The Effect of Previous Exposure to Malaria Infection and Clinical Malaria Episodes on the Immune Response to the Two-Dose Ad26.ZEBOV, MVA-BN-Filo Ebola Vaccine Regimen
Abstract
1. Introduction
2. Materials and Methods
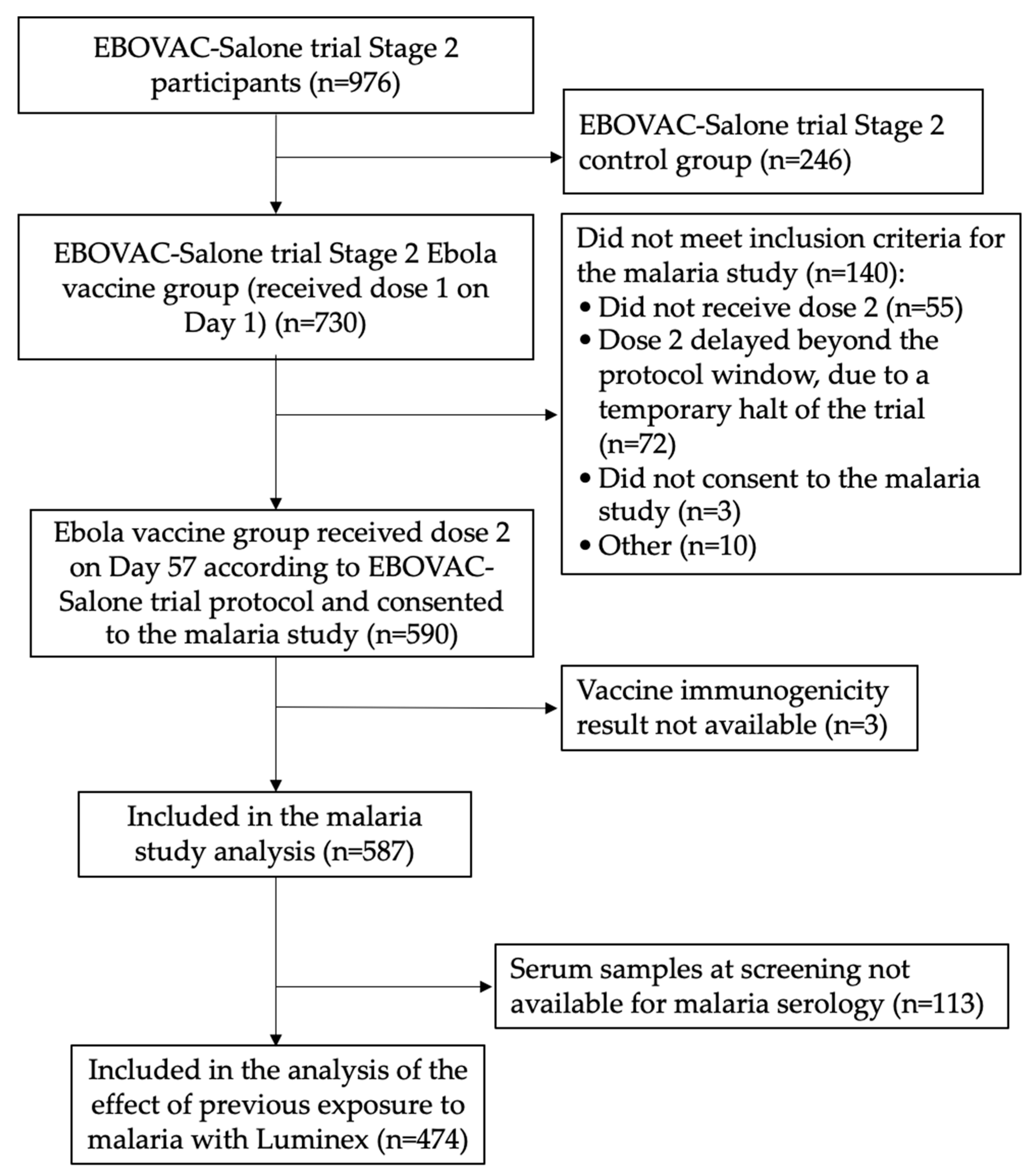
2.1. Study Design and Participants
2.2. Assessment of Exposure to Malaria Infection
2.3. Assessment of Vaccine-Induced Immune Responses
2.4. Sample Size Calculation and Statistical Analysis
3. Results
3.1. Study Participants
3.2. The Effect of Previous Exposure to Malaria on Vaccine Immunogenicity
3.3. The Effect of Clinical Episodes of Malaria after Vaccination on Vaccine Immunogenicity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Centers for Disease Control and Prevention (CDC). What Is Ebola Disease? Available online: https://www.cdc.gov/vhf/ebola/about.html (accessed on 31 July 2023).
