RNA Vaccines: Yeast as a Novel Antigen Vehicle
Abstract
:1. Introduction
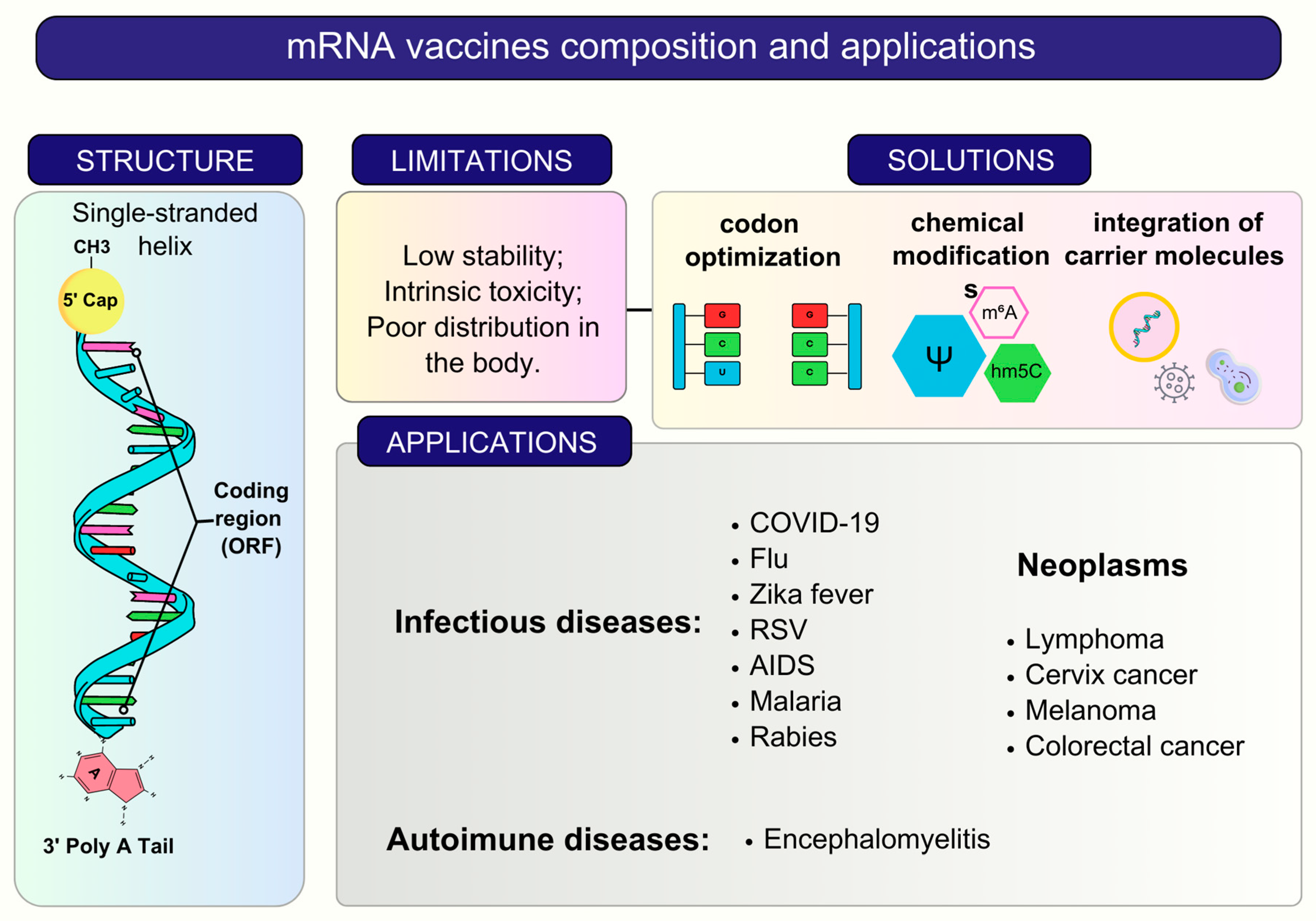
2. RNA Vaccines and Delivery Systems
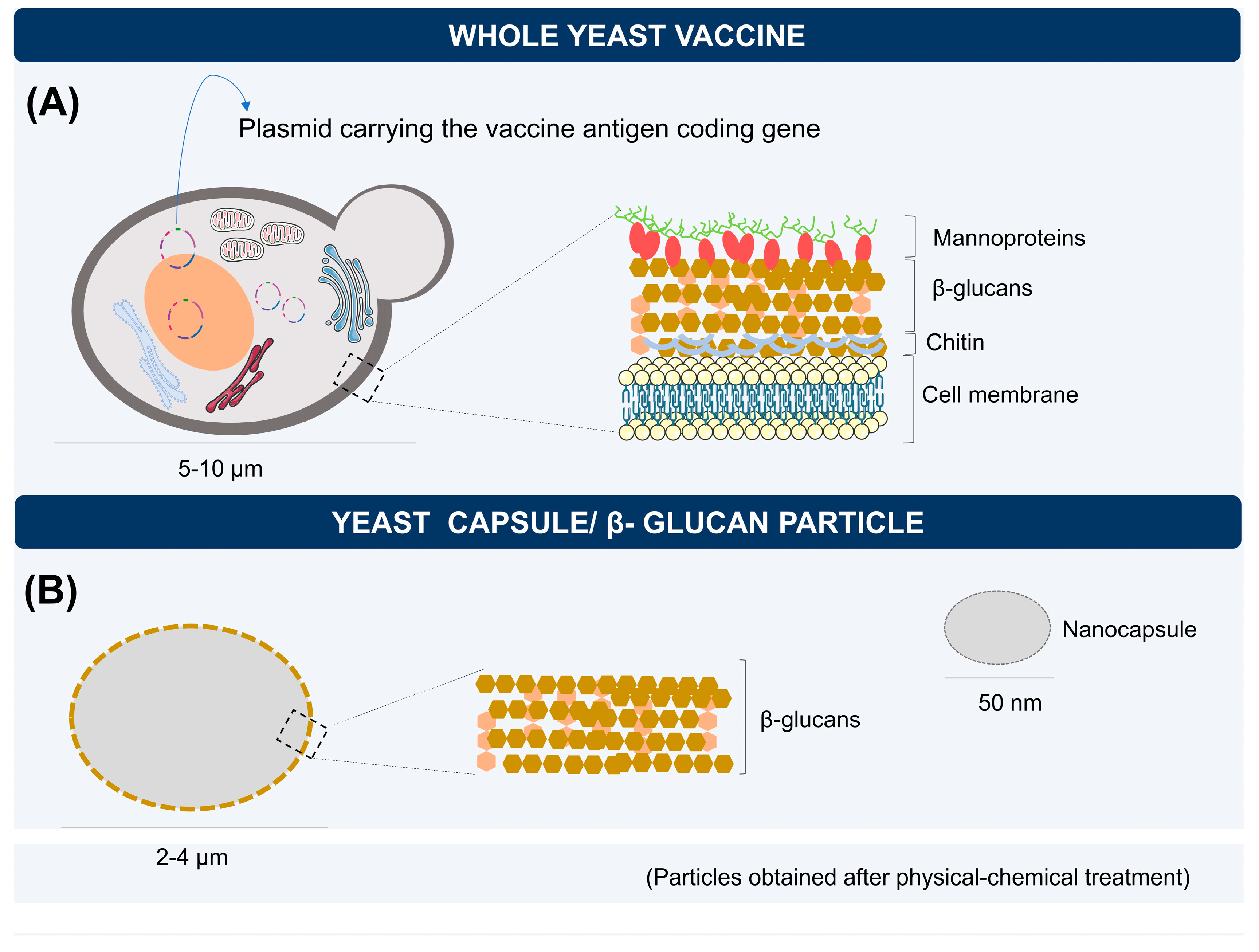
3. Yeasts as Vaccine Carriers: Characteristics and Immunological Aspects
4. Biodelivery of mRNA Vaccines by Yeasts
5. Capsules, Microparticles, and Nanoparticles of Yeast β-glucans for RNA Delivery
6. Routes of Administration Using Yeast for Delivery of Vaccines
| Route | Type of Antigen | Vehicle | Animals | Main Findings | Ref. |
|---|---|---|---|---|---|
| Tnf-α and Map4k4 (siRNA) | Yeast capsule | Male C57BL6/J mice | The orally absorbed Map4k4-siRNA-containing GeRPs underwent siRNA-mediated gene silencing and protected mice from LPS/d-GalN-induced lethality by inhibition of TNF-α and Il-1β production in macrophages. | [106] | |
| Oral | CD40 (shRNA) | Whole yeast | Female C57BL/6 mice | The shRNA carried by yeast effectively repressed the target gene (CD40) in vivo and had a significant effect on IL-6, IL-10, IL-12, and TNF-α expression. | [16] |
| IL-1β (shRNA) | Yeast microcapsule | Male C57BL/6 mice | Yeast microcapsule-mediated the delivery of IL-1β shRNA successfully, downregulated the intestinal inflammatory response in PTOA mice. | [82] | |
| miR365 antagomir | Yeast cell wall particle (YCWP) | Male C57BL/6 mice | The results showed that NPs-YCWP can effectively resist the corrosion of SGF, and was successfully engulfed by macrophages. | [107] | |
| rH9- DNA- RNA | Whole yeast | Chicken | Both DNA and RNA cassettes were successfully delivered by yeast and the vaccines elicited humoral and cellular immune responses. | [72] | |
| I.V. | - | Whole yeast | Female C57BL/6 mice | The results demonstrate that after P. pastoris inoculation, there was no pathology in the tested mice, and its dissemination to some tissues is quickly eliminated in the first hours. Thus, its use was considered safe for the development of vaccines, highlighting the intravenous route. | [96] |
| GI-5005 (HCV NS3-Core) | Whole yeast | BALB/cBy and C57BL/6J mice | The immunization with GI-5005 led to the induction of cytotoxic effector cells that can kill syngeneic tumor cells expressing NS3. | [108] | |
| S.C. | Yeast-CEA | Whole yeast | Female C57BL/6 mice | The study showed that the vaccine reduces tumor burden, and extends overall survival in CEA-transgenic mice. Furthermore, it was able to elicit increased antigen-specific T-cell responses after each vaccination. | [109] |
| GI-6301 (Yeast-Brachyury vaccine) | Whole yeast | Adults with advanced or metastatic chordoma (Phase I) | The vaccine was safe and immunogenic in humans. And two chordoma patients showed evidence of disease control (one mixed response and one partial response). | [110] | |
| Y-5A15 (Yeast/Aβ1-15) | Yeast surface display | APP/PS1 transgenic AD mice | The vaccine exerted favorable effects on cognition and neuropathology in the mice. Furthermore, it induced high titers of antibodies against Aβ. | [102] | |
| I.M. | Sulfated B-1,3–1,6-glucan from S. cerevisiae | Yeast cell wall particle modified | Chicken | The results showed increases in the levels of splenocytes, IL-2, IFN-Y and serum antibodies. | [98] |
| B-1,3–1,6-glucan with glyceraldehyde-3-phosphate dehydrogenase (rGAPDH) | Yeast cell wall particle modified | Fish | The results showed increases in survival rate, level of transcription of immunomodulators and production of antibodies. |
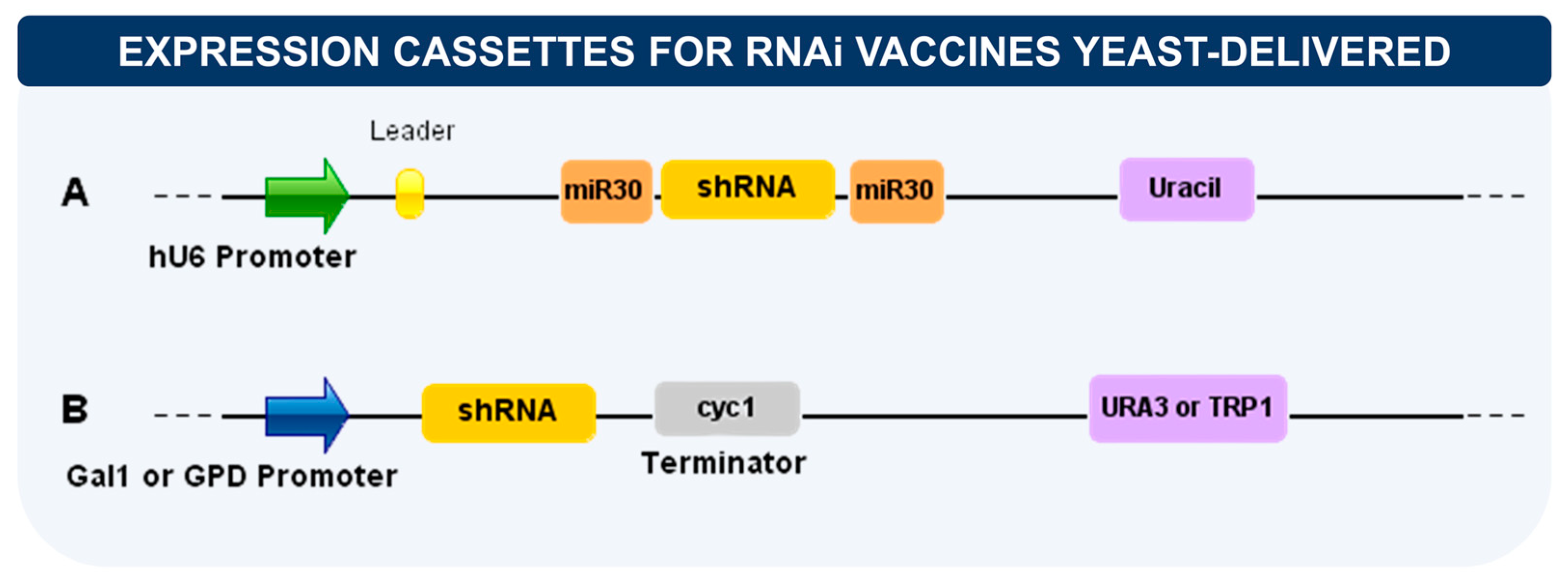
7. Delivery of RNA Interference (RNAi)
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pollard, A.J.; Bijker, E.M. A Guide to Vaccinology: From Basic Principles to New Developments. Nat. Rev. Immunol. 2021, 21, 83–100. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, R.B.; Ovsyannikova, I.G.; Palese, P.; Poland, G.A. Current Challenges in Vaccinology. Front. Immunol. 2020, 11, 1181. [Google Scholar] [CrossRef] [PubMed]
- Aljabali, A.A.A.; Bashatwah, R.M.; Obeid, M.A.; Mishra, V.; Mishra, Y.; Serrano-Aroca, Á.; Lundstrom, K.; Tambuwala, M.M. Current State of, Prospects for, and Obstacles to MRNA Vaccine Development. Drug Discov. Today 2023, 28, 103458. [Google Scholar] [CrossRef]
- Park, J.W.; Lagniton, P.N.P.; Liu, Y.; Xu, R.-H. MRNA Vaccines for COVID-19: What, Why and How. Int. J. Biol. Sci. 2021, 17, 1446–1460. [Google Scholar] [CrossRef]
