Neutralizing Activity against BQ.1.1, BN.1, and XBB.1 in Bivalent COVID-19 Vaccine Recipients: Comparison by the Types of Prior Infection and Vaccine Formulations
Abstract
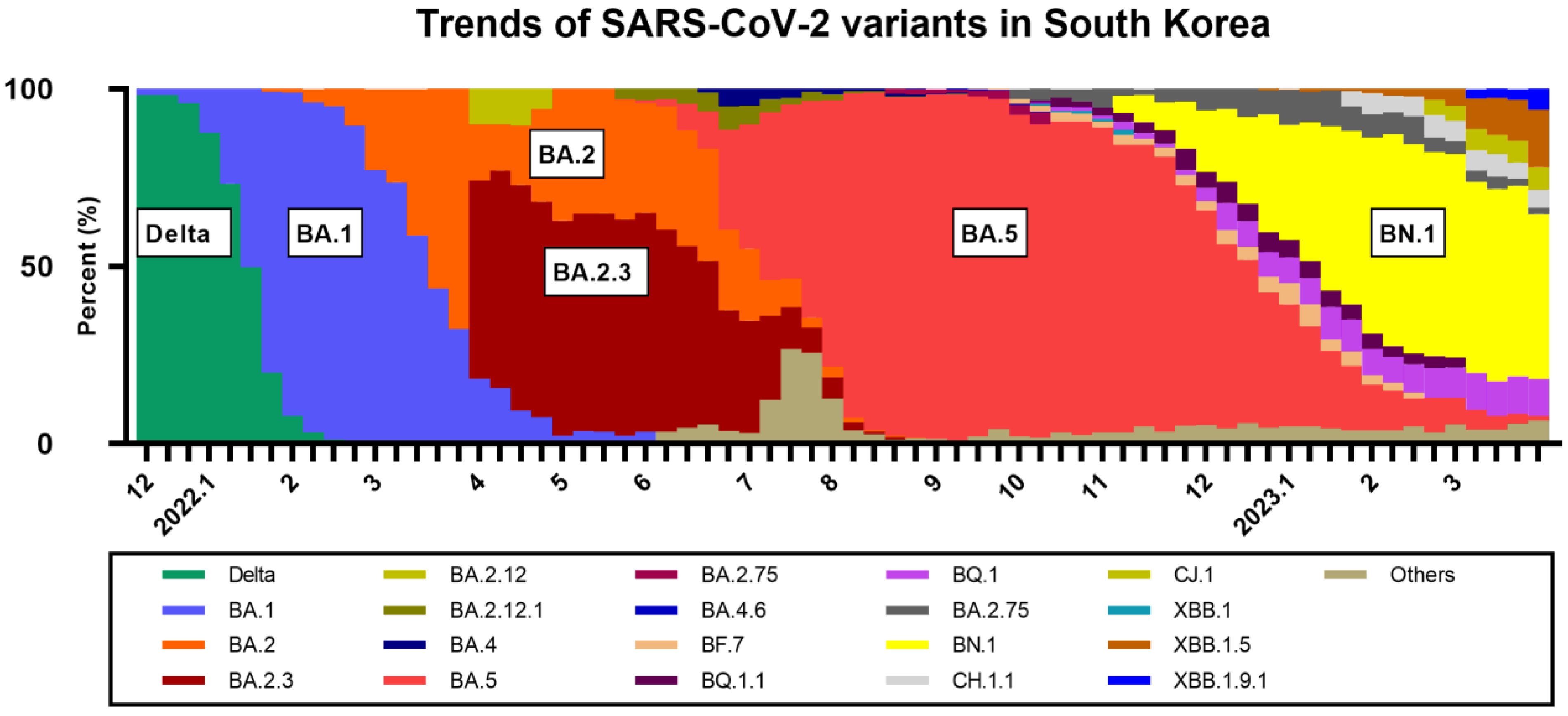
1. Introduction
2. Materials and Methods
2.1. Study Design and Procedure
2.2. Immunological Analysis
2.3. Statistical Analysis
