Just Keep Rolling?—An Encompassing Review towards Accelerated Vaccine Product Life Cycles
Abstract
1. Introduction
2. Vaccine Types
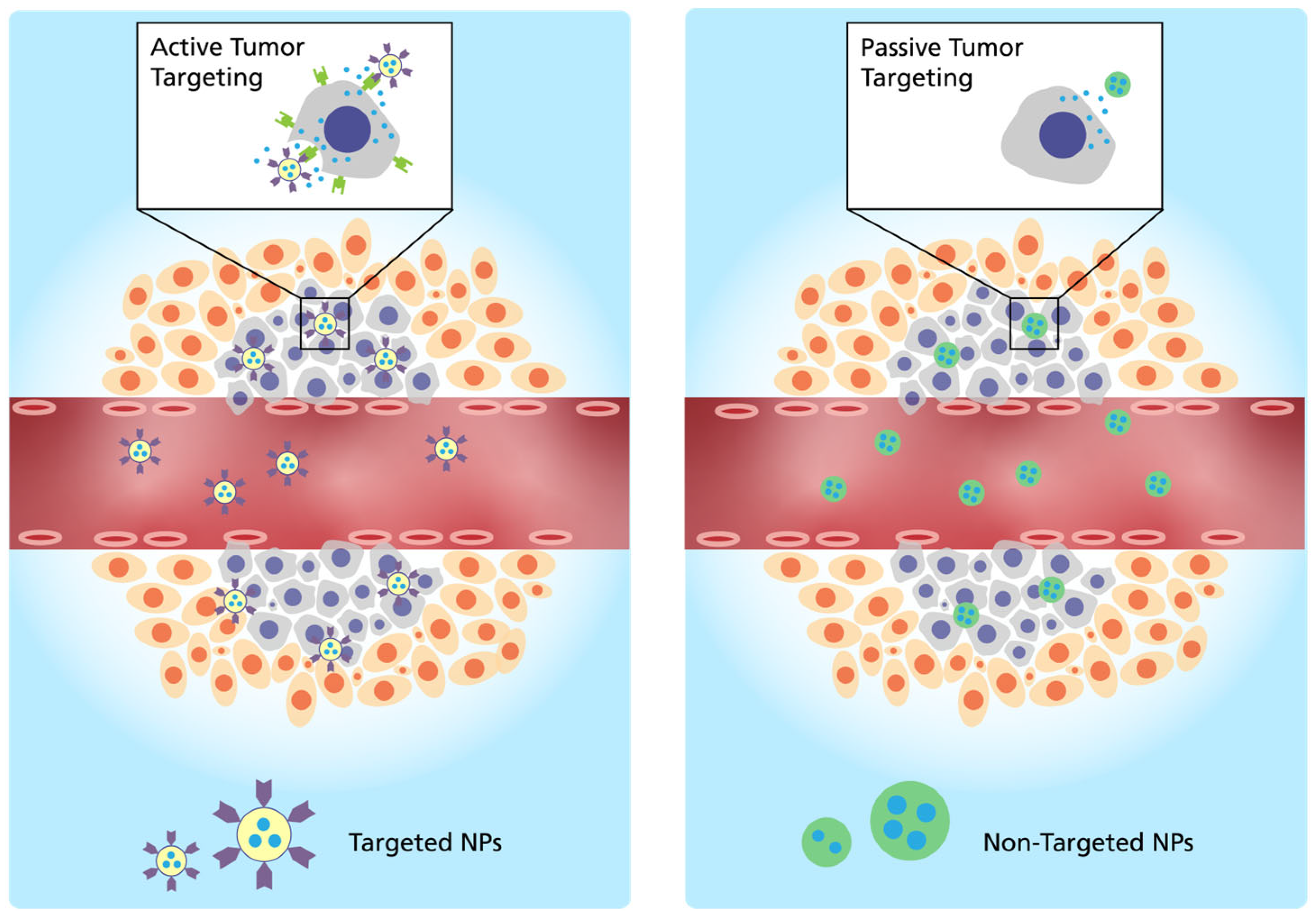
2.1. Administration and Targeting Mechanism
2.2. Endosomale Escape
2.3. RNA as the Active Pharmaceutical Ingredient
2.4. Role of Adjuvants and LNPs
2.5. Biomaterials Enabled Long-Term Storage
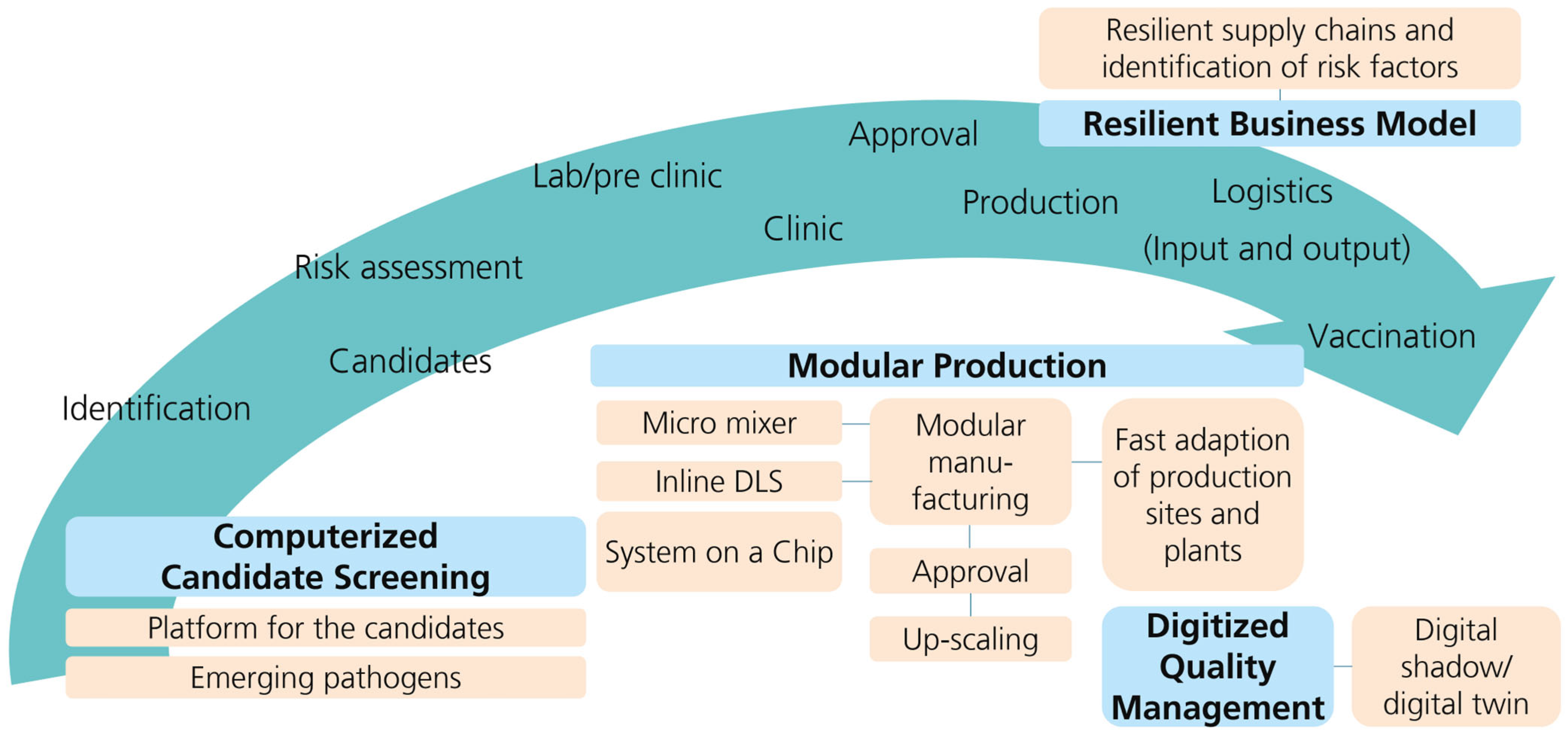
3. In Silico Tools for Vaccine Development
3.1. Epitope Prediction in Immunoinformatics
3.2. AI-based Protein Complex Predictions
