, J.; Vidor, E.; Bonnet, M.-C. Randomized controlled study of fractional doses of inactivated poliovirus vaccine administered intradermally with a needle in the Philippines. Int. J. Infect. Dis. 2012, 16, e110–e116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Lee, A.; Grigoryan, L.; Arunachalam, P.S.; Scott, M.K.D.; Trisal, M.; Wimmers, F.; Sanyal, M.; Weidenbacher, P.A.; Feng, Y.; et al. Mechanisms of innate and adaptive immunity to the Pfizer-BioNTech BNT162b2 vaccine. Nat. Immunol. 2022, 23, 543–555. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; Kaki, M.; Potluri, V.S.; Kahar, P.; Khanna, D. A comprehensive review of SARS-CoV-2 vaccines: Pfizer, Moderna & Johnson & Johnson. Hum. Vaccines Immunother. 2022, 18, 2002083. [Google Scholar] [CrossRef]
- Wadman, M. Public needs to prep for vaccine side effects. Science 2020, 370, 1022. [Google Scholar] [CrossRef] [PubMed]
- Scully, M.; Singh, D.; Lown, R.; Poles, A.; Solomon, T.; Levi, M.; Goldblatt, D.; Kotoucek, P.; Thomas, W.; Lester, W. Pathologic Antibodies to Platelet Factor 4 after ChAdOx1 nCoV-19 Vaccination. N. Engl. J. Med. 2021, 384, 2202–2211. [Google Scholar] [CrossRef]
- Ledford, H. COVID vaccines and blood clots: What researchers know so far. Nature 2021, 596, 479–481. [Google Scholar] [CrossRef]
- Wise, J. Covid-19: European countries suspend use of Oxford-AstraZeneca vaccine after reports of blood clots. BMJ 2021, 372, n699. [Google Scholar] [CrossRef]
- Barda, N.; Dagan, N.; Ben-Shlomo, Y.; Kepten, E.; Waxman, J.; Ohana, R.; Hernán, M.A.; Lipsitch, M.; Kohane, I.; Netzer, D.; et al. Safety of the BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Setting. N. Engl. J. Med. 2021, 385, 1078–1090. [Google Scholar] [CrossRef]
- Mason, K.L.; Huffnagle, G.B.; Noverr, M.C.; Kao, J.Y. Overview of Gut Immunology. In GI Microbiota and Regulation of the Immune System; Huffnagle, G.B., Noverr, M.C., Eds.; Springer: New York, NY, USA, 2008; pp. 1–14. [Google Scholar]
- Sugawara, T.; Ohsuka, Y.; Taya, K.; Yasui, Y.; Wada, N.; Sakano, M.; Koshida, R.; Fujii, F.; Shibata, S.; Hashimoto, G. Diarrhea as a minor adverse effect due to oral polio vaccine. Jpn. J. Infect. Dis. 2009, 62, 51–53. [Google Scholar] [CrossRef]
- Alfaro-Murillo, J.A.; Ávila-Agüero, M.L.; Fitzpatrick, M.C.; Crystal, C.J.; Falleiros-Arlant, L.-H.; Galvani, A.P. The case for replacing live oral polio vaccine with inactivated vaccine in the Americas. Lancet 2020, 395, 1163–1166. [Google Scholar] [CrossRef]
- Oshitani, H.; Kamigaki, T.; Suzuki, A. Major issues and challenges of influenza pandemic preparedness in developing countries. Emerg. Infect. Dis. 2008, 14, 875–880. [Google Scholar] [CrossRef] [PubMed]
- Brandau, D.T.; Jones, L.S.; Wiethoff, C.M.; Rexroad, J.; Middaugh, C.R. Thermal stability of vaccines. J. Pharm. Sci. 2003, 92, 218–231. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Srivastava, V.; Baindara, P.; Ahmad, A. Thermostable vaccines: An innovative concept in vaccine development. Exp. Rev. Vaccines 2022, 21, 811–824. [Google Scholar] [CrossRef]
- Lloyd, J.; Cheyne, J. The origins of the vaccine cold chain and a glimpse of the future. Vaccine 2017, 35, 2115–2120. [Google Scholar] [CrossRef]
- Uddin, M.N.; Roni, M.A. Challenges of Storage and Stability of mRNA-Based COVID-19 Vaccines. Vaccines 2021, 9, 1033. [Google Scholar] [CrossRef] [PubMed]