2.4. Ethics Statement
3. Results
3.1. Study Participants
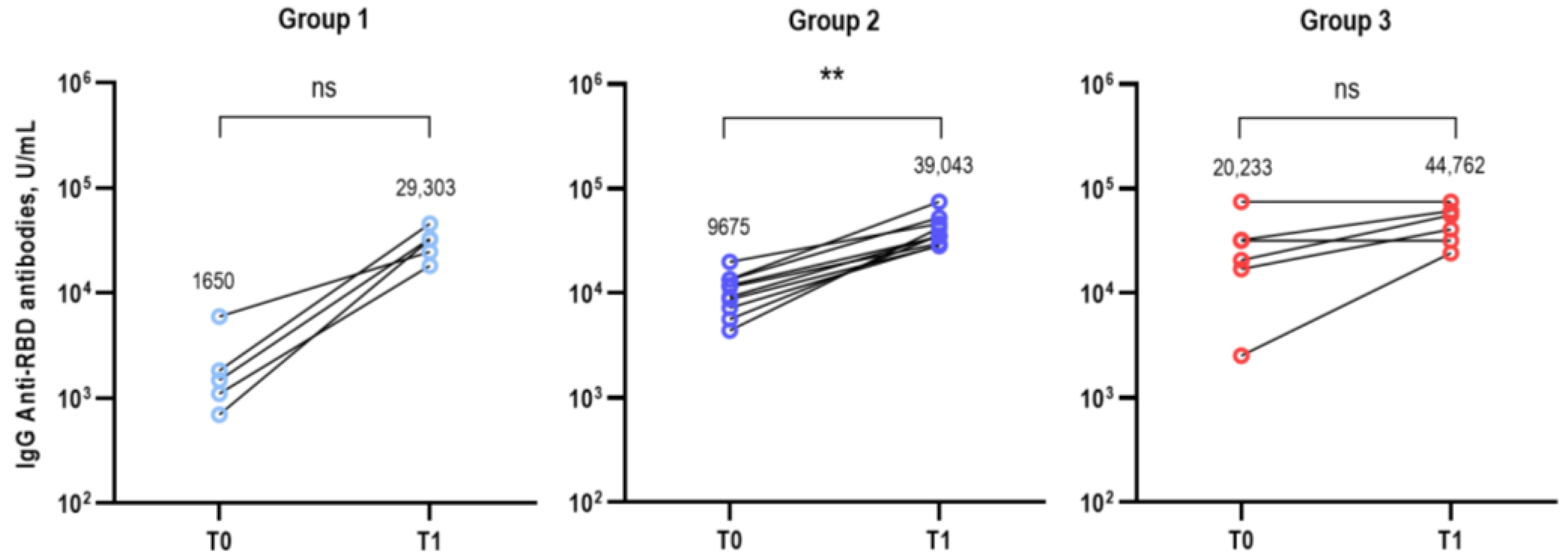
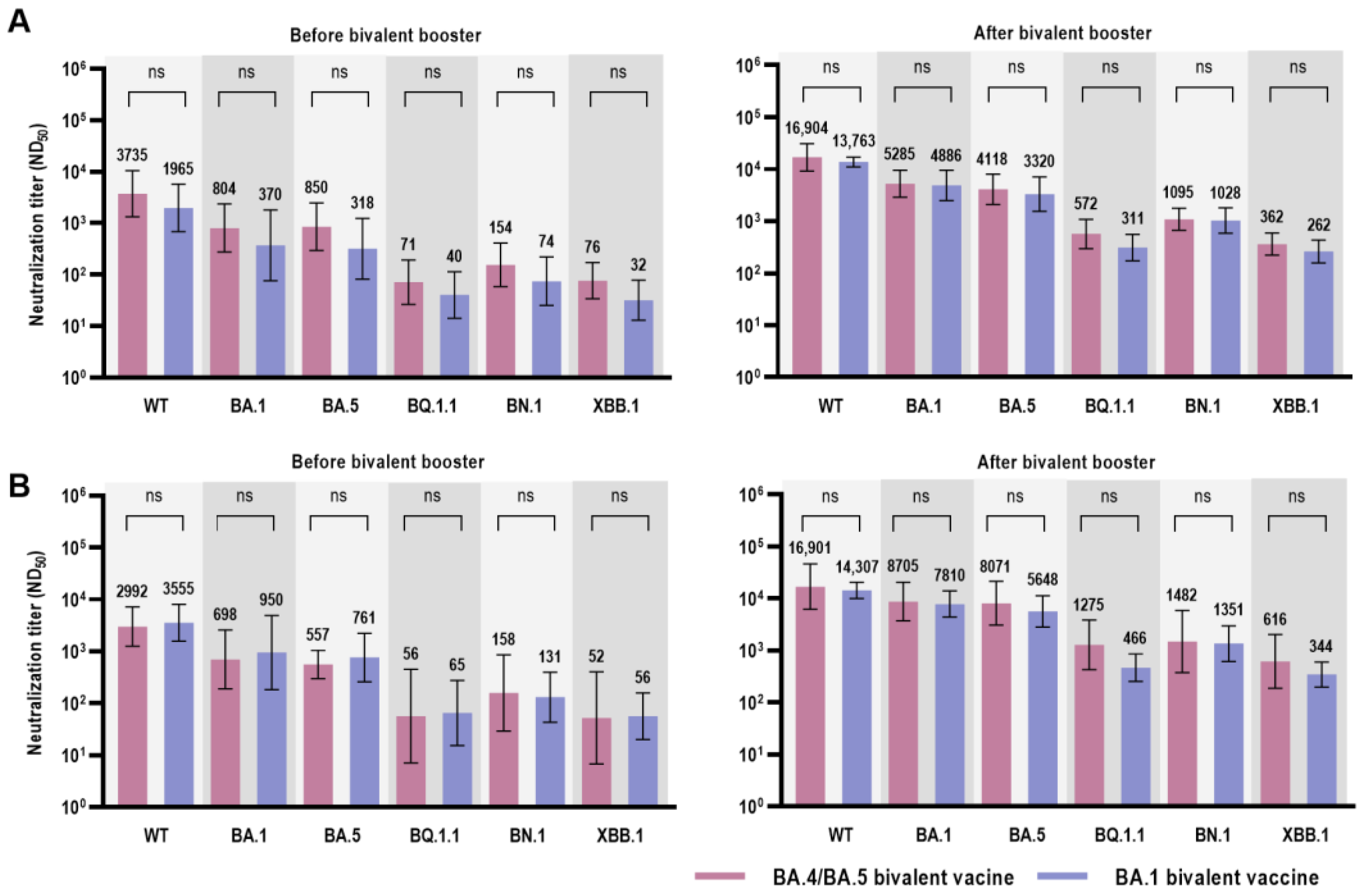
3.2. Immunological Analysis after Bivalent COVID-19 Vaccination
3.3. Reactogenicity of the Bivalent COVID-19 Vaccine
4. Discussions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Classification of Omicron (B.1.1.529): SARS-CoV-2 Variant of Concern. 2021. Available online: https://www.who.int/news/item/26-11-2021-classification-of-omicron-(b.1.1.529)-sars-cov-2-variant-of-concern (accessed on 30 June 2023).
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/region/wpro/country/kr (accessed on 30 June 2023).
