VP4/VP56/VP35 Virus-like Particles Effectively Protect Grass Carp (Ctenopharyngodon idella) against GCRV-II Infection
Abstract
:Highlights:
- 1.
- Three outer capsid proteins of GCRV-II self-assemble into VLPs in vitro.
- 2.
- VLPs vaccine have low toxicity and uniform particle size.
- 3.
- VLPs + astragalus polysaccharide improve protection and reduces tissue viral load.
- 4.
- VLPs vaccine induce immune response and regulate antioxidant immunity.
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals, Bacteria, Virus, and Gene Cloning of Full-LengthVP4/VP56/VP35
2.2. Recombinant Expression and Purification of the VP4/VP56/VP35 Proteins in Prokaryotic and Yeast Systems
2.3. SDS-PAGE Analysis and Western Blot (WB)
2.4. Assembly of GCRV Virus-like Particles (VLPs)
2.5. Pull-Down, Transmission Electron Microscopy (TEM), and Dynamic Light Scattering (DLS) Analysis
2.6. Hemolysis and Cytotoxicity Tests
2.7. Injection Immunization and Viral Challenge
2.8. Enzyme Activity Assay in Serum, Quantitative RT-PCR Analysis of Immune-Related Gene Expression, Viral Load Determination and Statistical Analysis
2.9. Hematoxylin and Eosin Staining
3. Results
3.1. Protein Expression, Purification, and Particle self-Assembly Analysis of VP4/VP56/VP35
3.2. VLPs Are Morphologically Stable, Uniform in Size, and Low in Toxicity
3.3. VLPs Enhance IgM Antibody Levels and Protective Efficacy While Alleviating Tissue Viral Load
3.4. VLPs Effectively Improve Serum Immune and Antioxidant Enzyme Activity against GCRV-II Infection
3.5. VLPs Facilitate the Expression of the Representative Immune Genes in Head Kidney
3.6. VLPs Strengthen the Protective Effect and Reduce Tissue Lesions in Grass Carp Tissues
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gao, T.; Gao, C.; Wu, S.; Wang, Y.; Yin, J.; Li, Y.; Zeng, W.; Bergmann, S.M.; Wang, Q. Recombinant baculovirus-produced grass carp reovirus virus-like particles as vaccine candidate that provides protective immunity against GCRV genotype II infection in grass carp. Vaccines 2021, 9, 53. [Google Scholar] [CrossRef]
- Li, Z.; Wang, G.; Yu, E.; Zhang, K.; Yu, D.; Gong, W.; Xie, J. Artificial substrata increase pond farming density of grass carp (Ctenopharyngodon idella) by increasing the bacteria that participate in nitrogen and phosphorus cycles in pond water. PeerJ 2019, 7, e7906. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Zhang, A.; Pei, Y.; Chu, P.; Li, Y.; Huang, R.; Liao, L.; Zhu, Z.; Wang, Y. Differences in responses of grass carp to different types of grass carp reovirus (GCRV) and the mechanism of hemorrhage revealed by transcriptome sequencing. BMC Genom. 2017, 18, 452. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Zhu, W.; Huo, X.; Qiao, M.; Yang, C.; Zhang, Y.; Su, J. Oral Lactobacillus casei expressing VP56310–500 and adjuvant flagellin C delivered by alginate-chitosan microcapsules remarkably enhances the immune protection against GCRV infection in grass carp. Aquaculture 2023, 567, 739301. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, P.; Guo, S.; Zhao, Z.; Wang, G.; Zhua, B. Dual-targeting polymer nanoparticles efficiently deliver DNA vaccine and induce robust prophylactic immunity against spring viremia of carp virus infection. Microbiol. Spectr. 2022, 10, e0308522. [Google Scholar] [CrossRef]
- Sun, H.; Shang, M.; Tang, Z.; Jiang, H.; Dong, H.; Zhou, X.; Lin, Z.; Shi, C.; Ren, P.; Zhao, L.; et al. Oral delivery of Bacillus subtilis spores expressing Clonorchis sinensis paramyosin protects grass carp from cercaria infection. Appl. Microbiol. Biotechnol. 2020, 104, 1633–1646. [Google Scholar] [CrossRef]
- Nooraei, S.; Bahrulolum, H.; Hoseini, Z.S.; Katalani, C.; Hajizade, A.; Easton, A.J.; Ahmadian, G. Virus-like particles: Preparation, immunogenicity and their roles as nanovaccines and drug nanocarriers. J. Nanobiotechnol. 2021, 19, 59. [Google Scholar] [CrossRef]
- Li, T.; Lin, H.; Zhang, Y.; Li, M.; Wang, D.; Che, Y.; Zhu, Y.; Li, S.; Zhang, J.; Ge, S.; et al. Improved characteristics and protective efficacy in an animal model of E. coli-derived recombinant double-layered rotavirus virus-like particles. Vaccine 2014, 32, 1921–1931. [Google Scholar] [CrossRef]
- Nan, L.; Liu, Y.; Ji, P.; Feng, H.; Chen, C.; Wang, J.; Liu, D.; Cui, Y.; Wang, Y.; Li, Y.; et al. Trigger factor assisted self-assembly of canine parvovirus VP2 protein into virus-like particles in Escherichia coli with high immunogenicity. Virol. J. 2018, 15, 103. [Google Scholar] [CrossRef]
- Blazevic, V.; Lappalainen, S.; Nurminen, K.; Huhti, L.; Vesikari, T. Norovirus VLPs and rotavirus VP6 protein as combined vaccine for childhood gastroenteritis. Vaccine 2011, 29, 8126–8133. [Google Scholar] [CrossRef]