4. Digitization and Regulation of Vaccine Quality Management
4.1. Challenges and Benefits of Digital Vaccine Quality Management
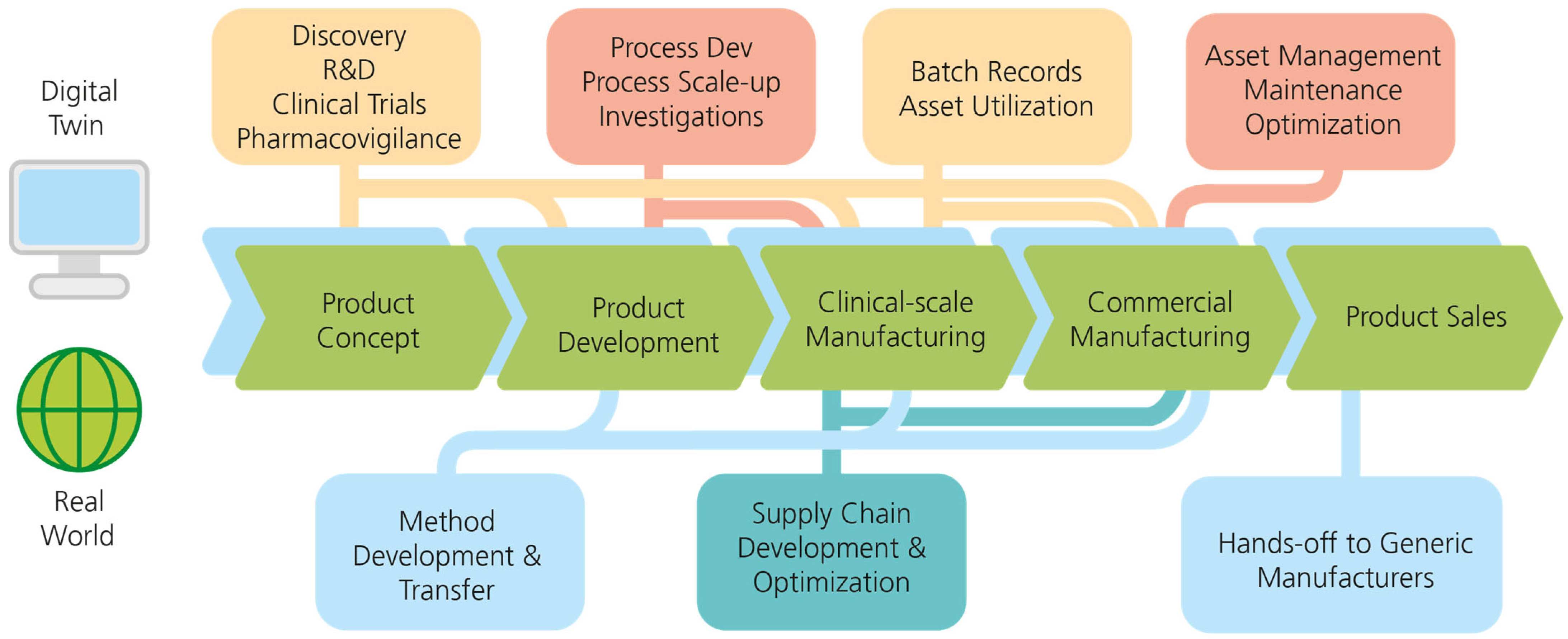
4.2. Digital Twin Implementations in Vaccine Product Life Cycles
4.3. Regulatory Considerations
- “Accelerated Assessment”: Medicinal products classified by the CHMP as therapeutic innovations or of major interest to public health and serving an unmet medical need; processing time for marketing authorization application from 210 to 150 days; e.g., Ebola vaccine, BioNTech Pfizer BNT162b2.