- Wu, N.; Joyal-Desmarais, K.; Ribeiro, P.A.B.; Vieira, A.M.; Stojanovic, J.; Sanuade, C.; Yip, D.; Bacon, S.L. Long-term effectiveness of COVID-19 vaccines against infections, hospitalisations, and mortality in adults: Findings from a rapid living systematic evidence synthesis and meta-analysis up to December, 2022. Lancet Respir. Med. 2023, 11, 439–452. [Google Scholar] [CrossRef]
- Kim, Y.Y.; Choe, Y.J.; Kim, J.; Kim, R.K.; Jang, E.J.; Lee, H.; Yi, S.; Lee, S.; Park, Y.J. Vaccine Effectiveness Against Severe Disease and Death for Patients With COVID-19 During the Delta-Dominant and Omicron-Emerging Periods: A K-COVE Study. J. Korean Med. Sci. 2023, 38, e87. [Google Scholar] [CrossRef]
- Bobrovitz, N.; Ware, H.; Ma, X.; Li, Z.; Hosseini, R.; Cao, C.; Selemon, A.; Whelan, M.; Premji, Z.; Issa, H.; et al. Protective effectiveness of previous SARS-CoV-2 infection and hybrid immunity against the omicron variant and severe disease: A systematic review and meta-regression. Lancet Infect. Dis. 2023, 23, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Iketani, S.; Li, Z.; Liu, L.; Guo, Y.; Huang, Y.; Bowen, A.D.; Liu, M.; Wang, M.; Yu, J.; et al. Alarming antibody evasion properties of rising SARS-CoV-2 BQ and XBB subvariants. Cell 2023, 186, 279–286.e278. [Google Scholar] [CrossRef] [PubMed]
- Hyun, H.; Jang, A.Y.; Park, H.; Heo, J.Y.; Seo, Y.B.; Nham, E.; Yoon, J.G.; Seong, H.; Noh, J.Y.; Cheong, H.J.; et al. Humoral and cellular immunogenicity of homologous and heterologous booster vaccination in Ad26.COV2.S-primed individuals: Comparison by breakthrough infection. Front. Immunol. 2023, 14, 1131229. [Google Scholar] [CrossRef]
- Zou, J.; Kurhade, C.; Patel, S.; Kitchin, N.; Tompkins, K.; Cutler, M.; Cooper, D.; Yang, Q.; Cai, H. Neutralization of BA.4–BA.5, BA.4.6, BA.2.75.2, BQ.1.1, and XBB.1 with Bivalent Vaccine. N. Engl. J. Med. 2023, 388, 853–854. [Google Scholar] [CrossRef]
- Miller, J.; Hachmann, N.P.; Collier, A.r.Y.; Lasrado, N.; Mazurek, C.R.; Patio, R.C.; Powers, O.; Surve, N.; Theiler, J.; Korber, B.; et al. Substantial Neutralization Escape by SARSCoV-2 Omicron Variants BQ.1.1 and XBB.1. N. Engl. J. Med. 2023, 388, 660–662. [Google Scholar] [CrossRef]
- Wang, Q.; Bowen, A.; Tam, A.R.; Valdez, R.; Stoneman, E.; Mellis, I.A.; Gordon, A.; Liu, L.; Ho, D.D. SARS-CoV-2 neutralising antibodies after bivalent versus monovalent booster. Lancet Infect. Dis. 2023, 23, 527–528. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. Coronavirus (COVID-19) Update: FDA Authorizes Moderna, Pfizer-BioNTech Bivalent COVID-19 Vaccines for Use as a Booster Dose. 2022. Available online: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-moderna-pfizer-biontech-bivalent-covid-19-vaccines-use (accessed on 12 April 2023).
- Korea Disease Control and Prevention Agency. Regular Briefing: Transition to Once-a-Year COVID-19 Vaccination (March 22). 2023. Available online: https://www.kdca.go.kr/board/board.es?mid=a20501010000&bid=0015&list_no=722117&cg_code=&act=view&nPage=1# (accessed on 27 July 2023).
- Nextstrain Team. Genomic Epidemiology of SARS-CoV-2 with Subsampling Focused Globally over the Past 6 Months. Available online: https://nextstrain.org/ncov/gisaid/global/6m (accessed on 27 July 2023).
- Korea Disease Control and Prevention Agency. Current Status of SARS-CoV-2 Variants in South Korea. Available online: https://www.kdca.go.kr/contents.es?mid=a20107030000 (accessed on 12 April 2023).
- Hodcroft, E.B. CoVariants: SARS-CoV-2 Mutations and Variants of Interest. Available online: https://covariants.org (accessed on 12 April 2023).