- International Committee on Taxonomy of Viruses. Genus: Ebolavirus. Available online: https://ictv.global/report/chapter/filoviridae/filoviridae/orthoebolavirus (accessed on 25 July 2023).
- Mehand, M.S.; Al-Shorbaji, F.; Millett, P.; Murgue, B. The WHO R&D Blueprint: 2018 review of emerging infectious diseases requiring urgent research and development efforts. Antivir. Res. 2018, 159, 63–67. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Ebola Virus Disease Distribution Map: Cases of Ebola Virus Disease in Africa Since 1976. Available online: https://www.cdc.gov/vhf/ebola/history/distribution-map.html (accessed on 25 July 2023).
- US Food and Drug Administration. First FDA-Approved Vaccine for the Prevention of Ebola Virus Disease, Marking a Critical Milestone in Public Health Preparedness and Response. Available online: https://www.fda.gov/news-events/press-announcements/first-fda-approved-vaccine-prevention-ebola-virus-disease-marking-critical-milestone-public-health (accessed on 25 July 2023).
- European Commission. Vaccine against Ebola: Commission Grants First-Ever Market Authorisation. Available online: https://ec.europa.eu/commission/presscorner/detail/en/ip_19_6246 (accessed on 25 July 2023).
- Merck. ERVEBO® (Ebola Zaire Vaccine, Live) Now Registered in Four African Countries, within 90 Days of Reference Country Approval and WHO Prequalification. Available online: https://www.merck.com/news/ervebo-ebola-zaire-vaccine-live-now-registered-in-four-african-countries-within-90-days-of-reference-country-approval-and-who-prequalification/ (accessed on 25 July 2023).
- Burki, T. Ebola virus vaccine receives prequalification. Lancet 2019, 394, 1893. [Google Scholar] [CrossRef]
- Johnson & Johnson. Johnson & Johnson Joins World Health Organization in Efforts to Prevent Spread of Ebola in West Africa. 13 May 2021. Available online: https://www.jnj.com/johnson-johnson-joins-world-health-organization-in-efforts-to-prevent-spread-of-ebola-in-west-africa (accessed on 25 July 2023).
- European Commission. Vaccine against Ebola: Commission Grants New Market Authorisations. Available online: https://ec.europa.eu/commission/presscorner/detail/%20en/ip_20_1248 (accessed on 25 July 2023).
- Johnson & Johnson. Ebola. Available online: https://healthforhumanityreport.jnj.com/2022/global-health-equity/pandemics-epidemics/ebola.html (accessed on 25 July 2023).
- World Health Organization. World Malaria Report 2022. Geneva: World Health Organization; 2022. Licence: CC BY-NC-SA 3.0 IGO. Available online: https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2022 (accessed on 25 July 2023).