- PRIME (“PRIority MEdicines”): Drugs that serve an unmet medical need or have a clear therapeutic advantage over already approved drugs for the same indication; receive adequate regulatory support to accelerate prior to the official submission of the regulatory dossier; e.g., Ebola and dengue vaccine, Biontech BNT211 cell therapy for the treatment of testicular cancer.
- “Conditional Approval”: The advantage associated with the market availability of the drug clearly outweighs the potential risks of incomplete data (life-threatening diseases, very rare diseases (“orphan medicines” with lack of data due to low incidence)); e.g., live-attenuated pandemic influenza vaccine H5N1.
- “Exceptional Circumstances”: Drugs directed against extremely rare target diseases, so that conclusive clinical evidence on safety and efficacy cannot be generated or the collection of the missing data would violate ethical standards; e.g., smallpox vaccine “Imvanex” and inactivated pandemic influenza vaccines.
- Article 58 procedure: For the scientific evaluation of vaccines not intended for marketing in the European Community to provide expert support for low- to middle-income countries in the regulation and licensing of medicines and to make the high European evaluation standards also available to these countries; e.g., malaria-hepatitis B combination vaccine “Mosquirix”.
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deutsche Akademie der Naturforscher Leopoldina e.V.; German National Academy of Sciences. Vaccine Development, Testing, and Approval. Available online: https://www.leopoldina.org/en/topics/vaccinations/vaccine-development-and-recommendations/ (accessed on 30 May 2023).
- Nord, L.B. Pharmamarkt in der Pandemie—Der Gewinner der Krise? Available online: https://www.nordlb.de/meine-nordlb/download/research-dokument-10227?cHash=da511f96a0d59f1c338c0d55f59a5f34 (accessed on 26 July 2023).
- Statista. Leading 10 Therapeutic Areas Worldwide by Sales in 2019. Available online: https://www.statista.com/statistics/407971/projected-revenue-of-top-therapeutic-areas-worldwide/ (accessed on 26 July 2023).
- VFA—Verband Forschender Arzneimittelhersteller, e.V. Europas Impfstoffindustrie. Wir Versorgen die Welt—Verlässlich und Innovativ. Available online: https://www.vfa.de/download/faktenblatt-impfstoffindustrie-europa.pdf (accessed on 26 July 2023).
- Frost & Sullivan. Growth Opportunities in Global Vaccines Market, Forecast to 2024: Increased Investment in Adult Immunization and Improved Mammalian Cell-Culture Expression Systems will Drive Growth and Profitability ME98-52. 2019. Available online: https://store.frost.com/growth-opportunities-in-global-vaccines-market-forecast-to-2024.html (accessed on 26 July 2023).
- Gahr, M.; Heininger, U.; Bartmann, P.; Huppertz, H.I.; Kinet, M.; Klein, R.; Korenke, C. Folgen der Monopolisierung in der Pharmaindustrie für die Bereitstellung von Impfstoffen. Monatsschrift Kinderheilkd. 2013, 161, 554–558. [Google Scholar] [CrossRef]
- Frost & Sullivan. Global Vaccine Growth Opportunities: Capability Expansion in Nucleic Acid-based Vaccines is Accelerating the Disruption in Vaccines Globally PCCF-52. 2022. Available online: https://store.frost.com/global-vaccine-growth-opportunities.html (accessed on 26 July 2023).
- Statista. Impfstoffe—Umsatz. Available online: https://de.statista.com/outlook/hmo/pharmazeutika/impfstoffe/weltweit#umsatz (accessed on 26 July 2023).
- Statista. Marktanteile führender Pharmaunternehmen im Segment Impfstoffe im Jahr 2017 und Prognose für das Jahr 2024. Available online: https://de.statista.com/statistik/daten/studie/312609/umfrage/marktanteile-fuehrender-pharmaunternehmen-im-segment-impfstoffe/ (accessed on 26 July 2023).
- Statista. Global Vaccine Market Revenues from 2014 to 2020 (in Billion U.S. Dollars). Available online: https://www.statista.com/statistics/265102/revenues-in-the-global-vaccine-market/ (accessed on 26 July 2023).
- U.S. Department of Health and Human Services. Vaccine Types. Available online: https://www.hhs.gov/immunization/basics/types/index.html (accessed on 5 June 2023).