- Mulder, A.M.; Carragher, B.; Towne, V.; Meng, Y.; Wang, Y.; Dieter, L.; Potter, C.S.; Washabaugh, M.W.; Sitrin, R.D.; Zhao, Q. Toolbox for non-intrusive structural and functional analysis of recombinant VLP based vaccines: A case study with hepatitis B vaccine. PLoS ONE 2012, 7, e33235. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Zhang, J.; Zhang, X.; Zhou, C.; Wang, Z.; Huang, S.; Wang, H.; Yang, C.; Jiang, H.; Cai, J.P.; et al. Efficacy and safety of a recombinant hepatitis E vaccine in healthy adults: A large-scale, randomised, double-blind placebo-controlled, phase 3 trial. Lancet 2010, 376, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, H.-J. Yeast as an expression system for producing virus-like particles: What factors do we need to consider? Lett. Appl. Microbiol. 2017, 64, 111–123. [Google Scholar] [CrossRef]
- Srivastava, V.; Nand, K.N.; Ahmad, A.; Kumar, R. Yeast-based virus-like particles as an emerging platform for vaccine development and delivery. Vaccines 2023, 11, 479. [Google Scholar] [CrossRef] [PubMed]
- McAleer, W.J.; Buynak, E.B.; Maigetter, R.Z.; Wampler, D.E.; Miller, W.J.; Hilleman, M.R. Human hepatitis B vaccine from recombinant yeast. Nature 1984, 307, 178–180. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Sings, H.L.; Bryan, J.T.; Wang, B.; Wang, Y.; Mach, H.; Kosinski, M.; Washabaugh, M.W.; Sitrin, R.; Barr, E. GARDASIL: Prophylactic human papillomavirus vaccine development—From bench top to bed-side. Clin. Pharmacol. Ther. 2007, 81, 259–264. [Google Scholar] [CrossRef]
- Huang, X.; Wang, X.; Zhang, J.; Xia, N.; Zhao, Q. Escherichia coli-derived virus-like particles in vaccine development. NPJ Vacc. 2017, 2, 3. [Google Scholar] [CrossRef]
- Zhao, Q.; Allen, M.J.; Wang, Y.; Wang, B.; Wang, N.; Shi, L.; Sitrin, R.D. Disassembly and reassembly improves morphology and thermal stability of human papillomavirus type 16 virus-like particles. Nanomedicine 2012, 8, 1182–1189. [Google Scholar] [CrossRef]
- Rao, Y.; Su, J. Insights into the antiviral immunity against grass carp (Ctenopharyngodon idella) reovirus (GCRV) in grass carp. J. Immunol. Res. 2015, 2015, 670437. [Google Scholar] [CrossRef]
- Jiang, H.; Bian, Q.; Zeng, W.; Ren, P.; Sun, H.; Lin, Z.; Tang, Z.; Zhou, X.; Wang, Q.; Wang, Y.; et al. Oral delivery of Bacillus subtilis spores expressing grass carp reovirus VP4 protein produces protection against grass carp reovirus infection. Fish Shellfish Immunol. 2019, 84, 768–780. [Google Scholar] [CrossRef]
- Zhang, A.; He, L.; Wang, Y. Prediction of GCRV virus-host protein interactome based on structural motif-domain interactions. BMC Bioinform. 2017, 18, 145. [Google Scholar] [CrossRef] [PubMed]