- Portugal, S.; Tipton, C.M.; Sohn, H.; Kone, Y.; Wang, J.; Li, S.; Skinner, J.; Virtaneva, K.; Sturdevant, D.E.; Porcella, S.F.; et al. Malaria-associated atypical memory B cells exhibit markedly reduced B cell receptor signaling and effector function. Elife 2015, 4, e07218. [Google Scholar] [CrossRef] [PubMed]
- Usen, S.; Milligan, P.; Ethevenaux, C.; Greenwood, B.; Mulholland, K. Effect of fever on the serum antibody response of Gambian children to Haemophilus influenzae type b conjugate vaccine. Pediatr. Infect Dis. J. 2000, 19, 444–449. [Google Scholar] [CrossRef] [PubMed]
- Bradley-Moore, A.M.; Greenwood, B.M.; Bradley, A.K.; Bartlett, A.; Bidwell, D.E.; Voller, A.; Craske, J.; Kirkwood, B.R.; Gilles, H.M. Malaria chemoprophylaxis with chloroquine in young Nigerian children. II. Effect on the immune response to vaccination. Ann. Trop Med. Parasitol. 1985, 79, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Nakalembe, M.; Banura, C.; Namujju, P.B.; Mirembe, F.M. Immunogenicity to the bivalent HPV-16/18 vaccine among adolescent African students exposed to helminths and malaria. J. Infect. Dev. Ctries 2015, 9, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Mahon, B.E.; Simon, J.; Widdowson, M.A.; Samai, M.; Rogier, E.; Legardy-Williams, J.; Liu, K.; Schiffer, J.; Lange, J.; DeByle, C.; et al. Baseline Asymptomatic Malaria Infection and Immunogenicity of Recombinant Vesicular Stomatitis Virus-Zaire Ebola Virus Envelope Glycoprotein. J. Infect. Dis. 2021, 224, 1907–1915. [Google Scholar] [CrossRef] [PubMed]
- Ishola, D.; The EBOVAC-Salone Malaria Infection Sub-Study Team. Asymptomatic Malaria Infection and the Immune Response to the 2-Dose Ad26.ZEBOV, MVA-BN-Filo Ebola Vaccine Regimen in Adults and Children. Clin. Infect. Dis. 2022, 75, 1585–1593. [Google Scholar] [CrossRef] [PubMed]
- Achan, J.; Reuling, I.J.; Yap, X.Z.; Dabira, E.; Ahmad, A.; Cox, M.; Nwakanma, D.; Tetteh, K.; Wu, L.; Bastiaens, G.J.H.; et al. Serologic Markers of Previous Malaria Exposure and Functional Antibodies Inhibiting Parasite Growth Are Associated with Parasite Kinetics Following a Plasmodium falciparum Controlled Human Infection. Clin. Infect. Dis. 2020, 70, 2544–2552. [Google Scholar] [CrossRef] [PubMed]
- Helb, D.A.; Tetteh, K.K.; Felgner, P.L.; Skinner, J.; Hubbard, A.; Arinaitwe, E.; Mayanja-Kizza, H.; Ssewanyana, I.; Kamya, M.R.; Beeson, J.G.; et al. Novel serologic biomarkers provide accurate estimates of recent Plasmodium falciparum exposure for individuals and communities. Proc. Natl. Acad. Sci. USA 2015, 112, E4438–E4447. [Google Scholar] [CrossRef] [PubMed]
- Baiden, F.; Fleck, S.; Leigh, B.; Ayieko, P.; Tindanbil, D.; Otieno, T.; Lawal, B.; Tehtor, M.; Rogers, M.; Odeny, L.; et al. Prevalence of malaria and helminth infections in rural communities in northern Sierra Leone, a baseline study to inform Ebola vaccine study protocols. PLoS ONE 2022, 17, e0270968. [Google Scholar] [CrossRef]
- Ishola, D.; Manno, D.; Afolabi, M.O.; Keshinro, B.; Bockstal, V.; Rogers, B.; Owusu-Kyei, K.; Serry-Bangura, A.; Swaray, I.; Lowe, B.; et al. Safety and long-term immunogenicity of the two-dose heterologous Ad26.ZEBOV and MVA-BN-Filo Ebola vaccine regimen in adults in Sierra Leone: A combined open-label, non-randomised stage 1, and a randomised, double-blind, controlled stage 2 trial. Lancet Infect. Dis. 2022, 22, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Manno, D.; Ayieko, P.; Ishola, D.; Afolabi, M.; Rogers, B.; Baiden, F.; Serry-Bangura, A.; Bah, O.; Kohn, B.; Swaray, I.; et al. Ebola Virus Glycoprotein IgG Seroprevalence in Community Previously Affected by Ebola, Sierra Leone. Emerg Infect. Dis. 2022, 28, 734–738. [Google Scholar] [CrossRef] [PubMed]
- Government of Sierra Leone, Ministry of Health and Sanitation, National Malaria Control Programme. Guidelines for Case Management of Malaria. Fourth Edition. 2015. Available online: https://www.afro.who.int/publications/guidelines-case-management-malaria-2015 (accessed on 25 July 2023).