- Lokugamage, M.P.; Vanover, D.; Beyersdorf, J.; Hatit, M.Z.C.; Rotolo, L.; Echeverri, E.S.; Peck, H.E.; Ni, H.; Yoon, J.-K.; Kim, Y.; et al. Optimization of lipid nanoparticles for the delivery of nebulized therapeutic mRNA to the lungs. Nat. Biomed. Eng. 2021, 5, 1059–1068. [Google Scholar] [CrossRef] [PubMed]
- Yin, B.; Chan, C.K.W.; Liu, S.; Hong, H.; Wong, S.H.D.; Lee, L.K.C.; Ho, L.W.C.; Zhang, L.; Leung, K.C.-F.; Choi, P.C.-L.; et al. Intrapulmonary Cellular-Level Distribution of Inhaled Nanoparticles with Defined Functional Groups and Its Correlations with Protein Corona and Inflammatory Response. ACS Nano 2019, 13, 14048–14069. [Google Scholar] [CrossRef]
- Sung, J.; Alghoul, Z.; Long, D.; Yang, C.; Merlin, D. Oral delivery of IL-22 mRNA-loaded lipid nanoparticles targeting the injured intestinal mucosa: A novel therapeutic solution to treat ulcerative colitis. Biomaterials 2022, 288, 121707. [Google Scholar] [CrossRef]
- Yang, C.; Long, D.; Sung, J.; Alghoul, Z.; Merlin, D. Orally Administered Natural Lipid Nanoparticle-Loaded 6-Shogaol Shapes the Anti-Inflammatory Microbiota and Metabolome. Pharmaceutics 2021, 13, 1355. [Google Scholar] [CrossRef]
- El-Mayta, R.; Zhang, R.; Shepherd, S.J.; Wang, F.; Billingsley, M.M.; Dudkin, V.; Klein, D.; Lu, H.D.; Mitchell, M.J. A Nanoparticle Platform for Accelerated In Vivo Oral Delivery Screening of Nucleic Acids. Adv. Ther. 2021, 4, 2000111. [Google Scholar] [CrossRef]
- Davies, N.; Hovdal, D.; Edmunds, N.; Nordberg, P.; Dahlén, A.; Dabkowska, A.; Arteta, M.Y.; Radulescu, A.; Kjellman, T.; Höijer, A.; et al. Functionalized lipid nanoparticles for subcutaneous administration of mRNA to achieve systemic exposures of a therapeutic protein. Mol. Ther. Nucleic Acids 2021, 24, 369–384. [Google Scholar] [CrossRef]
- An, D.; Schneller, J.L.; Frassetto, A.; Liang, S.; Zhu, X.; Park, J.-S.; Theisen, M.; Hong, S.-J.; Zhou, J.; Rajendran, R.; et al. Systemic Messenger RNA Therapy as a Treatment for Methylmalonic Acidemia. Cell Rep. 2017, 21, 3548–3558. [Google Scholar] [CrossRef]
- Ramaswamy, S.; Tonnu, N.; Tachikawa, K.; Limphong, P.; Vega, J.B.; Karmali, P.P.; Chivukula, P.; Verma, I.M. Systemic delivery of factor IX messenger RNA for protein replacement therapy. Proc. Natl. Acad. Sci. USA 2017, 114, E1941–E1950. [Google Scholar] [CrossRef]
- Richner, J.M.; Himansu, S.; Dowd, K.A.; Butler, S.L.; Salazar, V.; Fox, J.M.; Julander, J.G.; Tang, W.W.; Shresta, S.; Pierson, T.C.; et al. Modified mRNA Vaccines Protect against Zika Virus Infection. Cell 2017, 168, 1114–1125. [Google Scholar] [CrossRef]
- Schrom, E.; Huber, M.; Aneja, M.; Dohmen, C.; Emrich, D.; Geiger, J.; Hasenpusch, G.; Herrmann-Janson, A.; Kretzschmann, V.; Mykhailyk, O.; et al. Translation of Angiotensin-Converting Enzyme 2 upon Liver- and Lung-Targeted Delivery of Optimized Chemically Modified mRNA. Mol. Ther. Nucleic Acids 2017, 7, 350–365. [Google Scholar] [CrossRef]
- Alberer, M.; Gnad-Vogt, U.; Hong, H.S.; Mehr, K.T.; Backert, L.; Finak, G.; Gottardo, R.; Bica, M.A.; Garofano, A.; Koch, S.D.; et al. Safety and immunogenicity of a mRNA rabies vaccine in healthy adults: An open-label, non-randomised, prospective, first-in-human phase 1 clinical trial. Lancet 2017, 390, 1511–1520. [Google Scholar] [CrossRef]
- Hassett, K.J.; Benenato, K.E.; Jacquinet, E.; Lee, A.; Woods, A.; Yuzhakov, O.; Himansu, S.; Deterling, J.; Geilich, B.M.; Ketova, T.; et al. Optimization of Lipid Nanoparticles for Intramuscular Administration of mRNA Vaccines. Mol. Ther. Nucleic Acids 2019, 15, 1–11. [Google Scholar] [CrossRef]
- Sedic, M.; Senn, J.J.; Lynn, A.; Laska, M.; Smith, M.; Platz, S.J.; Bolen, J.; Hoge, S.; Bulychev, A.; Jacquinet, E.; et al. Safety Evaluation of Lipid Nanoparticle-Formulated Modified mRNA in the Sprague-Dawley Rat and Cynomolgus Monkey. Vet. Pathol. 2018, 55, 341–354. [Google Scholar] [CrossRef]
- Kularatne, R.N.; Crist, R.M.; Stern, S.T. The Future of Tissue-Targeted Lipid Nanoparticle-Mediated Nucleic Acid Delivery. Pharmaceuticals 2022, 15, 897. [Google Scholar] [CrossRef]
- Mucker, E.M.; Thiele-Suess, C.; Baumhof, P.; Hooper, J.W. Lipid nanoparticle delivery of unmodified mRNAs encoding multiple monoclonal antibodies targeting poxviruses in rabbits. Mol. Ther. Nucleic Acids 2022, 28, 847–858. [Google Scholar] [CrossRef]
- Liu, Y.; Qian, X.; Ran, C.; Li, L.; Fu, T.; Su, D.; Xie, S.; Tan, W. Aptamer-Based Targeted Protein Degradation. ACS Nano 2023, 17, 6150–6164. [Google Scholar] [CrossRef]
- Lin, C.; Mostafa, A.; Jans, A.; Wolters, J.C.; Mohamed, M.R.; van der Vorst, E.P.C.; Trautwein, C.; Bartneck, M. Targeting Ligand Independent Tropism of siRNA-LNP by Small Molecules for Directed Therapy of Liver or Myeloid Immune Cells. Adv. Healthc. Mater. 2023, 12, e2202670. [Google Scholar] [CrossRef]
- Qin, J.; Xue, L.; Gong, N.; Zhang, H.; Shepherd, S.J.; Haley, R.M.; Swingle, K.L.; Mitchell, M.J. RGD peptide-based lipids for targeted mRNA delivery and gene editing applications. RSC Adv. 2022, 12, 25397–25404. [Google Scholar] [CrossRef]