- Zong, Q.; Zhang, Y.; Lu, L. Preparation and immunogenicity of polyclonal antibodies against VP4, VP35 protein of type II grass carp reovirus. J. Fish. China 2016, 40, 355–362. [Google Scholar] [CrossRef]
- Jiang, L.; Liu, A.-Q.; Zhang, C.; Zhang, Y.-A.; Tu, J. Hsp90 regulates GCRV-II proliferation by interacting with VP35 as its receptor and chaperone. J. Virol. 2022, 96, e0117522. [Google Scholar] [CrossRef] [PubMed]
- Yin, G.; Jeney, G.; Racz, T.; Xu, P.; Jun, X.; Jeney, Z. Effect of two Chinese herbs (Astragalus radix and Scutellaria radix) on non-specific immune response of tilapia, Oreochromis niloticus. Aquaculture 2006, 253, 39–47. [Google Scholar] [CrossRef]
- Cho, W.C.; Leung, K.N. In vitro and in vivo anti-tumor effects of Astragalus membranaceus. Cancer Lett. 2007, 252, 43–54. [Google Scholar] [CrossRef]
- Huang, G.C.; Wu, L.S.; Chen, L.G.; Yang, L.L.; Wang, C.C. Immuno-enhancement effects of Huang Qi Liu Yi Tang in a murine model of cyclophosphamide-induced leucopenia. J. Ethnopharmacol. 2007, 109, 229–235. [Google Scholar] [CrossRef]
- Lin, S.M.; Jiang, Y.; Chen, Y.J.; Luo, L.; Doolgindachbaporn, S.; Yuangsoi, B. Effects of Astragalus polysaccharides (APS) and chitooligosaccharides (COS) on growth, immune response and disease resistance of juvenile largemouth bass, Micropterus salmoides. Fish Shellfish Immunol. 2017, 70, 40–47. [Google Scholar] [CrossRef]
- Kanellos, T.; Sylvester, I.D.; D’Mello, F.; Howard, C.R.; Mackie, A.; Dixon, P.F.; Chang, K.C.; Ramstad, A.; Midtlyng, P.J.; Russell, P.H. DNA vaccination can protect Cyprinus Carpio against spring viraemia of carp virus. Vaccine 2006, 24, 4927–4933. [Google Scholar] [CrossRef]
- Kim, C.H.; Johnson, M.C.; Drennan, J.D.; Simon, B.E.; Thomann, E.; Leong, J.A. DNA vaccines encoding viral glycoproteins induce nonspecific immunity and Mx protein synthesis in fish. J. Virol. 2000, 74, 7048–7054. [Google Scholar] [CrossRef]
- Yao, Q.; Bu, Z.; Vzorov, A.; Yang, C.; Compans, R.W. Virus-like particle and DNA-based candidate AIDS vaccines. Vaccine 2003, 21, 638–643. [Google Scholar] [CrossRef]
- Schwarz, B.; Uchida, M.; Douglas, T. Biomedical and catalytic opportunities of virus-like particles in nanotechnology. Adv. Virus Res. 2017, 97, 1–60. [Google Scholar] [CrossRef] [PubMed]
- Chroboczek, J.; Szurgot, I.; Szolajska, E. Virus-like particles as vaccine. Acta Biochim. Pol. 2014, 61, 531–539. [Google Scholar] [CrossRef]
- Palomares, L.A.; Ramírez, O.T. Challenges for the production of virus-like particles in insect cells: The case of rotavirus-like particles. Biochem. Eng. J. 2009, 45, 158–167. [Google Scholar] [CrossRef]