- Chaudhary, N.; Weissman, D.; Whitehead, K.A. MRNA Vaccines for Infectious Diseases: Principles, Delivery and Clinical Translation. Nat. Rev. Drug Discov. 2021, 20, 817–838. [Google Scholar] [CrossRef] [PubMed]
- Kauffman, K.J.; Webber, M.J.; Anderson, D.G. Materials for Non-Viral Intracellular Delivery of Messenger RNA Therapeutics. J. Control. Release 2016, 240, 227–234. [Google Scholar] [CrossRef]
- Kim, J.; Eygeris, Y.; Gupta, M.; Sahay, G. Self-Assembled MRNA Vaccines. Adv. Drug Deliv. Rev. 2021, 170, 83–112. [Google Scholar] [CrossRef]
- de Moura, I.A.; Silva, A.J.D.; de Macêdo, L.S.; da Conceição Viana Invenção, M.; de Sousa, M.M.G.; de Freitas, A.C. Enhancing the Effect of Nucleic Acid Vaccines in the Treatment of HPV-Related Cancers: An Overview of Delivery Systems. Pathogens 2022, 11, 1444. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, P. Yeast-Based Vaccines: New Perspective in Vaccine Development and Application. FEMS Yeast Res. 2019, 19, foz007. [Google Scholar] [CrossRef] [Green Version]
- Silva, A.J.D.; de Macêdo, L.S.; Leal, L.R.S.; de Jesus, A.L.S.; Freitas, A.C. Yeasts as a Promising Delivery Platform for DNA and RNA Vaccines. FEMS Yeast Res. 2021, 21, foab018. [Google Scholar] [CrossRef]
- Walch, B.; Breinig, T.; Schmitt, M.J.; Breinig, F. Delivery of Functional DNA and Messenger RNA to Mammalian Phagocytic Cells by Recombinant Yeast. Gene Ther. 2012, 19, 237–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berner, V.K.; Sura, M.E.; Hunter, K.W. Conjugation of Protein Antigen to Microparticulate β-Glucan from Saccharomyces Cerevisiae: A New Adjuvant for Intradermal and Oral Immunizations. Appl. Microbiol. Biotechnol. 2008, 80, 1053–1061. [Google Scholar] [CrossRef] [PubMed]
- Kiflmariam, M.G.; Yang, H.; Zhang, Z. Gene Delivery to Dendritic Cells by Orally Administered Recombinant Saccharomyces Cerevisiae in Mice. Vaccine 2013, 31, 1360–1363. [Google Scholar] [CrossRef]
- Liu, D.; Lu, S.; Zhang, L.; Ji, M.; Liu, S.; Wang, S.; Liu, R. An Indoleamine 2, 3-Dioxygenase SiRNA Nanoparticle-Coated and Trp2-Displayed Recombinant Yeast Vaccine Inhibits Melanoma Tumor Growth in Mice. J. Control. Release 2018, 273, 1–12. [Google Scholar] [CrossRef]
- Seif, M.; Hoppstädter, J.; Breinig, F.; Kiemer, A.K. Yeast-Mediated MRNA Delivery Polarizes Immuno-Suppressive Macrophages towards an Immuno-Stimulatory Phenotype. Eur. J. Pharm. Biopharm. 2017, 117, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, T.; Wang, L.; Shao, S.; Chen, Z.; Zhang, Z. In Vivo Targeted Delivery of CD40 ShRNA to Mouse Intestinal Dendritic Cells by Oral Administration of Recombinant Sacchromyces Cerevisiae. Gene Ther. 2014, 21, 709–714. [Google Scholar] [CrossRef]
- Bilusic, M.; Heery, C.R.; Arlen, P.M.; Rauckhorst, M.; Apelian, D.; Tsang, K.Y.; Tucker, J.A.; Jochems, C.; Schlom, J.; Gulley, J.L.; et al. Phase I Trial of a Recombinant Yeast-CEA Vaccine (GI-6207) in Adults with Metastatic CEA-Expressing Carcinoma. Cancer Immunol. Immunother. 2014, 63, 225–234. [Google Scholar] [CrossRef] [Green Version]
- Cohn, A.; Morse, M.A.; O’Neil, B.; Whiting, S.; Coeshott, C.; Ferraro, J.; Bellgrau, D.; Apelian, D.; Rodell, T.C. Whole Recombinant Saccharomyces Cerevisiae Yeast Expressing Ras Mutations as Treatment for Patients With Solid Tumors Bearing Ras Mutations: Results From a Phase 1 Trial. J. Immunother. 2018, 41, 141–150. [Google Scholar] [CrossRef] [Green Version]
- Heery, C.R.; Singh, B.H.; Rauckhorst, M.; Marté, J.L.; Donahue, R.N.; Grenga, I.; Rodell, T.C.; Dahut, W.; Arlen, P.M.; Madan, R.A.; et al. Phase I Trial of a Yeast-Based Therapeutic Cancer Vaccine (GI-6301) Targeting the Transcription Factor Brachyury. Cancer Immunol. Res. 2015, 3, 1248–1256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schlake, T.; Thess, A.; Fotin-Mleczek, M.; Kallen, K.-J. Developing MRNA-Vaccine Technologies. RNA Biol. 2012, 9, 1319–1330. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Maruggi, G.; Shan, H.; Li, J. Advances in MRNA Vaccines for Infectious Diseases. Front. Immunol. 2019, 10, 594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rijkers, G.T.; Weterings, N.; Obregon-Henao, A.; Lepolder, M.; Dutt, T.S.; Van Overveld, F.J.; Henao-Tamayo, M. Antigen Presentation of MRNA-Based and Virus-Vectored SARS-CoV-2 Vaccines. Vaccines 2021, 9, 848. [Google Scholar] [CrossRef]