- Nakamura, T.; Kawai, M.; Sato, Y.; Maeki, M.; Tokeshi, M.; Harashima, H. The Effect of Size and Charge of Lipid Nanoparticles Prepared by Microfluidic Mixing on Their Lymph Node Transitivity and Distribution. Mol. Pharm. 2020, 17, 944–953. [Google Scholar] [CrossRef]
- Silva, F.; Cabral Campello, M.P.; Paulo, A. Radiolabeled Gold Nanoparticles for Imaging and Therapy of Cancer. Materials 2020, 14, 4. [Google Scholar] [CrossRef]
- Luozhong, S.; Yuan, Z.; Sarmiento, T.; Chen, Y.; Gu, W.; McCurdy, C.; Gao, W.; Li, R.; Wilkens, S.; Jiang, S. Phosphatidylserine Lipid Nanoparticles Promote Systemic RNA Delivery to Secondary Lymphoid Organs. Nano Lett. 2022, 22, 8304–8311. [Google Scholar] [CrossRef]
- Akinc, A.; Maier, M.A.; Manoharan, M.; Fitzgerald, K.; Jayaraman, M.; Barros, S.; Ansell, S.; Du, X.; Hope, M.J.; Madden, T.D.; et al. The Onpattro story and the clinical translation of nanomedicines containing nucleic acid-based drugs. Nat. Nanotechnol. 2019, 14, 1084–1087. [Google Scholar] [CrossRef]
- Pattipeiluhu, R.; Arias-Alpizar, G.; Basha, G.; Chan, K.Y.T.; Bussmann, J.; Sharp, T.H.; Moradi, M.-A.; Sommerdijk, N.; Harris, E.N.; Cullis, P.R.; et al. Anionic Lipid Nanoparticles Preferentially Deliver mRNA to the Hepatic Reticuloendothelial System. Adv. Mater. 2022, 34, e2201095. [Google Scholar] [CrossRef]
- Qiu, M.; Tang, Y.; Chen, J.; Muriph, R.; Ye, Z.; Huang, C.; Evans, J.; Henske, E.P.; Xu, Q. Lung-selective mRNA delivery of synthetic lipid nanoparticles for the treatment of pulmonary lymphangioleiomyomatosis. Proc. Natl. Acad. Sci. USA 2022, 119, e2116271119. [Google Scholar] [CrossRef]
- Almeida, M.S.d.; Susnik, E.; Drasler, B.; Taladriz-Blanco, P.; Petri-Fink, A.; Rothen-Rutishauser, B. Understanding nanoparticle endocytosis to improve targeting strategies in nanomedicine. Chem. Soc. Rev. 2021, 50, 5397–5434. [Google Scholar] [CrossRef]
- Varkouhi, A.K.; Scholte, M.; Storm, G.; Haisma, H.J. Endosomal escape pathways for delivery of biologicals. J. Control. Release 2011, 151, 220–228. [Google Scholar] [CrossRef]
- Chan, C.-L.; Ewert, K.K.; Majzoub, R.N.; Hwu, Y.-K.; Liang, K.S.; Leal, C.; Safinya, C.R. Optimizing cationic and neutral lipids for efficient gene delivery at high serum content. J. Gene Med. 2014, 16, 84–96. [Google Scholar] [CrossRef]
- Junglas, B.; Axt, A.; Siebenaller, C.; Sonel, H.; Hellmann, N.; Weber, S.A.L.; Schneider, D. Membrane destabilization and pore formation induced by the Synechocystis IM30 protein. Biophys. J. 2022, 121, 3411–3421. [Google Scholar] [CrossRef]
- Bus, T.; Traeger, A.; Schubert, U.S. The great escape: How cationic polyplexes overcome the endosomal barrier. J. Mater. Chem. B 2018, 6, 6904–6918. [Google Scholar] [CrossRef]
- Su, D.; Coste, M.; Diaconu, A.; Barboiu, M.; Ulrich, S. Cationic dynamic covalent polymers for gene transfection. J. Mater. Chem. B 2020, 8, 9385–9403. [Google Scholar] [CrossRef]
- Lee, J.; Sands, I.; Zhang, W.; Zhou, L.; Chen, Y. DNA-inspired nanomaterials for enhanced endosomal escape. Proc. Natl. Acad. Sci. USA 2021, 118, e2104511118. [Google Scholar] [CrossRef]
- Ali, L.M.A.; Gary-Bobo, M. Photochemical Internalization of siRNA for Cancer Therapy. Cancers 2022, 14, 3597. [Google Scholar] [CrossRef]
- Rappaport, A.R.; Hong, S.-J.; Scallan, C.D.; Gitlin, L.; Akoopie, A.; Boucher, G.R.; Egorova, M.; Espinosa, J.A.; Fidanza, M.; Kachura, M.A.; et al. Low-dose self-amplifying mRNA COVID-19 vaccine drives strong protective immunity in non-human primates against SARS-CoV-2 infection. Nat. Commun. 2022, 13, 3289. [Google Scholar] [CrossRef]
- Vogel, A.B.; Lambert, L.; Kinnear, E.; Busse, D.; Erbar, S.; Reuter, K.C.; Wicke, L.; Perkovic, M.; Beissert, T.; Haas, H.; et al. Self-Amplifying RNA Vaccines Give Equivalent Protection against Influenza to mRNA Vaccines but at Much Lower Doses. Mol. Ther. 2018, 26, 446–455. [Google Scholar] [CrossRef]
- Blakney, A.K.; Ip, S.; Geall, A.J. An Update on Self-Amplifying mRNA Vaccine Development. Vaccines 2021, 9, 97. [Google Scholar] [CrossRef]
- Sahin, U.; Karikó, K.; Türeci, Ö. mRNA-based therapeutics—Developing a new class of drugs. Nat. Rev. Drug Discov. 2014, 13, 759–780. [Google Scholar] [CrossRef]
- Shuman, S. Catalytic activity of vaccinia mRNA capping enzyme subunits coexpressed in Escherichia coli. J. Biol. Chem. 1990, 265, 11960–11966. [Google Scholar] [CrossRef]
- Holtkamp, S.; Kreiter, S.; Selmi, A.; Simon, P.; Koslowski, M.; Huber, C.; Türeci, O.; Sahin, U. Modification of antigen-encoding RNA increases stability, translational efficacy, and T-cell stimulatory capacity of dendritic cells. Blood 2006, 108, 4009–4017. [Google Scholar] [CrossRef]
- Gebre, M.S.; Brito, L.A.; Tostanoski, L.H.; Edwards, D.K.; Carfi, A.; Barouch, D.H. Novel approaches for vaccine development. Cell 2021, 184, 1589–1603. [Google Scholar] [CrossRef]
- Arranta Bio. An Integrated Solution for mRNA Vaccine Manufacturing. Available online: https://arrantabio.com/mrna-manufacturing-vaccines/ (accessed on 22 June 2023).