- Redding, D.W.; Atkinson, P.M.; Cunningham, A.A.; Lo Iacono, G.; Moses, L.M.; Wood, J.L.N.; Jones, K.E. Impacts of environmental and socio-economic factors on emergence and epidemic potential of Ebola in Africa. Nat. Commun. 2019, 10, 4531. [Google Scholar] [CrossRef] [PubMed]
- Henao-Restrepo, A.M.; Longini, I.M.; Egger, M.; Dean, N.E.; Edmunds, W.J.; Camacho, A.; Carroll, M.W.; Doumbia, M.; Draguez, B.; Duraffour, S.; et al. Efficacy and effectiveness of an rVSV-vectored vaccine expressing Ebola surface glycoprotein: Interim results from the Guinea ring vaccination cluster-randomised trial. Lancet 2015, 386, 857–866. [Google Scholar] [CrossRef] [PubMed]
- Henao-Restrepo, A.M.; Camacho, A.; Longini, I.M.; Watson, C.H.; Edmunds, W.J.; Egger, M.; Carroll, M.W.; Dean, N.E.; Diatta, I.; Doumbia, M.; et al. Efficacy and effectiveness of an rVSV-vectored vaccine in preventing Ebola virus disease: Final results from the Guinea ring vaccination, open-label, cluster-randomised trial (Ebola Ca Suffit!). Lancet 2017, 389, 505–518. [Google Scholar] [CrossRef]
- Hennekens, C.; Buring, J.; Mayrent, S.L. Epidemiology in Medicine; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 1987; Volume 7, p. 169. [Google Scholar]

| Long-Term and Recent Exposure to Malaria at Screening | N (%) | Post-Dose 1 EBOV GP-Specific Binding Antibody GMC, EU/mL | GMR 1 (95% CI) | p |
|---|---|---|---|---|
| All participants 2 | N = 474 | |||
| Low | 144 (30.4) | 361 (306–426) | 1 | 0.39 |
| Intermediate | 213 (44.9) | 324 (283–371) | 0.88 (0.72–1.08) | |
| High | 117 (24.7) | 402 (337–481) | 0.99 (0.78–1.25) | |
| By age group | ||||
| 1–3 years | N = 96 | |||
| Low | 37 (38.5) | 783 (612–1002) | 1 | 0.75 |
| Intermediate | 28 (29.2) | 777 (575–1050) | 0.94 (0.65–1.37) | |
| High | 31 (32.3) | 694 (491–981) | 0.86 (0.55–1.35) | |
| 4–11 years | N = 116 | |||
| Low | 32 (27.6) | 288 (208–400) | 1 | 0.06 |
| Intermediate | 56 (48.3) | 453 (367–560) | 1.35 (0.92–1.98) | |
| High | 28 (24.1) | 358 (247–520) | 0.89 (0.58–1.37) | |
| 12–17 years | N = 115 | |||
| Low | 33 (28.7) | 365 (263–506) | 1 | 0.83 |
| Intermediate | 54 (47.0) | 308 (241–393) | 0.91 (0.62–1.33) | |
| High | 28 (24.3) | 323 (233–447) | 0.90 (0.59–1.38) | |
| ≥18 years | N = 147 | |||
| Low | 42 (28.6) | 214 (163–283) | 1 | 0.28 |
| Intermediate | 75 (51.0) | 189 (152–235) | 0.92 (0.67–1.25) | |
| High | 30 (20.4) | 314 (230–428) | 1.19 (0.85–1.67) | |
| Long-Term and Recent Exposure to Malaria at Screening | N (%) | Post-Dose 2 EBOV GP-Specific Binding Antibody GMC, EU/mL | GMR 1 (95% CI) | p |
|---|---|---|---|---|
| All participants 2 | N = 466 | |||
| Low | 143 (30.7) | 8717 (7102–10,699) | 1 | 0.70 |
| Intermediate | 206 (44.2) | 7927 (6629–9479) | 0.94 (0.72–1.23) | |
| High | 117 (25.1) | 9331 (7392–11,778) | 1.12 (0.82–1.51) | |