- Matsumura, T.; Takano, T.; Takahashi, Y. Immune Responses Related to the Immunogenicity and Reactogenicity of COVID-19 MRNA Vaccines. Int. Immunol. 2023, 35, 213–220. [Google Scholar] [CrossRef]
- Krienke, C.; Kolb, L.; Diken, E.; Streuber, M.; Kirchhoff, S.; Bukur, T.; Akilli-Öztürk, Ö.; Kranz, L.M.; Berger, H.; Petschenka, J.; et al. A Noninflammatory MRNA Vaccine for Treatment of Experimental Autoimmune Encephalomyelitis. Science 2021, 371, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Zhang, Y.; Huang, L. MRNA Vaccine for Cancer Immunotherapy. Mol. Cancer 2021, 20, 41. [Google Scholar] [CrossRef]
- Wadhwa, A.; Aljabbari, A.; Lokras, A.; Foged, C.; Thakur, A. Opportunities and Challenges in the Delivery of MRNA-Based Vaccines. Pharmaceutics 2020, 12, 102. [Google Scholar] [CrossRef] [Green Version]
- Tenchov, R.; Bird, R.; Curtze, A.E.; Zhou, Q. Lipid Nanoparticles─From Liposomes to MRNA Vaccine Delivery, a Landscape of Research Diversity and Advancement. ACS Nano 2021, 15, 16982–17015. [Google Scholar] [CrossRef]
- Xu, T.; Yu, S.; Zhang, J.; Wu, S. Dysregulated Tumor-Associated Macrophages in Carcinogenesis, Progression and Targeted Therapy of Gynecological and Breast Cancers. J. Hematol. Oncol. 2021, 14, 181. [Google Scholar] [CrossRef]
- Freund, I.; Eigenbrod, T.; Helm, M.; Dalpke, A. RNA Modifications Modulate Activation of Innate Toll-Like Receptors. Genes 2019, 10, 92. [Google Scholar] [CrossRef] [Green Version]
- Vaidyanathan, S.; Azizian, K.T.; Haque, A.K.M.A.; Henderson, J.M.; Hendel, A.; Shore, S.; Antony, J.S.; Hogrefe, R.I.; Kormann, M.S.D.; Porteus, M.H.; et al. Uridine Depletion and Chemical Modification Increase Cas9 MRNA Activity and Reduce Immunogenicity without HPLC Purification. Mol. Ther. Nucleic Acids 2018, 12, 530–542. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, S.; Satapathy, S.R.; Dutta, T. Delivery Strategies for MRNA Vaccines. Pharm. Med. 2022, 36, 11–20. [Google Scholar] [CrossRef]
- Schoenmaker, L.; Witzigmann, D.; Kulkarni, J.A.; Verbeke, R.; Kersten, G.; Jiskoot, W.; Crommelin, D.J.A. MRNA-Lipid Nanoparticle COVID-19 Vaccines: Structure and Stability. Int. J. Pharm. 2021, 601, 120586. [Google Scholar] [CrossRef]
- Oude Blenke, E.; Örnskov, E.; Schöneich, C.; Nilsson, G.A.; Volkin, D.B.; Mastrobattista, E.; Almarsson, Ö.; Crommelin, D.J.A. The Storage and In-Use Stability of MRNA Vaccines and Therapeutics: Not A Cold Case. J. Pharm. Sci. 2023, 112, 386–403. [Google Scholar] [CrossRef]
- Eygeris, Y.; Gupta, M.; Kim, J.; Sahay, G. Chemistry of Lipid Nanoparticles for RNA Delivery. Acc. Chem. Res. 2022, 55, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Shende, P.; Basarkar, V. Recent Trends and Advances in Microbe-Based Drug Delivery Systems. DARU J. Pharm. Sci. 2019, 27, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Aldosari, B.N.; Alfagih, I.M.; Almurshedi, A.S. Lipid Nanoparticles as Delivery Systems for RNA-Based Vaccines. Pharmaceutics 2021, 13, 206. [Google Scholar] [CrossRef]
- Ayad, C.; Libeau, P.; Lacroix-Gimon, C.; Ladavière, C.; Verrier, B. LipoParticles: Lipid-Coated PLA Nanoparticles Enhanced In Vitro MRNA Transfection Compared to Liposomes. Pharmaceutics 2021, 13, 377. [Google Scholar] [CrossRef]
- Bochicchio, S.; Dalmoro, A.; Barba, A.; Grassi, G.; Lamberti, G. Liposomes as SiRNA Delivery Vectors. CDM 2015, 15, 882–892. [Google Scholar] [CrossRef] [PubMed]
- Soler Besumbes, E.; Fornaguera, C.; Monge, M.; García-Celma, M.J.; Carrión, J.; Solans, C.; Dols-Perez, A. PLGA Cationic Nanoparticles, Obtained from Nano-Emulsion Templating, as Potential DNA Vaccines. Eur. Polym. J. 2019, 120, 109229. [Google Scholar] [CrossRef]
- Blakney, A.K.; McKay, P.F.; Hu, K.; Samnuan, K.; Jain, N.; Brown, A.; Thomas, A.; Rogers, P.; Polra, K.; Sallah, H.; et al. Polymeric and Lipid Nanoparticles for Delivery of Self-Amplifying RNA Vaccines. J. Control. Release 2021, 338, 201–210. [Google Scholar] [CrossRef]
- Bose, R.J.; Kim, M.; Chang, J.H.; Paulmurugan, R.; Moon, J.J.; Koh, W.-G.; Lee, S.-H.; Park, H. Biodegradable Polymers for Modern Vaccine Development. J. Ind. Eng. Chem. 2019, 77, 12–24. [Google Scholar] [CrossRef]
- Chowdhury, S.; Toth, I.; Stephenson, R.J. Dendrimers in Vaccine Delivery: Recent Progress and Advances. Biomaterials 2022, 280, 121303. [Google Scholar] [CrossRef] [PubMed]