- Jung, H.N.; Lee, S.-Y.; Lee, S.; Youn, H.; Im, H.-J. Lipid nanoparticles for delivery of RNA therapeutics: Current status and the role of in vivo imaging. Theranostics 2022, 12, 7509–7531. [Google Scholar] [CrossRef]
- Nooraei, S.; Sarkar Lotfabadi, A.; Akbarzadehmoallemkolaei, M.; Rezaei, N. Immunogenicity of Different Types of Adjuvants and Nano-Adjuvants in Veterinary Vaccines: A Comprehensive Review. Vaccines 2023, 11, 453. [Google Scholar] [CrossRef]
- Kobiyama, K.; Ishii, K.J. Making innate sense of mRNA vaccine adjuvanticity. Nat. Immunol. 2022, 23, 474–476. [Google Scholar] [CrossRef]
- Schoenmaker, L.; Witzigmann, D.; Kulkarni, J.A.; Verbeke, R.; Kersten, G.; Jiskoot, W.; Crommelin, D.J.A. mRNA-lipid nanoparticle COVID-19 vaccines: Structure and stability. Int. J. Pharm. 2021, 601, 120586. [Google Scholar] [CrossRef]
- Li, C.; Lee, A.; Grigoryan, L.; Arunachalam, P.S.; Scott, M.K.D.; Trisal, M.; Wimmers, F.; Sanyal, M.; Weidenbacher, P.A.; Feng, Y.; et al. Mechanisms of innate and adaptive immunity to the Pfizer-BioNTech BNT162b2 vaccine. Nat. Immunol. 2022, 23, 543–555. [Google Scholar] [CrossRef]
- Alameh, M.-G.; Tombácz, I.; Bettini, E.; Lederer, K.; Ndeupen, S.; Sittplangkoon, C.; Wilmore, J.R.; Gaudette, B.T.; Soliman, O.Y.; Pine, M.; et al. Lipid nanoparticles enhance the efficacy of mRNA and protein subunit vaccines by inducing robust T follicular helper cell and humoral responses. Immunity 2021, 54, 2877–2892. [Google Scholar] [CrossRef]
- D’Amico, C.; Fontana, F.; Cheng, R.; Santos, H.A. Development of vaccine formulations: Past, present, and future. Drug Deliv. Transl. Res. 2021, 11, 353–372. [Google Scholar] [CrossRef]
- Li, H.; Bian, Y.-L.; Schreurs, N.; Zhang, X.-G.; Raza, S.H.A.; Fang, Q.; Wang, L.-Q.; Hu, J.-H. Effects of five cryoprotectants on proliferation and differentiation-related gene expression of frozen-thawed bovine calf testicular tissue. Reprod. Domest. Anim. 2018, 53, 1211–1218. [Google Scholar] [CrossRef]
- Young, R.E.; Hofbauer, S.I.; Riley, R.S. Overcoming the challenge of long-term storage of mRNA-lipid nanoparticle vaccines. Mol. Ther. 2022, 30, 1792–1793. [Google Scholar] [CrossRef]
- Janjua, T.I.; Cao, Y.; Yu, C.; Popat, A. Clinical translation of silica nanoparticles. Nat. Rev. Mater. 2021, 6, 1072–1074. [Google Scholar] [CrossRef]
- Hosseinpour, S.; Cao, Y.; Liu, J.; Xu, C.; Walsh, L.J. Efficient transfection and long-term stability of rno-miRNA-26a-5p for osteogenic differentiation by large pore sized mesoporous silica nanoparticles. J. Mater. Chem. B 2021, 9, 2275–2284. [Google Scholar] [CrossRef]
- Jia, F.; Huang, W.; Yin, Y.; Jiang, Y.; Yang, Q.; Huang, H.; Nie, G.; Wang, H. Stabilizing RNA Nanovaccines with Transformable Hyaluronan Dynamic Hydrogel for Durable Cancer Immunotherapy. Adv. Funct. Mater. 2023, 33, 2204636. [Google Scholar] [CrossRef]
- Shahjin, F.; Patel, M.; Machhi, J.; Cohen, J.D.; Nayan, M.U.; Yeapuri, P.; Zhang, C.; Waight, E.; Hasan, M.; Abdelmoaty, M.M.; et al. Multipolymer microsphere delivery of SARS-CoV-2 antigens. Acta Biomater. 2023, 158, 493–509. [Google Scholar] [CrossRef]
- Voigt, E.A.; Gerhardt, A.; Hanson, D.; Jennewein, M.F.; Battisti, P.; Reed, S.; Singh, J.; Mohamath, R.; Bakken, J.; Beaver, S.; et al. A self-amplifying RNA vaccine against COVID-19 with long-term room-temperature stability. NPJ Vaccines 2022, 7, 136. [Google Scholar] [CrossRef]
- Oli, A.N.; Obialor, W.O.; Ifeanyichukwu, M.O.; Odimegwu, D.C.; Okoyeh, J.N.; Emechebe, G.O.; Adejumo, S.A.; Ibeanu, G.C. Immunoinformatics and Vaccine Development: An Overview. Immunotargets Ther. 2020, 9, 13–30. [Google Scholar] [CrossRef]
- Kazi, A.; Chuah, C.; Majeed, A.B.A.; Leow, C.H.; Lim, B.H.; Leow, C.Y. Current progress of immunoinformatics approach harnessed for cellular- and antibody-dependent vaccine design. Pathog. Glob. Health 2018, 112, 123–131. [Google Scholar] [CrossRef]