- Wang, D.; Liu, X.; Wei, M.; Qian, C.; Song, S.; Chen, J.; Wang, Z.; Xu, Q.; Yang, Y.; He, M.; et al. Rational design of a multi-valent human papillomavirus vaccine by capsomere-hybrid co-assembly of virus-like particles. Nat. Commun. 2020, 11, 2841. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Li, S.; Yu, H.; Xia, N.; Modis, Y. Virus-like particle-based human vaccines: Quality assessment based on structural and functional properties. Trends Biotechnol. 2013, 31, 654–663. [Google Scholar] [CrossRef]
- Bian, T.; Gardin, A.; Gemen, J.; Houben, L.; Perego, C.; Lee, B.; Elad, N.; Chu, Z.; Pavan, G.M.; Klajn, R. Electrostatic co-assembly of nanoparticles with oppositely charged small molecules into static and dynamic superstructures. Nat. Chem. 2021, 13, 940–949. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Chávez, Á.J.; Moreno-Fierros, L.; Bustos-Jaimes, I. Therapy with multi-epitope virus-like particles of B19 parvovirus reduce tumor growth and lung metastasis in an aggressive breast cancer mouse model. Vaccine 2019, 37, 7256–7268. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Kang, H.; Tuo, T.; Wang, L.; Xia, Y.; Zhang, Y.; Zhou, L.; Ge, X.; Han, J.; Guo, X.; et al. Astragalus polysaccharide alleviated the inhibition of CSFV C-strain replication caused by PRRSV via the TLRs/NF-kappaB/TNF-alpha pathways. Virus Res. 2022, 319, 198854. [Google Scholar] [CrossRef]
- Jin, Q.; Cheng, L.; Zhu, Y.; Zhao, X.; Zhang, W.; Gao, X.; Xiong, T.; Guo, L. Immune-related effects of compound astragalus polysaccharide and sulfated epimedium polysaccharide on newborn piglets. Anim. Biotechnol. 2021, 34, 1–12. [Google Scholar] [CrossRef]
- Cui, L.C.; Guan, X.T.; Liu, Z.M.; Tian, C.Y.; Xu, Y.G. Recombinant lactobacillus expressing G protein of spring viremia of carp virus (SVCV) combined with ORF81 protein of koi herpesvirus (KHV): A promising way to induce protective immunity against SVCV and KHV infection in cyprinid fish via oral vaccination. Vaccine 2015, 33, 3092–3099. [Google Scholar] [CrossRef]
- Ouchida, R.; Mori, H.; Hase, K.; Takatsu, H.; Kurosaki, T.; Tokuhisa, T.; Ohno, H.; Wang, J.Y. Critical role of the IgM Fc receptor in IgM homeostasis, B-cell survival, and humoral immune responses. Proc. Natl. Acad. Sci. USA 2012, 109, E2699–E2706. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.; Ding, J.L. Natural antibodies bridge innate and adaptive immunity. J. Immunol. 