- Hraber, P.; Bradfute, S.; Clarke, E.; Ye, C.; Pitard, B. Amphiphilic Block Copolymer Delivery of a DNA Vaccine against Zika Virus. Vaccine 2018, 36, 6911–6917. [Google Scholar] [CrossRef]
- Ita, K. Polyplexes for Gene and Nucleic Acid Delivery: Progress and Bottlenecks. Eur. J. Pharm. Sci. 2020, 150, 105358. [Google Scholar] [CrossRef] [PubMed]
- Asmamaw Mengstie, M. Viral Vectors for the in Vivo Delivery of CRISPR Components: Advances and Challenges. Front. Bioeng. Biotechnol. 2022, 10, 895713. [Google Scholar] [CrossRef]
- Couto, L.B.; High, K.A. Viral Vector-Mediated RNA Interference. Curr. Opin. Pharmacol. 2010, 10, 534–542. [Google Scholar] [CrossRef]
- Lundstrom, K. Viral Vectors Applied for RNAi-Based Antiviral Therapy. Viruses 2020, 12, 924. [Google Scholar] [CrossRef]
- Bai, F.; Li, Z.; Umezawa, A.; Terada, N.; Jin, S. Bacterial Type III Secretion System as a Protein Delivery Tool for a Broad Range of Biomedical Applications. Biotechnol. Adv. 2018, 36, 482–493. [Google Scholar] [CrossRef] [PubMed]
- Lage, H. Bacterial Vectors for RNAi Delivery to Cancer Cells. In Encyclopedia of Molecular Cell Biology and Molecular Medicine; Meyers, R.A., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2013; pp. 1–19. ISBN 978-3-527-60090-8. [Google Scholar]
- Lin, I.; Van, T.; Smooker, P. Live-Attenuated Bacterial Vectors: Tools for Vaccine and Therapeutic Agent Delivery. Vaccines 2015, 3, 940–972. [Google Scholar] [CrossRef] [Green Version]
- Alexander, E. Yeasts in Nanotechnology-Enabled Oral Vaccine and Gene Delivery. Bioengineered 2021, 12, 8325–8335. [Google Scholar] [CrossRef]
- Tan, Y.; Chen, L.; Li, K.; Lou, B.; Liu, Y.; Liu, Z. Yeast as Carrier for Drug Delivery and Vaccine Construction. J. Control. Release 2022, 346, 358–379. [Google Scholar] [CrossRef]
- Silva, A.J.D.; da Silva Rocha, C.K.; de Freitas, A.C. Standardization and Key Aspects of the Development of Whole Yeast Cell Vaccines. Pharmaceutics 2022, 14, 2792. [Google Scholar] [CrossRef]
- Juturu, V.; Wu, J.C. Heterologous Protein Expression in Pichia Pastoris: Latest Research Progress and Applications. ChemBioChem 2018, 19, 7–21. [Google Scholar] [CrossRef]
- Kumar, R. Investigating the Long-Term Stability of Protein Immunogen(s) for Whole Recombinant Yeast-Based Vaccines. FEMS Yeast Res. 2018, 18, foy071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, R.; Srivastava, V.; Baindara, P.; Ahmad, A. Thermostable Vaccines: An Innovative Concept in Vaccine Development. Expert Rev. Vaccines 2022, 21, 811–824. [Google Scholar] [CrossRef]
- Kumar, R.; Kharbikar, B.N. Lyophilized Yeast Powder for Adjuvant Free Thermostable Vaccine Delivery. Appl. Microbiol. Biotechnol. 2021, 105, 3131–3143. [Google Scholar] [CrossRef] [PubMed]
- Bal, J.; Luong, N.N.; Park, J.; Song, K.-D.; Jang, Y.-S.; Kim, D.-H. Comparative Immunogenicity of Preparations of Yeast-Derived Dengue Oral Vaccine Candidate. Microb. Cell Factories 2018, 17, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, G.D. Dectin-1: A Signalling Non-TLR Pattern-Recognition Receptor. Nat. Rev. Immunol. 2006, 6, 33–43. [Google Scholar] [CrossRef]
- Salazar, F.; Brown, G.D. Antifungal Innate Immunity: A Perspective from the Last 10 Years. J. Innate. Immun. 2018, 10, 373–397. [Google Scholar] [CrossRef]
- Erwig, L.P.; Gow, N.A.R. Interactions of Fungal Pathogens with Phagocytes. Nat. Rev. Microbiol. 2016, 14, 163–176. [Google Scholar] [CrossRef]
- Bazan, S.B.; Breinig, T.; Schmitt, M.J.; Breinig, F. Heat Treatment Improves Antigen-Specific T Cell Activation after Protein Delivery by Several but Not All Yeast Genera. Vaccine 2014, 32, 2591–2598. [Google Scholar] [CrossRef] [PubMed]
- Bian, G.; Cheng, Y.; Wang, Z.; Hu, Y.; Zhang, X.; Wu, M.; Chen, Z.; Shi, B.; Sun, S.; Shen, Y.; et al. Whole Recombinant Hansenula Polymorpha Expressing Hepatitis B Virus Surface Antigen (Yeast-HBsAg) Induces Potent HBsAg-Specific Th1 and Th2 Immune Responses. Vaccine 2009, 28, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Facciolà, A.; Visalli, G.; Laganà, A.; Di Pietro, A. An Overview of Vaccine Adjuvants: Current Evidence and Future Perspectives. Vaccines 2022, 10, 819. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, I.; Garg, R.; Van Drunen Littel-van Den Hurk, S. Selection of Adjuvants for Vaccines Targeting Specific Pathogens. Expert Rev. Vaccines 2019, 18, 505–521. [Google Scholar] [CrossRef]