| By age group | ||||
| 1–3 years | N = 96 | |||
| Low | 37 (38.5) | 23,263 (17,681–30,607) | 1 | 0.90 |
| Intermediate | 28 (29.2) | 24,544 (17,102–35,225) | 1.00 (0.60–1.65) | |
| High | 31 (32.3) | 19,313 (10,757–34,676) | 0.89 (0.50–1.59) | |
| 4–11 years | N = 115 | |||
| Low | 32 (27.8) | 11,046 (7571–16,116) | 1 | 0.45 |
| Intermediate | 55 (47.8) | 11,069 (8284–14,791) | 1.06 (0.65–1.71) | |
| High | 28 (24.4) | 7472 (4794–11,644) | 0.76 (0.41–1.42) | |
| 12–17 years | N = 112 | |||
| Low | 33 (29.5) | 8038 (4998–12,926) | 1 | 0.31 |
| Intermediate | 51 (45.5) | 11,561 (8392–15,927) | 1.48 (0.82–2.65) | |
| High | 28 (25.0) | 9803 (7338–13,096) | 1.21 (0.69–2.12) | |
| ≥18 years | N = 143 | |||
| Low | 41 (28.7) | 3190 (2576–3950) | 1 | 0.06 |
| Intermediate | 72 (50.3) | 3029 (2372–3869) | 0.94 (0.69–1.29) | |
| High | 30 (21.0) | 5170 (3676–7271) | 1.50 (1.01–2.24) | |
| Clinical Malaria Post-Dose 1 Vaccination | N (%) | Post-Dose 1 EBOV GP-Specific Binding Antibody GMC, EU/mL | GMR (95% CI) | p |
|---|---|---|---|---|
| All participants | N = 587 | |||
| None | 412 (70.2) | 371 (338–407) | 1 | 0.02 |
| At least one episode | 175 (29.8) | 323 (275–379) | 0.82 (0.69–0.98) 2 | |
| By age group | ||||
| 1–3 years | N = 125 | |||
| None | 74 (59.2) | 750 (630–892) | 1 | 0.23 |
| At least one episode | 51 (40.8) | 618 (460–830) | 0.82 (0.58–1.16) | |
| 4–11 years | N = 133 | |||
| None | 99 (74.4) | 413 (342–498) | 1 | 0.22 |
| At least one episode | 34 (25.6) | 331 (254–431) | 0.80 (0.58–1.11) | |
| 12–17 years | N = 141 | |||
| None | 120 (85.1) | 312 (264–368) | 1 | 0.83 |
| At least one episode | 21 (14.9) | 327 (209–510) | 1.05 (0.65–1.69) | |
| ≥18 years | N = 188 | |||
| None | 119 (63.3) | 260 (220–308) | 1 | 0.05 |
| At least one episode | 69 (36.7) | 197 (156–249) | 0.76 (0.57–1.00) | |
| Clinical Malaria Post-Dose 1 and 2 Vaccinations | N (%) | Post-Dose 2 EBOV GP-Specific Binding Antibody GMC, EU/mL | GMR (95% CI) | p |
|---|---|---|---|---|
| All participants | N = 579 | |||
| None | 350 (60.5) | 8489 (7498–9610) | 1 | 0.69 |
| At least one episode | 229 (39.5) | 9133 (7678–10,863) | 1.04 (0.87–1.24) 2 | |
| By age group | ||||
| 1–3 years | N = 125 | |||
| None | 54 (43.2) | 22,601 (18,039–28,317) | 1 | 0.88 |
| At least one episode | 71 (56.8) | 21,909 (16,020–29,963) | 0.97 (0.66–1.43) | |
| 4–11 years | N = 132 | |||
| None | 92 (69.7) | 9470 (7576–11,839) | 1 | 0.24 |
| At least one episode | 40 (30.3) | 12,062 (8469–17,178) | 1.27 (0.84–1.94) | |
| 12–17 years | N = 138 | |||
| None | 106 (76.8) | 9428 (7549–11,775) | 1 | 0.74 |
| At least one episode | 32 (23.2) | 10,186 (6864–15,117) | 1.08 (0.69–1.70) | |
| ≥18 years | N = 184 | |||
| None | 98 (53.3) | 3987 (3275–4852) | 1 | 0.65 |