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.R.; Zhu, Y.; Li, B.; Huang, C.L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef]
- Feng, S.; Phillips, D.J.; White, T.; Sayal, H.; Aley, P.K.; Bibi, S.; Dold, C.; Fuskova, M.; Gilbert, S.C.; Hirsch, I.; et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 2032–2040. [Google Scholar] [CrossRef]
- Uraki, R.; Ito, M.; Kiso, M.; Yamayoshi, S.; Iwatsuki-Horimoto, K.; Sakai-Tagawa, Y.; Furusawa, Y.; Imai, M.; Koga, M.; Yamamoto, S.; et al. Efficacy of antivirals and bivalent mRNA vaccines against SARS-CoV-2 isolate CH.1.1. Lancet Infect. Dis. 2023, 23, 525–526. [Google Scholar] [CrossRef] [PubMed]
- Wheatley, A.K.; Fox, A.; Tan, H.X.; Juno, J.A.; Davenport, M.P.; Subbarao, K.; Kent, S.J. Immune imprinting and SARS-CoV-2 vaccine design. Trends Immunol. 2021, 42, 956–959. [Google Scholar] [CrossRef] [PubMed]
- Arbel, R.; Peretz, A.; Sergienko, R.; Friger, M.; Beckenstein, T.; Duskin-Bitan, H.; Yaron, S.; Hammerman, A.; Bilenko, N.; Netzer, D. Effectiveness of a bivalent mRNA vaccine booster dose to prevent severe COVID-19 outcomes: A retrospective cohort study. Lancet Infect. Dis. 2023, 23, 914–921. [Google Scholar] [CrossRef] [PubMed]
- Lasrado, N.; Collier, A.-r.Y.; Miller, J.; Hachmann, N.P.; Liu, J.; Sciacca, M.; Wu, C.; Anand, T.; Bondzie, E.A.; Fisher, J.L.; et al. Waning Immunity Against XBB.1.5 Following Bivalent mRNA Boosters. bioRxiv 2023. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Perez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Chakraborti, S.; Gill, J.; Goswami, R.; Kumar, S.; Chandele, A.; Sharma, A. Structural Profiles of SARS-CoV-2 Variants in India. Curr. Microbiol. 2022, 80, 1. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Jian, F.; Wang, J.; Yu, Y.; Song, W.; Yisimayi, A.; Wang, J.; An, R.; Chen, X.; Zhang, N.; et al. Imprinted SARS-CoV-2 humoral immunity induces convergent Omicron RBD evolution. Nature 2023, 614, 521–529. [Google Scholar] [CrossRef]
- Kurhade, C.; Zou, J.; Xia, H.; Liu, M.; Chang, H.C.; Ren, P.; Xie, X.; Shi, P.Y. Low neutralization of SARS-CoV-2 Omicron BA.2.75.2, BQ.1.1 and XBB.1 by parental mRNA vaccine or a BA.5 bivalent booster. Nat. Med. 2023, 29, 344–347. [Google Scholar] [CrossRef]
- Yue, C.; Song, W.; Wang, L.; Jian, F.; Chen, X.; Gao, F.; Shen, Z.; Wang, Y.; Wang, X.; Cao, Y. ACE2 binding and antibody evasion in enhanced transmissibility of XBB.1.5. Lancet Infect. Dis. 2023, 23, 278–280. [Google Scholar] [CrossRef]
- Uriu, K.; Ito, J.; Zahradnik, J.; Fujita, S.; Kosugi, Y.; Schreiber, G.; Genotype to Phenotype Japan, C.; Sato, K. Enhanced transmissibility, infectivity, and immune resistance of the SARS-CoV-2 omicron XBB.1.5 variant. Lancet Infect. Dis. 2023, 23, 280–281. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Statement on the Antigen Composition of COVID-19 Vaccines. Available online: https://www.who.int/news/item/18-05-2023-statement-on-the-antigen-composition-of-covid-19-vaccines (accessed on 30 June 2023).