- Stephen, J.; Scales, H.E.; Benson, R.A.; Erben, D.; Garside, P.; Brewer, J.M. Neutrophil Swarming and Extracellular Trap Formation Play a Significant Role in Alum Adjuvant Activity. NPJ Vaccines 2017, 2, 1. [Google Scholar] [CrossRef]
- Ghimire, T.R. The Mechanisms of Action of Vaccines Containing Aluminum Adjuvants: An in Vitro vs in Vivo Paradigm. SpringerPlus 2015, 4, 181. [Google Scholar] [CrossRef]
- Liu, H.; Jia, Z.; Yang, C.; Song, M.; Jing, Z.; Zhao, Y.; Wu, Z.; Zhao, L.; Wei, D.; Yin, Z.; et al. Aluminum Hydroxide Colloid Vaccine Encapsulated in Yeast Shells with Enhanced Humoral and Cellular Immune Responses. Biomaterials 2018, 167, 32–43. [Google Scholar] [CrossRef]
- Bazan, S.B.; Geginat, G.; Breinig, T.; Schmitt, M.J.; Breinig, F. Uptake of Various Yeast Genera by Antigen-Presenting Cells and Influence of Subcellular Antigen Localization on the Activation of Ovalbumin-Specific CD8 T Lymphocytes. Vaccine 2011, 29, 8165–8173. [Google Scholar] [CrossRef]
- Brown, G.D. Innate Antifungal Immunity: The Key Role of Phagocytes. Annu. Rev. Immunol. 2011, 29, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Breinig, F.; Breinig, T.; Schmitt, M.J. MRNA Delivery to Human Dendritic Cells by Recombinant Yeast and Activation of Antigen-Specific Memory T Cells. In Synthetic Messenger RNA and Cell Metabolism Modulation; Rabinovich, P.M., Ed.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2013; Volume 969, pp. 163–184. ISBN 978-1-62703-259-9. [Google Scholar]
- Zhang, H.; Xie, R.; Zhang, H.; Sun, R.; Li, S.; Xia, C.; Li, Z.; Zhang, L.; Guo, Y.; Huang, J. Recombinant Hemagglutinin Protein and DNA-RNA-Combined Nucleic Acid Vaccines Harbored by Yeast Elicit Protective Immunity against H9N2 Avian Influenza Infection. Poult. Sci. 2023, 102, 102662. [Google Scholar] [CrossRef]
- Evstafieva, A.G.; Beletsky, A.V.; Borovjagin, A.V.; Bogdanov, A.A. Internal Ribosome Entry Site Ofencephalomyocarditis Virus RNA Is Unable to Direct Translation in Saccharomyces Cerevisiae. FEBS Lett. 1993, 335, 273–276. [Google Scholar] [CrossRef] [Green Version]
- Türkanoğlu Özçelik, A.; Yılmaz, S.; Inan, M. Pichia Pastoris Promoters. In Recombinant Protein Production in Yeast; Gasser, B., Mattanovich, D., Eds.; Methods in Molecular Biology; Springer: New York, NY, USA, 2019; Volume 1923, pp. 97–112. ISBN 978-1-4939-9023-8. [Google Scholar]
- Yang, J.; Cai, H.; Liu, J.; Zeng, M.; Chen, J.; Cheng, Q.; Zhang, L. Controlling AOX1 Promoter Strength in Pichia Pastoris by Manipulating Poly (DA:DT) Tracts. Sci. Rep. 2018, 8, 1401. [Google Scholar] [CrossRef] [Green Version]
- Peng, B.; Wood, R.J.; Nielsen, L.K.; Vickers, C.E. An Expanded Heterologous GAL Promoter Collection for Diauxie-Inducible Expression in Saccharomyces Cerevisiae. ACS Synth. Biol. 2018, 7, 748–751. [Google Scholar] [CrossRef] [PubMed]
- Bazan, S.B.; Walch-Rückheim, B.; Schmitt, M.J.; Breinig, F. Maturation and Cytokine Pattern of Human Dendritic Cells in Response to Different Yeasts. Med. Microbiol. Immunol. 2018, 207, 75–81. [Google Scholar] [CrossRef]
- He, L.; Bai, Y.; Xia, L.; Pan, J.; Sun, X.; Zhu, Z.; Ding, J.; Qi, C.; Tang, C. Oral Administration of a Whole Glucan Particle (WGP)-Based Therapeutic Cancer Vaccine Targeting Macrophages Inhibits Tumor Growth. Cancer Immunol. Immunother. 2022, 71, 2007–2028. [Google Scholar] [CrossRef] [PubMed]
- De Smet, R.; Allais, L.; Cuvelier, C.A. Recent Advances in Oral Vaccine Development: Yeast-Derived β-Glucan Particles. Hum. Vaccines Immunother. 2014, 10, 1309–1318. [Google Scholar] [CrossRef] [Green Version]
- Tipper, D.J.; Szomolanyi-Tsuda, E. Scaffolded Antigens in Yeast Cell Particle Vaccines Provide Protection against Systemic Polyoma Virus Infection. J. Immunol. Res. 2016, 2016, 2743292. [Google Scholar] [CrossRef] [Green Version]
- Dinarvand, R.; Cesar De Morais, P.; D’Emanuele, A. Nanoparticles for Targeted Delivery of Active Agents against Tumor Cells. J. Drug Deliv. 2012, 2012, 528123. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Peng, H.; Feng, M.; Zhang, W.; Li, Y. Yeast Microcapsule-Mediated Oral Delivery of IL-1β ShRNA for Post-Traumatic Osteoarthritis Therapy. Mol. Ther. Nucleic Acids 2021, 23, 336–346. [Google Scholar] [CrossRef]