- Chooi, W.H.; Ng, P.W.; Hussain, Z.; Ming, L.C.; Ibrahim, B.; Koh, D. Vaccine contamination: Causes and control. Vaccine 2022, 40, 1699–1701. [Google Scholar] [CrossRef]
- Volkin, D.B.; Burke, C.J.; Sanyal, G.; Middaugh, C.R. Analysis of vaccine stability. Dev. Biol. Stand. 1996, 87, 135–142. [Google Scholar]
- Azman, A.S.; Luquero, F.J.; Ciglenecki, I.; Grais, R.F.; Sack, D.A.; Lessler, J. The Impact of a One-Dose versus Two-Dose Oral Cholera Vaccine Regimen in Outbreak Settings: A Modeling Study. PLoS Med. 2015, 12, e1001867. [Google Scholar] [CrossRef] [Green Version]
- Vela Ramirez, J.E.; Sharpe, L.A.; Peppas, N.A. Current state and challenges in developing oral vaccines. Adv. Drug Deliv. Rev. 2017, 114, 116–131. [Google Scholar] [CrossRef]
- Zhao, T.; Li, J.; Fu, Y.; Ye, H.; Liu, X.; Li, G.; Yang, X.; Yang, J. Influence of gut microbiota on mucosal IgA antibody response to the polio vaccine. NPJ Vaccines 2020, 5, 47. [Google Scholar] [CrossRef]
- Srivastava, V.; Nand, K.N.; Ahmad, A.; Kumar, R. Yeast-Based Virus-like Particles as an Emerging Platform for Vaccine Development and Delivery. Vaccines 2023, 11, 479. [Google Scholar] [CrossRef]
- Cao, P.; Xu, Z.P.; Li, L. Tailoring functional nanoparticles for oral vaccine delivery: Recent advances and future perspectives. Compos. Part B Eng. 2022, 236, 109826. [Google Scholar] [CrossRef]
- Setlow, P. Germination of spores of Bacillus species: What we know and do not know. J. Bacteriol. 2014, 196, 1297–1305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cascio, V.; Gittings, D.; Merloni, K.; Hurton, M.; Laprade, D.; Austriaco, N. S-Adenosyl-L-methionine protects the probiotic yeast, Saccharomyces boulardii, from acid-induced cell death. BMC Microbiol. 2013, 13, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, C.; Cha, J.-H.; Kim, M.-G.; Shin, J.; Woo, S.H.; Kim, S.H.; Kim, J.W.; Ji, S.-C.; Lee, K.-J. The effects of dietary Bacillus subtilis on immune response, hematological parameters, growth performance, and resistance of juvenile olive flounder (Paralichthys olivaceus) against Streptococcus iniae. J. World Aquacult. Soc. 2020, 51, 551–562. [Google Scholar] [CrossRef]
- Liu, M.; Clemons, K.V.; Bigos, M.; Medovarska, I.; Brummer, E.; Stevens, D.A. Immune responses induced by heat killed Saccharomyces cerevisiae: A vaccine against fungal infection. Vaccine 2011, 29, 1745–1753. [Google Scholar] [CrossRef] [Green Version]
- Xu, B.; Zhang, W.; Chen, Y.; Xu, Y.; Wang, B.; Zong, L. Eudragit® L100-coated mannosylated chitosan nanoparticles for oral protein vaccine delivery. Int. J. Biol. Macromol. 2018, 113, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Church, J.A.; Parker, E.P.; Kirkpatrick, B.D.; Grassly, N.C.; Prendergast, A.J. Interventions to improve oral vaccine performance: A systematic review and meta-analysis. Lancet Infect. Dis. 2019, 19, 203–214. [Google Scholar] [CrossRef] [Green Version]
- Jana, P.; Shyam, M.; Singh, S.; Jayaprakash, V.; Dev, A. Biodegradable polymers in drug delivery and oral vaccination. Eur. Polym. J. 2021, 142, 110155. [Google Scholar] [CrossRef]
- Karandikar, S.; Mirani, A.; Waybhase, V.; Patravale, V.B.; Patankar, S. Nanovaccines for oral delivery-formulation strategies and challenges. In Nanostructures for Oral Medicine; Elsevier: Amsterdam, The Netherlands, 2017; pp. 263–293. [Google Scholar]