- Chen, J.; Liu, H.; Yang, J.; Chou, K.-C. Prediction of linear B-cell epitopes using amino acid pair antigenicity scale. Amino Acids 2007, 33, 423–428. [Google Scholar] [CrossRef]
- Saha, S.; Raghava, G.P.S. Prediction of continuous B-cell epitopes in an antigen using recurrent neural network. Proteins 2006, 65, 40–48. [Google Scholar] [CrossRef]
- Singh, H.; Ansari, H.R.; Raghava, G.P.S. Improved Method for Linear B-Cell Epitope Prediction Using Antigen’s Primary Sequence. PLoS ONE 2013, 8, e62216. [Google Scholar] [CrossRef]
- Kringelum, J.V.; Lundegaard, C.; Lund, O.; Nielsen, M. Reliable B cell epitope predictions: Impacts of method development and improved benchmarking. PLoS Comput. Biol. 2012, 8, e1002829. [Google Scholar] [CrossRef]
- Ponomarenko, J.; Bui, H.-H.; Li, W.; Fusseder, N.; Bourne, P.E.; Sette, A.; Peters, B. ElliPro: A new structure-based tool for the prediction of antibody epitopes. BMC Bioinform. 2008, 9, 514. [Google Scholar] [CrossRef]
- Tong, J.C.; Ren, E.C. Immunoinformatics: Current trends and future directions. Drug Discov. Today 2009, 14, 684–689. [Google Scholar] [CrossRef]
- Schaap-Johansen, A.-L.; Vujović, M.; Borch, A.; Hadrup, S.R.; Marcatili, P. T Cell Epitope Prediction and Its Application to Immunotherapy. Front. Immunol. 2021, 12, 712488. [Google Scholar] [CrossRef]
- Meysman, P.; Barton, J.; Bravi, B.; Cohen-Lavi, L.; Karnaukhov, V.; Lilleskov, E.; Montemurro, A.; Nielsen, M.; Mora, T.; Pereira, P.; et al. Benchmarking solutions to the T-cell receptor epitope prediction problem: IMMREP22 workshop report. ImmunoInformatics 2022, 9, 100024. [Google Scholar]
- Chen, B.; Khodadoust, M.S.; Olsson, N.; Wagar, L.E.; Fast, E.; Liu, C.L.; Muftuoglu, Y.; Sworder, B.J.; Diehn, M.; Levy, R.; et al. Predicting HLA class II antigen presentation through integrated deep learning. Nat. Biotechnol. 2019, 37, 1332–1343. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Kryshtafovych, A.; Moult, J.; Albrecht, R.; Chang, G.A.; Chao, K.; Fraser, A.; Greenfield, J.; Hartmann, M.D.; Herzberg, O.; Josts, I.; et al. Computational models in the service of X-ray and cryo-electron microscopy structure determination. Proteins 2021, 89, 1633–1646. [Google Scholar] [CrossRef]
- Tai, L.; Zhu, Y.; Ren, H.; Huang, X.; Zhang, C.; Sun, F. 8 Å structure of the outer rings of the Xenopus laevis nuclear pore complex obtained by cryo-EM and AI. Protein Cell 2022, 13, 760–777. [Google Scholar] [CrossRef]
- Baek, M.; DiMaio, F.; Anishchenko, I.; Dauparas, J.; Ovchinnikov, S.; Lee, G.R.; Wang, J.; Cong, Q.; Kinch, L.N.; Schaeffer, R.D.; et al. Accurate prediction of protein structures and interactions using a three-track neural network. Science 2021, 373, 871–876. [Google Scholar] [CrossRef]
- Wu, R.; Ding, F.; Wang, R.; Shen, R.; Zhang, X.; Luo, S.; Su, C.; Wu, Z.; Xie, Q.; Berger, B.; et al. High-resolution de novo structure prediction from primary sequence. bioRxiv 2022. [Google Scholar] [CrossRef]
- Lin, Z.; Akin, H.; Rao, R.; Hie, B.; Zhu, Z.; Lu, W.; Smetanin, N.; Verkuil, R.; Kabeli, O.; Shmueli, Y.; et al. Evolutionary-scale prediction of atomic-level protein structure with a language model. Science 2023, 379, 1123–1130. [Google Scholar] [CrossRef]
- Evans, R.; O’Neill, M.; Pritzel, A.; Antropova, N.; Senior, A.; Green, T.; Žídek, A.; Bates, R.; Blackwell, S.; Yim, J.; et al. Protein complex prediction with AlphaFold-Multimer. bioRxiv 2021. [Google Scholar] [CrossRef]
- Callaway, E. ‘The entire protein universe’: AI predicts shape of nearly every known protein. Nature 2022, 608, 15–16. [Google Scholar] [CrossRef]
- Williams, J.A.; Biancucci, M.; Lessen, L.; Tian, S.; Balsaraf, A.; Chen, L.; Chesterman, C.; Maruggi, G.; Vandepaer, S.; Huang, Y.; et al. Structural and computational design of a SARS-CoV-2 spike antigen with improved expression and immunogenicity. Sci. Adv. 2023, 9, eadg0330. [Google Scholar] [CrossRef]
- Federal Ministry of Health. Current Vaccination Status. Available online: https://impfdashboard.de/en/ (accessed on 17 May 2023).