- Liu, H.; Meng, Z.; Wang, H.; Zhang, S.; Huang, Z.; Geng, X.; Guo, R.; Wu, Z.; Hong, Z. Robust Immune Responses Elicited by a Hybrid Adjuvant Based on β-Glucan Particles from Yeast for the Hepatitis B Vaccine. ACS Appl. Bio. Mater. 2021, 4, 3614–3622. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Liang, M.; Huang, J.; Fan, Y.; Long, H.; Chen, Q.; Ren, Z.; Wu, C.; Wang, Y. Single-Helical Formyl β-Glucan Effectively Deliver CpG DNA with Poly(DA) to Macrophages for Enhanced Vaccine Effects. Int. J. Biol. Macromol. 2022, 223, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Soto, E.R.; Caras, A.C.; Kut, L.C.; Castle, M.K.; Ostroff, G.R. Glucan Particles for Macrophage Targeted Delivery of Nanoparticles. J. Drug Deliv. 2012, 2012, 143524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muta, T. Molecular Basis for Invertebrate Innate Immune Recognition of (1→3)-β-D-Glucan as A Pathogen-Associated Molecular Pattern. CPD 2006, 12, 4155–4161. [Google Scholar] [CrossRef]
- Soto, E.R.; Ostroff, G.R. Characterization of Multilayered Nanoparticles Encapsulated in Yeast Cell Wall Particles for DNA Delivery. Bioconjugate Chem. 2008, 19, 840–848. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Ostroff, G.R.; Lee, C.K.; Specht, C.A.; Levitz, S.M. Robust Stimulation of Humoral and Cellular Immune Responses Following Vaccination with Antigen-Loaded β-Glucan Particles. mBio 2010, 1, e00164-10. [Google Scholar] [CrossRef] [Green Version]
- Cheng, T.; Yan, T.; Wu, J.; Wang, Q.; Zhang, H. Yeast β-D-Glucan Functionalized Graphene Oxide for Macrophage-Targeted Delivery of CpG Oligodeoxynucleotides and Synergistically Enhanced Antitumor Immunity. Int. J. Biol. Macromol. 2023, 234, 123432. [Google Scholar] [CrossRef]
- Lee, K.; Min, D.; Choi, Y.; Yoon, S.; Jang, J.; Hwang, J.; Jeon, H.; Cho, Y.W.; Choi, J. Self-Assembling β-Glucan Nanomedicine for the Delivery of SiRNA. Biomedicines 2020, 8, 497. [Google Scholar] [CrossRef]
- Hwang, J.; Lee, K.; Gilad, A.A.; Choi, J. Synthesis of Beta-Glucan Nanoparticles for the Delivery of Single Strand DNA. Biotechnol. Bioproc. E 2018, 23, 144–149. [Google Scholar] [CrossRef]
- Xu, J.; Ma, Q.; Zhang, Y.; Fei, Z.; Sun, Y.; Fan, Q.; Liu, B.; Bai, J.; Yu, Y.; Chu, J.; et al. Yeast-Derived Nanoparticles Remodel the Immunosuppressive Microenvironment in Tumor and Tumor-Draining Lymph Nodes to Suppress Tumor Growth. Nat. Commun. 2022, 13, 110. [Google Scholar] [CrossRef]
- Zinkhan, S.; Ogrina, A.; Balke, I.; Reseviča, G.; Zeltins, A.; De Brot, S.; Lipp, C.; Chang, X.; Zha, L.; Vogel, M.; et al. The Impact of Size on Particle Drainage Dynamics and Antibody Response. J. Control. Release 2021, 331, 296–308. [Google Scholar] [CrossRef]
- Guimarães, L.E.; Baker, B.; Perricone, C.; Shoenfeld, Y. Vaccines, Adjuvants and Autoimmunity. Pharmacol. Res. 2015, 100, 190–209. [Google Scholar] [CrossRef]
- Zhang, T.; Hu, Y.; Wang, T.; Cai, P. Dihydroartemisinin Inhibits the Viability of Cervical Cancer Cells by Upregulating Caveolin 1 and Mitochondrial Carrier Homolog 2: Involvement of P53 Activation and NAD(P)H:Quinone Oxidoreductase 1 Downregulation. Int. J. Mol. Med. 2017, 40, 21–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becerril-García, M.Á.; Flores-Maldonado, O.E.; González, G.M.; García-González, G.; Hernández-Bello, R.; Palma-Nicolás, J.P. Safety Profile of Intravenous Administration of Live Pichia Pastoris Cells in Mice. FEMS Yeast Res. 2022, 22, foac023. [Google Scholar] [CrossRef] [PubMed]
- Diao, J.; Ye, H.; Yu, X.; Fan, Y.; Xu, L.; Li, T.; Wang, Y. Adjuvant and Immunostimulatory Effects of LPS and β-Glucan on Immune Response in Japanese Flounder, Paralichthys Olivaceus. Vet. Immunol. Immunopathol. 2013, 156, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yang, R.; Zhang, L.; Meng, X.; Fei, C.; Zhang, K.; Wang, X.; Zheng, W.; Xiao, S.; Zhang, S.; et al. Sulfated Glucan Can Improve the Immune Efficacy of Newcastle Disease Vaccine in Chicken. Int. J. Biol. Macromol. 2014, 70, 193–198. [Google Scholar] [CrossRef]
- Jin, Y.; Li, P.; Wang, F. β-Glucans as Potential Immunoadjuvants: A Review on the Adjuvanticity, Structure-Activity Relationship and Receptor Recognition Properties. Vaccine 2018, 36, 5235–5244. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wang, F. Polysaccharides: Candidates of Promising Vaccine Adjuvants. Drug Discov. Ther. 2015, 9, 88–93. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Lu, S.; Zhang, L.; Huang, Y.; Ji, M.; Sun, X.; Liu, X.; Liu, R. Yeast-Based Aβ1-15 Vaccine Elicits Strong Immunogenicity and Attenuates Neuropathology and Cognitive Deficits in Alzheimer’s Disease Transgenic Mice. Vaccines 2020, 8, 351. [Google Scholar] [CrossRef]