- Souza, C.C.; Guimarães, J.M.; Pereira, S.D.S.; Mariúba, L.A.M. The multifunctionality of expression systems in Bacillus subtilis: Emerging devices for the production of recombinant proteins. Exp. Biol. Med. 2021, 246, 2443–2453. [Google Scholar] [CrossRef]
- Paccez, J.D.; Luiz, W.B.; Sbrogio-Almeida, M.E.; Ferreira, R.C.; Schumann, W.; Ferreira, L.C. Stable episomal expression system under control of a stress inducible promoter enhances the immunogenicity of Bacillus subtilis as a vector for antigen delivery. Vaccine 2006, 24, 2935–2943. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, S.M.; Clemente, J.J.; Henriques, A.O.; Gomes, R.J.; Carrondo, M.J.; Cunha, A.E. A procedure for high-yield spore production by Bacillus subtilis. Biotechnol. Prog. 2005, 21, 1026–1031. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Huang, L.; Mou, C.; Zhang, E.; Wang, Y.; Cao, Y.; Yang, Q. Mucosal immune responses induced by oral administration recombinant Bacillus subtilis expressing the COE antigen of PEDV in newborn piglets. Biosci. Rep. 2019, 39, BSR20182028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Potocki, W.; Negri, A.; Peszyńska-Sularz, G.; Hinc, K.; Obuchowski, M.; Iwanicki, A. IL-1 Fragment Modulates Immune Response Elicited by Recombinant Bacillus subtilis Spores Presenting an Antigen/Adjuvant Chimeric Protein. Mol. Biotechnol. 2018, 60, 810–819. [Google Scholar] [CrossRef] [Green Version]
- Florence, A.T. Nanoparticle uptake by the oral route: Fulfilling its potential? Drug Discov. Today: Technol. 2005, 2, 75–81. [Google Scholar] [CrossRef]
- Angulo, C.; Sanchez, V.; Delgado, K.; Monreal-Escalante, E.; Hernández-Adame, L.; Angulo, M.; Tello-Olea, M.; Reyes-Becerril, M. Oral organic nanovaccines against bacterial and viral diseases. Microb. Pathog. 2022, 169, 105648. [Google Scholar] [CrossRef]
- Marasini, N.; Skwarczynski, M.; Toth, I. Oral delivery of nanoparticle-based vaccines. Exp. Rev. Vaccines 2014, 13, 1361–1376. [Google Scholar] [CrossRef]
- Ma, T.; Wang, L.; Yang, T.; Ma, G.; Wang, S. Homogeneous PLGA-lipid nanoparticle as a promising oral vaccine delivery system for ovalbumin. Asian J. Pharm. Sci. 2014, 9, 129–136. [Google Scholar] [CrossRef] [Green Version]
- Torchilin, V.P. Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discov. 2005, 4, 145–160. [Google Scholar] [CrossRef]
- Isanaka, S.; Garba, S.; Plikaytis, B.; Malone McNeal, M.; Guindo, O.; Langendorf, C.; Adehossi, E.; Ciglenecki, I.; Grais, R.F. Immunogenicity of an oral rotavirus vaccine administered with prenatal nutritional support in Niger: A cluster randomized clinical trial. PLoS Med. 2021, 18, e1003720. [Google Scholar] [CrossRef]
- Yousefpour, P.; Ni, K.; Irvine, D.J. Targeted modulation of immune cells and tissues using engineered biomaterials. Nat. Rev. Bioeng. 2023, 1, 107–124. [Google Scholar] [CrossRef]
- Zhu, Q.; Talton, J.; Zhang, G.; Cunningham, T.; Wang, Z.; Waters, R.C.; Kirk, J.; Eppler, B.; Klinman, D.M.; Sui, Y.; et al. Large intestine–targeted, nanoparticle-releasing oral vaccine to control genitorectal viral infection. Nat. Med. 2012, 18, 1291–1296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, Z.; Liu, W.; Liu, H.; Li, C.; Zhang, Y.; Meng, X.; Tang, T.; Xi, T.; Xing, Y. Oral Helicobacter pylori vaccine-encapsulated acid-resistant HP55/PLGA nanoparticles promote immune protection. Eur. J. Pharm. Biopharm. 2017, 111, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Kono, K. Dendrimer-based bionanomaterials produced by surface modification, assembly and hybrid formation. Polym. J. 2012, 44, 531–540. [Google Scholar] [CrossRef] [Green Version]