- Oğuz, F.; Atmaca, H. mRNA as a Therapeutics: Understanding mRNA Vaccines. Adv. Pharm. Bull. 2021, 12, 274–282. [Google Scholar] [CrossRef]
- Rodionov, N.; Tatarnikova, L. Digital twin technology as a modern approach to quality management. E3S Web Conf. 2021, 284, 4013. [Google Scholar] [CrossRef]
- Forbes. Meet Your Digital Twin: The Coming Revolution in Drug Development. Available online: https://www.forbes.com/sites/ganeskesari/2021/09/29/meet-your-digital-twin-the-coming-revolution-in-drug-development/ (accessed on 17 May 2023).
- Helgers, H.; Hengelbrock, A.; Schmidt, A.; Strube, J. Digital Twins for Continuous mRNA Production. Processes 2021, 9, 1967. [Google Scholar] [CrossRef]
- IBM. What is A Digital Twin? Available online: https://www.ibm.com/topics/what-is-a-digital-twin (accessed on 17 May 2023).
- Maher, C. Quality & Speed: Employing Digital Twins to Accelerate the Product Lifecycle. Available online: https://www.ispeboston.org/download/educational_presentations/2022/2022-02-16-Digital-Tools-Charlie-Maher.pdf (accessed on 17 May 2023).
- Siemens. Als Digital Enterprise die Digitale Transformation Beschleunigen. Available online: https://www.siemens.com/de/de/produkte/automatisierung/themenfelder/digital-enterprise.html?gclid=EAIaIQobChMI6ITaq7WN_AIVBp3VCh3tGAfuEAMYAyAAEgKFYPD_BwE&acz=1 (accessed on 17 May 2023).
- U.S. Food and Drug Administration. Smart Design and Manufacturing Pilot. Available online: https://www.fda.gov/emergency-preparedness-and-response/ocet-advanced-manufacturing/smart-design-and-manufacturing-pilot (accessed on 17 May 2023).
- Grabski, E.; Hildt, E.; Wagner, R. Zulassungsverfahren für Humanimpfstoffe in Deutschland und Europa und das Präqualifizierungsprogramm der WHO. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2020, 63, 4–15. [Google Scholar] [CrossRef]
- kpibench GmbH. kpibench Qualitätsmanagement: Digitale Qualitätssicherung in Echtzeit. Available online: https://www.kpibench.com/de/2021/02/kpibench-qualitatsmanagement-digitale-qualitats-sicherung-in-echtzeit/ (accessed on 17 May 2023).
- Van der Vorst, J.G.A.J. Supply Chain Management: Theory and Practices. In The Emerging World of Chains and Networks, Bridging Theory and Practice (p. 348). Available online: https://edepot.wur.nl/357992 (accessed on 17 July 2023).
- Kazancoglu, Y.; Sezer, M.D.; Ozbiltekin-Pala, M.; Kucukvar, M. Investigating the role of stakeholder engagement for more resilient vaccine supply chains during COVID-19. Oper. Manag. Res. 2022, 15, 428–439. [Google Scholar] [CrossRef]
- Bown, C.P.; Bollyky, T.J. How COVID-19 vaccine supply chains emerged in the midst of a pandemic. World Econ. 2022, 45, 468–522. [Google Scholar] [CrossRef]
- Weintraub, R.L.; Subramanian, L.; Karlage, A.; Ahmad, I.; Rosenberg, J. COVID-19 Vaccine To Vaccination: Why Leaders Must Invest In Delivery Strategies Now. Health Aff. 2021, 40, 33–41. [Google Scholar] [CrossRef]
- Wrapp, D.; Wang, N.; Corbett, K.S.; Goldsmith, J.A.; Hsieh, C.-L.; Abiona, O.; Graham, B.S.; McLellan, J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 2020, 367, 1260–1263. [Google Scholar] [CrossRef]
- Higgins, M.K. Can We AlphaFold Our Way Out of the Next Pandemic? J. Mol. Biol. 2021, 433, 167093. [Google Scholar] [CrossRef]
- Betsch, C.; Schmid, P.; Korn, L.; Steinmeyer, L.; Heinemeier, D.; Eitze, S.; Küpke, N.K.; Böhm, R. Impfverhalten psychologisch erklären, messen und verändern. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2019, 62, 400–409. [Google Scholar] [CrossRef]
- Wagner, C.S.; Cai, X.; Zhang, Y.; Fry, C.V. One-year in: COVID-19 research at the international level in CORD-19 data. PLoS ONE 2022, 17, e0261624. [Google Scholar] [CrossRef]
- Liu, W.; Huangfu, X.; Wang, H. Citation advantage of COVID-19-related publications. J. Inf. Sci. 2023, 016555152311743. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stiefel, J.; Zimmer, J.; Schloßhauer, J.L.; Vosen, A.; Kilz, S.; Balakin, S. Just Keep Rolling?—An Encompassing Review towards Accelerated Vaccine Product Life Cycles. Vaccines 2023, 11, 1287. https://doi.org/10.3390/vaccines11081287
Stiefel J, Zimmer J, Schloßhauer JL, Vosen A, Kilz S, Balakin S. Just Keep Rolling?—An Encompassing Review towards Accelerated Vaccine Product Life Cycles. Vaccines. 2023; 11(8):1287. https://doi.org/10.3390/vaccines11081287
Chicago/Turabian StyleStiefel, Janis, Jan Zimmer, Jeffrey L. Schloßhauer, Agnes Vosen, Sarah Kilz, and Sascha Balakin. 2023. "Just Keep Rolling?—An Encompassing Review towards Accelerated Vaccine Product Life Cycles" Vaccines 11, no. 8: 1287. https://doi.org/10.3390/vaccines11081287
APA StyleStiefel, J., Zimmer, J., Schloßhauer, J. L., Vosen, A., Kilz, S., & Balakin, S. (2023). Just Keep Rolling?—An Encompassing Review towards Accelerated Vaccine Product Life Cycles. Vaccines, 11(8), 1287. https://doi.org/10.3390/vaccines11081287