- Li, M.; Wang, Y.; Sun, Y.; Cui, H.; Zhu, S.J.; Qiu, H.-J. Mucosal Vaccines: Strategies and Challenges. Immunol. Lett. 2020, 217, 116–125. [Google Scholar] [CrossRef]
- Alu, A.; Chen, L.; Lei, H.; Wei, Y.; Tian, X.; Wei, X. Intranasal COVID-19 Vaccines: From Bench to Bed. eBioMedicine 2022, 76, 103841. [Google Scholar] [CrossRef]
- Baker, J.R.; Farazuddin, M.; Wong, P.T.; O’Konek, J.J. The Unfulfilled Potential of Mucosal Immunization. J. Allergy Clin. Immunol. 2022, 150, 1–11. [Google Scholar] [CrossRef]
- Kour, P.; Rath, G.; Sharma, G.; Goyal, A.K. Recent Advancement in Nanocarriers for Oral Vaccination. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1102–1114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aouadi, M.; Tesz, G.J.; Nicoloro, S.M.; Wang, M.; Chouinard, M.; Soto, E.; Ostroff, G.R.; Czech, M.P. Orally Delivered SiRNA Targeting Macrophage Map4k4 Suppresses Systemic Inflammation. Nature 2009, 458, 1180–1184. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Peng, H.; Zhang, W.; Li, Y.; Liu, L.; Leng, T. Yeast Cell Wall Particle Mediated Nanotube-RNA Delivery System Loaded with MiR365 Antagomir for Post-Traumatic Osteoarthritis Therapy via Oral Route. Theranostics 2020, 10, 8479–8493. [Google Scholar] [CrossRef]
- Haller, A.A.; Lauer, G.M.; King, T.H.; Kemmler, C.; Fiolkoski, V.; Lu, Y.; Bellgrau, D.; Rodell, T.C.; Apelian, D.; Franzusoff, A.; et al. Whole Recombinant Yeast-Based Immunotherapy Induces Potent T Cell Responses Targeting HCV NS3 and Core Proteins. Vaccine 2007, 25, 1452–1463. [Google Scholar] [CrossRef] [PubMed]
- Wansley, E.K.; Chakraborty, M.; Hance, K.W.; Bernstein, M.B.; Boehm, A.L.; Guo, Z.; Quick, D.; Franzusoff, A.; Greiner, J.W.; Schlom, J.; et al. Vaccination with a Recombinant Saccharomyces Cerevisiae Expressing a Tumor Antigen Breaks Immune Tolerance and Elicits Therapeutic Antitumor Responses. Clin. Cancer Res. 2008, 14, 4316–4325. [Google Scholar] [CrossRef] [Green Version]
- DeMaria, P.J.; Bilusic, M.; Park, D.M.; Heery, C.R.; Donahue, R.N.; Madan, R.A.; Bagheri, M.H.; Strauss, J.; Shen, V.; Marté, J.L.; et al. Randomized, Double-Blind, Placebo-Controlled Phase II Study of Yeast-Brachyury Vaccine (GI-6301) in Combination with Standard-of-Care Radiotherapy in Locally Advanced, Unresectable Chordoma. Oncology 2021, 26, e847–e858. [Google Scholar] [CrossRef]
- Bumcrot, D.; Manoharan, M.; Koteliansky, V.; Sah, D.W.Y. RNAi Therapeutics: A Potential New Class of Pharmaceutical Drugs. Nat. Chem. Biol. 2006, 2, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Zhong, L.; Weng, Y.; Peng, L.; Huang, Y.; Zhao, Y.; Liang, X.-J. Therapeutic SiRNA: State of the Art. Signal Transduct. Target. Ther. 2020, 5, 101. [Google Scholar] [CrossRef]
- Karagiannis, T.C.; El-Osta, A. RNA Interference and Potential Therapeutic Applications of Short Interfering RNAs. Cancer Gene Ther. 2005, 12, 787–795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahmoodi Chalbatani, G.; Dana, H.; Gharagouzloo, E.; Grijalvo, S.; Eritja, R.; Logsdon, C.D.; Memari, F.; Miri, S.R.; Rezvani Rad, M.; Marmari, V. Small Interfering RNAs (SiRNAs) in Cancer Therapy: A Nano-Based Approach. Int. J. Nanomed. 2019, 14, 3111–3128. [Google Scholar] [CrossRef] [Green Version]
- Huang, D.T.-N.; Lu, C.-Y.; Shao, P.-L.; Chang, L.-Y.; Wang, J.-Y.; Chang, Y.-H.; Lai, M.-J.; Chi, Y.-H.; Huang, L.-M. In Vivo Inhibition of Influenza A Virus Replication by RNA Interference Targeting the PB2 Subunit via Intratracheal Delivery. PLoS ONE 2017, 12, e0174523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prakash, O.; Khan, D.N.; Singh, T.; Shukla, S.; Prakash, S.; Amita, J. Effect of SiRNA Targeting Dengue Virus Genes on Replication of Dengue Virus: An in Vitro Experimental Study. VirusDisease 2021, 32, 518–525. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, F.; Limani, S.W.; Mnyandu, N.; Maepa, M.B.; Ely, A.; Arbuthnot, P. Advances with RNAi-Based Therapy for Hepatitis B Virus Infection. Viruses 2020, 12, 851. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y.; Takeshita, F.; Kuwano, K.; Ochiya, T. RNAi Therapeutic Platforms for Lung Diseases. Pharmaceuticals 2013, 6, 223–250. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Liu, C.; Bai, S.; Lu, Z.; Liu, G. Broadening the Horizons of RNA Delivery Strategies in Cancer Therapy. Bioengineering 2022, 9, 576. [Google Scholar] [CrossRef]