- Teo, I.; Toms, S.M.; Marteyn, B.; Barata, T.S.; Simpson, P.; Johnston, K.A.; Schnupf, P.; Puhar, A.; Bell, T.; Tang, C.; et al. Preventing acute gut wall damage in infectious diarrhoeas with glycosylated dendrimers. EMBO Mol. Med. 2012, 4, 866–881. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Feng, W.; Chen, Y.; Shi, J. Inorganic nanoparticles in clinical trials and translations. Nano Today 2020, 35, 100972. [Google Scholar] [CrossRef]
- Wang, T.; Zou, M.; Jiang, H.; Ji, Z.; Gao, P.; Cheng, G. Synthesis of a novel kind of carbon nanoparticle with large mesopores and macropores and its application as an oral vaccine adjuvant. Eur. J. Pharm. Sci. 2011, 44, 653–659. [Google Scholar] [CrossRef]
- Wu, X.; Farooq, M.A.; Li, T.; Geng, T.; Kutoka, P.T.; Wang, B. Cationic chitosan-modified silica nanoparticles for oral delivery of protein vaccine. J. Biomed. Mater. Res. A 2021, 109, 2111–2119. [Google Scholar] [CrossRef]

| Oral Vaccines | Injection Vaccines | |
|---|---|---|
| Administration Route | Oral (non-invasive) | Mostly intramuscular or subcutaneous (invasive) |
| The Site Producing a Protective Response | Mucosal and systemic | Systemic |
| Secretory Immunoglobulin | Mainly IgA | IgG |
| Cost | Relatively low | High |
| Manufacturing Procedure | Relatively simple and do not require an extensive purification process | Extensive purification process needed with higher standard aseptic equipment |
| Distribution | Easy to distribute | Professional healthcare workers and specific locations required |
| Dosage | Higher doses due to degradation in the stomach and intestine | Relatively accurate |
| Disease | Vaccine Name | Antigens | References |
|---|---|---|---|
| Polio | Polio Sabin | Live-attenuated Sabin strains 1,2,3 | [11,31] |
| Cholera | Dukoral | Inactivated strains (types) of V. cholerae serotype O1 and recombinant cholera toxin B subunit (rCTB) | [32,33] |
| Vaxchora | Weakened cholera bacterium Vibrio cholerae (serogroup O1) | [34,35] | |
| Rotavirus | RotaTeq | Live rotavirus strains containing antigen G1, G2, G3, G4 and P1(8) | [36,37] |
| Rotarix | Weakened human rotavirus RIX4414 strain | [38,39] | |
| Typhoid fever | Vivotif | Live attenuated strain Salmonella typhi Ty21a (1,2) | [40,41] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwong, K.W.-Y.; Xin, Y.; Lai, N.C.-Y.; Sung, J.C.-C.; Wu, K.-C.; Hamied, Y.K.; Sze, E.T.-P.; Lam, D.M.-K. Oral Vaccines: A Better Future of Immunization. Vaccines 2023, 11, 1232. https://doi.org/10.3390/vaccines11071232
Kwong KW-Y, Xin Y, Lai NC-Y, Sung JC-C, Wu K-C, Hamied YK, Sze ET-P, Lam DM-K. Oral Vaccines: A Better Future of Immunization. Vaccines. 2023; 11(7):1232. https://doi.org/10.3390/vaccines11071232
Chicago/Turabian StyleKwong, Keith Wai-Yeung, Ying Xin, Nelson Cheuk-Yin Lai, Johnny Chun-Chau Sung, Kam-Chau Wu, Yusuf Khwaja Hamied, Eric Tung-Po Sze, and Dominic Man-Kit Lam. 2023. "Oral Vaccines: A Better Future of Immunization" Vaccines 11, no. 7: 1232. https://doi.org/10.3390/vaccines11071232
APA StyleKwong, K. W.-Y., Xin, Y., Lai, N. C.-Y., Sung, J. C.-C., Wu, K.-C., Hamied, Y. K., Sze, E. T.-P., & Lam, D. M.-K. (2023). Oral Vaccines: A Better Future of Immunization. Vaccines, 11(7), 1232. https://doi.org/10.3390/vaccines11071232