- Yamasoba, D.; Uriu, K.; Plianchaisuk, A.; Kosugi, Y.; Pan, L.; Zahradnik, J.; Ito, J.; Sato, K. Virological characteristics of the SARS-CoV-2 omicron XBB.1.16 variant. Lancet Infect. Dis. 2023, 23, 655–656. [Google Scholar] [CrossRef] [PubMed]

| Group 1 | Group 2 | Group 3 | |
|---|---|---|---|
| SARS-CoV-2 Infection-Naïve (n = 5) | Prior BA.1/BA.2-Infected (n = 10) | Prior BA.5-Infected (n = 6) | |
| Demographics | |||
| Male, n (%) | 2 (40.0) | 2 (20.0) | 3 (50.0) |
| Median age (IQR), y | 65 (61–71) | 63 (62–67) | 64 (50–67) |
| Median BMI (IQR), kg/m2 | 21.8 (21.2–23.7) | 25.4 (21.3–26.0) | 24.8 (24.4–30.5) |
| Medical comorbidities, n (%) | 2 (40.0) | 5 (50.0) | 2 (33.3) |
| Chronic heart disease | 0 (0) | 1 (10.0) | 1 (16.7) |
| Chronic lung disease | 0 (0) | 0 (0) | 0 (0) |
| Chronic liver disease | 0 (0) | 0 (0) | 0 (0) |
| Chronic renal disease | 0 (0) | 0 (0) | 0 (0) |
| Rheumatologic disease | 0 (0) | 1 (10.0) | 0 (0) |
| Hypertension | 1 (20.0) | 1 (10.0) | 1 (16.7) |
| Diabetes mellitus | 2 (40.0) | 2 (20.0) | 2 (33.3) |
| Dyslipidemia | 0 (0) | 0 (0) | 0 (0) |
| Previous vaccination, n (%) | |||
| ChAd/ChAd/BNT/NVX | 3 (60.0) | 7 (70.0) | 3 (50.0) |
| ChAd/ChAd/M/NVX | 1 (20.0) | 2 (20.0) | 2 (33.3) |
| BNT/BNT/BNT/NVX | 1 (20.0) | 0 (0) | 0 (0) |
| BNT/BNT/NVX | 0 (0) | 0 (0) | 1 (16.7) |
| M/M/NVX | 0 (0) | 1 (10.0) | 0 (0) |
| Median interval between previous dose and bivalent vaccination (IQR), days | 190 (188–219) | 216 (189–237) | 230 (221–272) |
| Bivalent vaccine, n (%) | |||
| BA.4/5 | 2 (40.0) | 4 (40.0) | 6 (100) |
| BA.1 | 3 (60.0) | 6 (60.0) | 0 (0) |
| Group 1 | Group 2 | Group 3 | p-Value | |||
|---|---|---|---|---|---|---|
| SARS-CoV-2 Infection-Naïve (n = 5) | Prior BA.1/BA.2-Infected (n = 10) | Prior BA.5-Infected (n = 6) | Group 1 vs. Group 2 | Group 2 vs. Group 3 | Group 3 vs. Group 1 | |
| Before Bivalent COVID-19 Vaccine, GMT (95% CI) | ||||||
| Wild type | 481 (100–2300) | 3318 (2061–5342) | 9591 (1987–46,290) | 0.028 | 0.056 | 0.009 |
| BA.1 | 49 (11–224) | 840 (339–2084) | 2401 (920–6264) | 0.003 | 0.093 | 0.004 |
| BA.5 | 91 (9–939) | 672 (376–1200) | 1849 (290–11,799) | 0.055 | 0.042 | 0.017 |
| BQ.1.1 | 13 (6–27) | 61 (25–152) | 160 (37–693) | 0.024 | 0.072 | 0.022 |
| BN.1 | 17 (4–73) | 141 (69–290) | 378 (169–847) | 0.007 | 0.056 | 0.009 |
| XBB.1 | 11 (8–15) | 55 (26–115) | 176 (93–334) | 0.016 | 0.007 | 0.004 |
| After Bivalent COVID-19 Vaccine, GMT (95% CI) | ||||||
| Wild type | 9272 (4319–19,905) | 15,293 (11,082–21,104) | 24,204 (8558–68,450) | 0.099 | 0.492 | 0.178 |
| BA.1 | 1636 (850–3150) | 8156 (5627–11,823) | 6058 (2520–14,561) | 0.001 | 0.264 | 0.009 |
| BA.5 | 915 (506–1655) | 6515 (4154–10,217) | 4861 (2393–9875) | 0.001 | 0.492 | 0.004 |