| At least one episode | 86 (46.7) | 3741 (3104–4509) | 0.94 (0.72–1.23) | |
| Clinical Malaria Post-Dose 2 Vaccination | N (%) | Post-Dose 2 EBOV GP-Specific Binding Antibody GMC, EU/mL | GMR (95% CI) | p |
|---|---|---|---|---|
| All participants | N = 579 | |||
| None | 481 (83.1) | 8372 (7511–9333) | 1 | 0.83 1 |
| At least one episode | 98 (16.9) | 10,775 (8189–14,178) | 1.03 (0.79–1.33) 1 | |
| By age group | ||||
| 1–3 years | N = 125 | |||
| None | 82 (65.6) | 24,019 (19,917–28,966) | 1 | 0.29 |
| At least one episode | 43 (34.4) | 19,117 (11,996–30,467) | 0.80 (0.48–1.32) | |
| 4–11 years | N = 132 | |||
| None | 123 (93.2) | 10,051 (8273–12,212) | 1 | 0.59 |
| At least one episode | 9 (6.8) | 12,299 (5419–27,914) | 1.22 (0.53–2.85) | |
| 12–17 years | N = 138 | |||
| None | 122 (88.4) | 9474 (7678–11,689) | 1 | 0.71 |
| At least one episode | 16 (11.6) | 10,607 (6589–17,076) | 1.12 (0.66–1.89) | |
| ≥18 years | N = 184 | |||
| None | 154 (83.7) | 3743 (3217–4356) | 1 | 0.27 |
| At least one episode | 30 (16.3) | 4591 (3397–6205) | 1.23 (0.87–1.72) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manno, D.; Patterson, C.; Drammeh, A.; Tetteh, K.; Kroma, M.T.; Otieno, G.T.; Lawal, B.J.; Soremekun, S.; Ayieko, P.; Gaddah, A.; et al. The Effect of Previous Exposure to Malaria Infection and Clinical Malaria Episodes on the Immune Response to the Two-Dose Ad26.ZEBOV, MVA-BN-Filo Ebola Vaccine Regimen. Vaccines 2023, 11, 1317. https://doi.org/10.3390/vaccines11081317
Manno D, Patterson C, Drammeh A, Tetteh K, Kroma MT, Otieno GT, Lawal BJ, Soremekun S, Ayieko P, Gaddah A, et al. The Effect of Previous Exposure to Malaria Infection and Clinical Malaria Episodes on the Immune Response to the Two-Dose Ad26.ZEBOV, MVA-BN-Filo Ebola Vaccine Regimen. Vaccines. 2023; 11(8):1317. https://doi.org/10.3390/vaccines11081317
Chicago/Turabian StyleManno, Daniela, Catriona Patterson, Abdoulie Drammeh, Kevin Tetteh, Mattu Tehtor Kroma, Godfrey Tuda Otieno, Bolarinde Joseph Lawal, Seyi Soremekun, Philip Ayieko, Auguste Gaddah, and et al. 2023. "The Effect of Previous Exposure to Malaria Infection and Clinical Malaria Episodes on the Immune Response to the Two-Dose Ad26.ZEBOV, MVA-BN-Filo Ebola Vaccine Regimen" Vaccines 11, no. 8: 1317. https://doi.org/10.3390/vaccines11081317
APA StyleManno, D., Patterson, C., Drammeh, A., Tetteh, K., Kroma, M. T., Otieno, G. T., Lawal, B. J., Soremekun, S., Ayieko, P., Gaddah, A., Kamara, A. B., Baiden, F., Afolabi, M. O., Tindanbil, D., Owusu-Kyei, K., Ishola, D., Deen, G. F., Keshinro, B., Njie, Y., ... Watson-Jones, D. (2023). The Effect of Previous Exposure to Malaria Infection and Clinical Malaria Episodes on the Immune Response to the Two-Dose Ad26.ZEBOV, MVA-BN-Filo Ebola Vaccine Regimen. Vaccines, 11(8), 1317. https://doi.org/10.3390/vaccines11081317