- Sioud, M. Engineering Better Immunotherapies via RNA Interference. Hum. Vaccines Immunother. 2014, 10, 3165–3174. [Google Scholar] [CrossRef] [Green Version]
- Setten, R.L.; Rossi, J.J.; Han, S. The Current State and Future Directions of RNAi-Based Therapeutics. Nat. Rev. Drug Discov. 2019, 18, 421–446. [Google Scholar] [CrossRef]
- Xiang, S.; Fruehauf, J.; Li, C.J. Short Hairpin RNA–Expressing Bacteria Elicit RNA Interference in Mammals. Nat. Biotechnol. 2006, 24, 697–702. [Google Scholar] [CrossRef]
- Duman-Scheel, M. Saccharomyces Cerevisiae (Baker’s Yeast) as an Interfering RNA Expression and Delivery System. Curr. Drug Targets 2019, 20, 942–952. [Google Scholar] [CrossRef] [Green Version]
- Barreby, E.; Sulen, A.; Aouadi, M. Glucan-Encapsulated SiRNA Particles (GeRPs) for Specific Gene Silencing in Adipose Tissue Macrophages. In Lipid-Activated Nuclear Receptors; Gage, M.C., Pineda-Torra, I., Eds.; Methods in Molecular Biology; Springer: New York, NY, USA, 2019; Volume 1951, pp. 49–57. ISBN 978-1-4939-9129-7. [Google Scholar]
- Hapairai, L.K.; Mysore, K.; Chen, Y.; Harper, E.I.; Scheel, M.P.; Lesnik, A.M.; Sun, L.; Severson, D.W.; Wei, N.; Duman-Scheel, M. Lure-and-Kill Yeast Interfering RNA Larvicides Targeting Neural Genes in the Human Disease Vector Mosquito Aedes Aegypti. Sci. Rep. 2017, 7, 13223. [Google Scholar] [CrossRef] [Green Version]
- Mysore, K.; Hapairai, L.K.; Sun, L.; Harper, E.I.; Chen, Y.; Eggleson, K.K.; Realey, J.S.; Scheel, N.D.; Severson, D.W.; Wei, N.; et al. Yeast Interfering RNA Larvicides Targeting Neural Genes Induce High Rates of Anopheles Larval Mortality. Malar. J. 2017, 16, 461. [Google Scholar] [CrossRef] [Green Version]
- Hilderbrand, S.A.; Weissleder, R. Near-Infrared Fluorescence: Application to in Vivo Molecular Imaging. Curr. Opin. Chem. Biol. 2010, 14, 71–79. [Google Scholar] [CrossRef]
- Tesz, G.J.; Aouadi, M.; Prot, M.; Nicoloro, S.M.; Boutet, E.; Amano, S.U.; Goller, A.; Wang, M.; Guo, C.-A.; Salomon, W.E.; et al. Glucan Particles for Selective Delivery of SiRNA to Phagocytic Cells in Mice. Biochem. J. 2011, 436, 351–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zakria, H.M.; Han, B.; Yue, F.; Mu, L.; Fang, Y.; Li, X.; Xu, K.; Zhang, Z. Significant Body Mass Increase by Oral Administration of a Cascade of ShIL21-MSTN Yeast-Based DNA Vaccine in Mice. Biomed. Pharmacother. 2019, 118, 109147. [Google Scholar] [CrossRef] [PubMed]
- Drinnenberg, I.A.; Weinberg, D.E.; Xie, K.T.; Mower, J.P.; Wolfe, K.H.; Fink, G.R.; Bartel, D.P. RNAi in Budding Yeast. Science 2009, 326, 544–550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vandermies, M.; Fickers, P. Bioreactor-Scale Strategies for the Production of Recombinant Protein in the Yeast Yarrowia Lipolytica. Microorganisms 2019, 7, 40. [Google Scholar] [CrossRef] [Green Version]


| Delivery System | Advantages | Disadvantages | References |
|---|---|---|---|
| Lipid-Based |
|
| [36,37,38,39] |
| Polymer-Based |
|
| [40,41,42,43,44] |
| Viral Vectors |
|
| [45,46,47] |
| Bacterial Vectors |
|
| [48,49,50] |
| Yeast |
|
| [10,11,51,52] |
| Routes | Advantages | Limitations |
|---|---|---|
| Oral |
|
|
| S.C. |
|
|
| I.V. |
|
|
| I.M. |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, A.J.D.; de Sousa, M.M.G.; de Macêdo, L.S.; de França Neto, P.L.; de Moura, I.A.; Espinoza, B.C.F.; Invenção, M.D.C.V.; de Pinho, S.S.; da Gama, M.A.T.M.; de Freitas, A.C. RNA Vaccines: Yeast as a Novel Antigen Vehicle. Vaccines 2023, 11, 1334. https://doi.org/10.3390/vaccines11081334
Silva AJD, de Sousa MMG, de Macêdo LS, de França Neto PL, de Moura IA, Espinoza BCF, Invenção MDCV, de Pinho SS, da Gama MATM, de Freitas AC. RNA Vaccines: Yeast as a Novel Antigen Vehicle. Vaccines. 2023; 11(8):1334. https://doi.org/10.3390/vaccines11081334
Chicago/Turabian StyleSilva, Anna Jéssica Duarte, Mylenna Máyra Gois de Sousa, Larissa Silva de Macêdo, Pedro Luiz de França Neto, Ingrid Andrêssa de Moura, Benigno Cristofer Flores Espinoza, Maria Da Conceição Viana Invenção, Samara Sousa de Pinho, Marco Antonio Turiah Machado da Gama, and Antonio Carlos de Freitas. 2023. "RNA Vaccines: Yeast as a Novel Antigen Vehicle" Vaccines 11, no. 8: 1334. https://doi.org/10.3390/vaccines11081334