| BQ.1.1 | 115 (73–183) | 697 (397–1222) | 628 (512–770) | 0.001 | 0.713 | 0.004 |
| BN.1 | 490 (251–958) | 1402 (818–2404) | 1289 (921–1804) | 0.028 | 0.492 | 0.004 |
| XBB.1 | 144 (79–261) | 434 (271–695) | 355 (201–626) | 0.005 | 0.562 | 0.009 |
| BNT162b2 (BA.4/BA.5 Bivalent) | mRNA-1273.214 (BA.1 Bivalent) | p-Value | |
|---|---|---|---|
| Before Bivalent COVID-19 Vaccine, GMT (95% CI) | |||
| Wild type | 2992 (1252–7148) | 3555 (1573–8035) | 0.914 |
| BA.1 | 698 (189–2579) | 950 (184–4916) | 0.914 |
| BA.5 | 557 (298–1042) | 761 (260–2222) | 0.762 |
| BQ.1.1 | 57 (7–450) | 65 (15–274) | 0.657 |
| BN.1 | 158 (29–855) | 131 (43–398) | 0.762 |
| XBB.1 | 52 (7–400) | 56 (20–158) | 0.814 |
| After Bivalent COVID-19 Vaccine, GMT (95% CI) | |||
| Wild type | 16,901 (6204–46,047) | 14,307 (10,047–20,372) | 0.762 |
| BA.1 | 8705 (3722–20,358) | 7810 (4362–13,984) | 0.914 |
| BA.5 | 8071 (3060–21,287) | 5648 (2842–11,224) | 0.476 |
| BQ.1.1 | 1275 (424–3837) | 466 (254–855) | 0.067 |
| BN.1 | 1482 (373–5894) | 1351 (613–2977) | 0.762 |
| XBB.1 | 616 (187–2023) | 344 (198–599) | 0.352 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hyun, H.-J.; Choi, M.-J.; Nham, E.; Seong, H.; Yoon, J.-G.; Noh, J.-Y.; Cheong, H.-J.; Kim, W.-J.; Yoon, S.-K.; Park, S.-J.; et al. Neutralizing Activity against BQ.1.1, BN.1, and XBB.1 in Bivalent COVID-19 Vaccine Recipients: Comparison by the Types of Prior Infection and Vaccine Formulations. Vaccines 2023, 11, 1320. https://doi.org/10.3390/vaccines11081320
Hyun H-J, Choi M-J, Nham E, Seong H, Yoon J-G, Noh J-Y, Cheong H-J, Kim W-J, Yoon S-K, Park S-J, et al. Neutralizing Activity against BQ.1.1, BN.1, and XBB.1 in Bivalent COVID-19 Vaccine Recipients: Comparison by the Types of Prior Infection and Vaccine Formulations. Vaccines. 2023; 11(8):1320. https://doi.org/10.3390/vaccines11081320
Chicago/Turabian StyleHyun, Hak-Jun, Min-Joo Choi, Eliel Nham, Hye Seong, Jin-Gu Yoon, Ji-Yun Noh, Hee-Jin Cheong, Woo-Joo Kim, Sun-Kyung Yoon, Se-Jin Park, and et al. 2023. "Neutralizing Activity against BQ.1.1, BN.1, and XBB.1 in Bivalent COVID-19 Vaccine Recipients: Comparison by the Types of Prior Infection and Vaccine Formulations" Vaccines 11, no. 8: 1320. https://doi.org/10.3390/vaccines11081320
APA StyleHyun, H.-J., Choi, M.-J., Nham, E., Seong, H., Yoon, J.-G., Noh, J.-Y., Cheong, H.-J., Kim, W.-J., Yoon, S.-K., Park, S.-J., Gwak, W.-S., Lee, J.-W., Kim, B.-G., & Song, J.-Y. (2023). Neutralizing Activity against BQ.1.1, BN.1, and XBB.1 in Bivalent COVID-19 Vaccine Recipients: Comparison by the Types of Prior Infection and Vaccine Formulations. Vaccines, 11(8), 1320. https://doi.org/10.3390/vaccines11081320