2015, 194, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Manson, J.J.; Mauri, C.; Ehrenstein, M.R. Natural serum IgM maintains immunological homeostasis and prevents autoimmunity. Springer Semin. Immunopathol. 2005, 26, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Huo, X.; Wang, Z.; Xiao, X.; Yang, C.; Su, J. Oral administration of nanopeptide CMCS-20H conspicuously boosts immunity and precautionary effect against bacterial infection in fish. Front. Immunol. 2021, 12, 811616. [Google Scholar] [CrossRef]
- Huang, X.; Ma, Y.; Wang, Y.; Niu, C.; Liu, Z.; Yao, X.; Jiang, X.; Pan, R.; Jia, S.; Li, D.; et al. Oral probiotic vaccine expressing koi herpesvirus (KHV) ORF81 protein delivered by chitosan-alginate capsules is a promising strategy for mass oral vaccination of carps against KHV infection. J. Virol. 2021, 95, 15–21. [Google Scholar] [CrossRef]
- Chen, F.F.; Lin, H.B.; Li, J.C.; Wang, Y.; Li, J.; Zhang, D.G.; Yu, W.Y. Grass carp (Ctenopharyngodon idellus) invariant chain of the MHC class II chaperone protein associates with the class I molecule. Fish Shellfish Immunol. 2017, 63, 1–8. [Google Scholar] [CrossRef]
- Li, X.; Du, H.; Liu, L.; You, X.; Wu, M.; Liao, Z. MHC class II alpha, beta and MHC class II-associated invariant chains from Chinese sturgeon (Acipenser sinensis) and their response to immune stimulation. Fish Shellfish Immunol. 2017, 70, 1–12. [Google Scholar] [CrossRef]
- Sallusto, F.; Lanzavecchia, A. Understanding dendritic cell and T-lymphocyte traffic through the analysis of chemokine receptor expression. Immunol. Rev. 2000, 177, 134–140. [Google Scholar] [CrossRef]
- Chen, T.; Zhou, J.; Qu, Z.; Zou, Q.; Liu, X.; Su, J.; Fu, X.; Yuan, G. Administration of dietary recombinant hepcidin on grass carp (Ctenopharyngodon idella) against Flavobacterium columnare infection under cage aquaculture conditions. Fish Shellfish Immunol. 2020, 99, 27–34. [Google Scholar] [CrossRef]
- Jiang, Z.; Sun, Z.; Hu, J.; Li, D.; Xu, X.; Li, M.; Feng, Z.; Zeng, S.; Mao, H.; Hu, C. Grass carp Mex3A promotes ubiquitination and degradation of RIG-I to inhibit innate immune response. Front. Immunol. 2022, 13, 909315. [Google Scholar] [CrossRef]
- Su, J. The discovery of type IV interferon system revolutionizes interferon family and opens up a new frontier in jawed vertebrate immune defense. Sci. China Life Sci. 2022, 65, 2335–2337. [Google Scholar] [CrossRef] [PubMed]
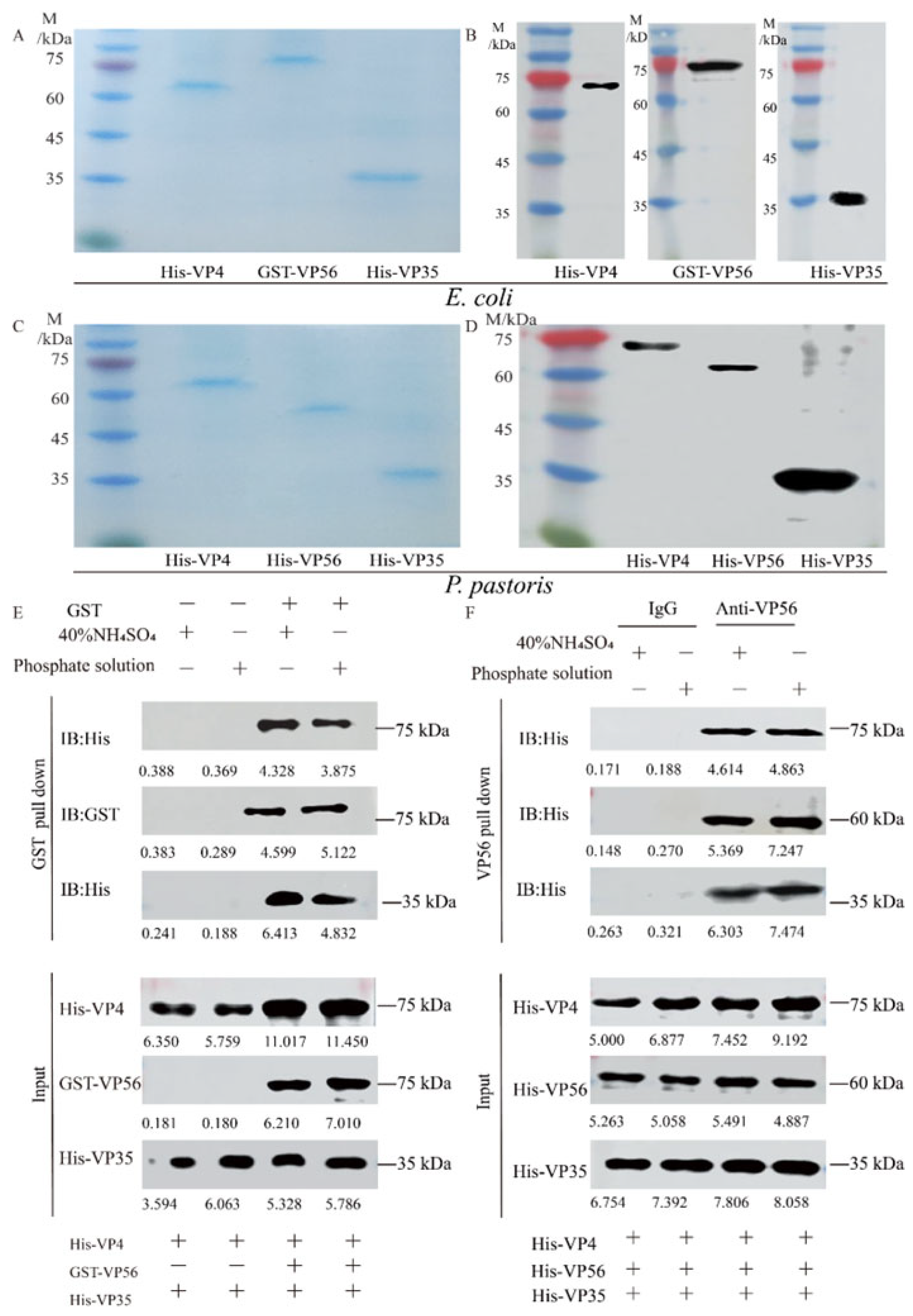


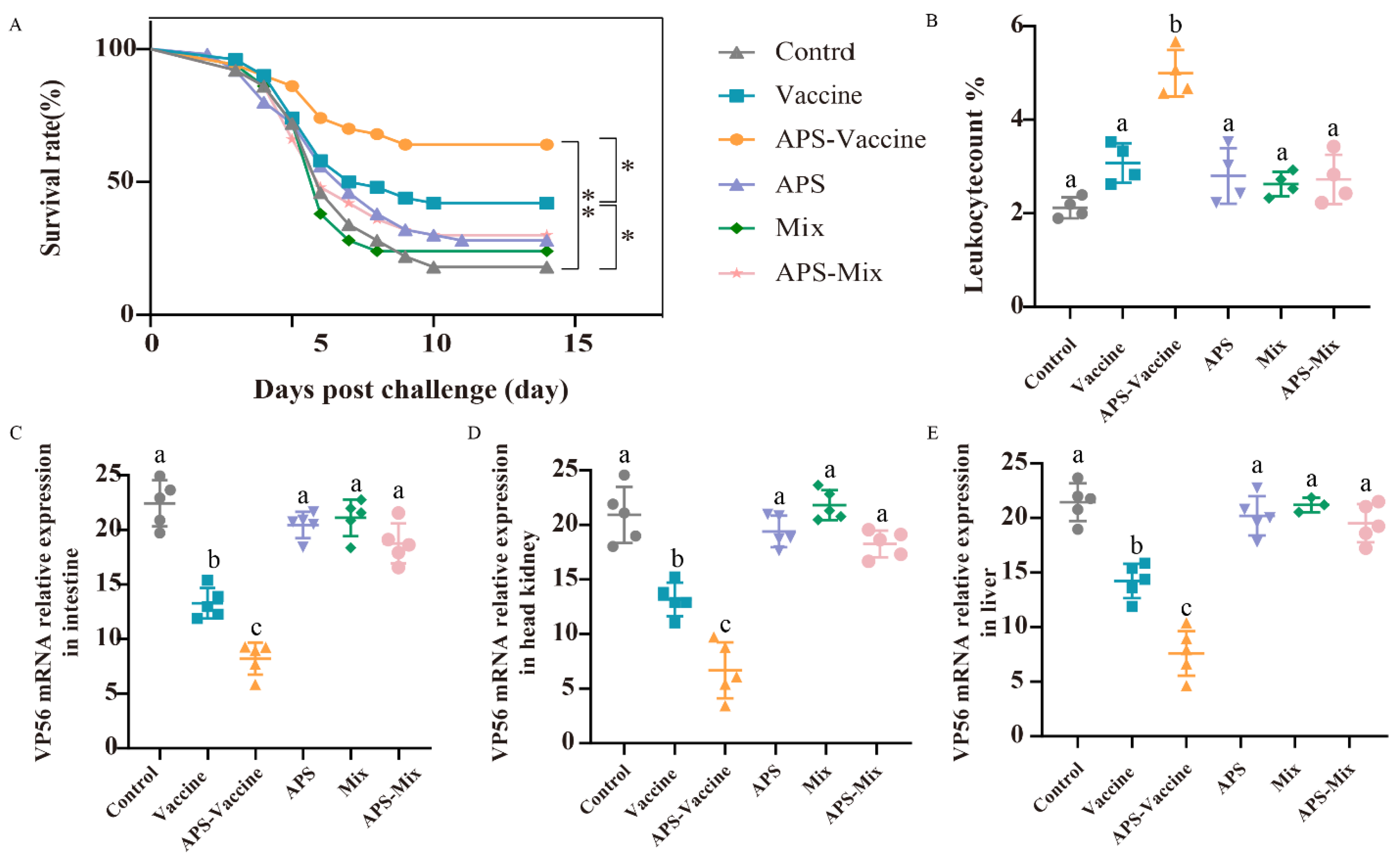
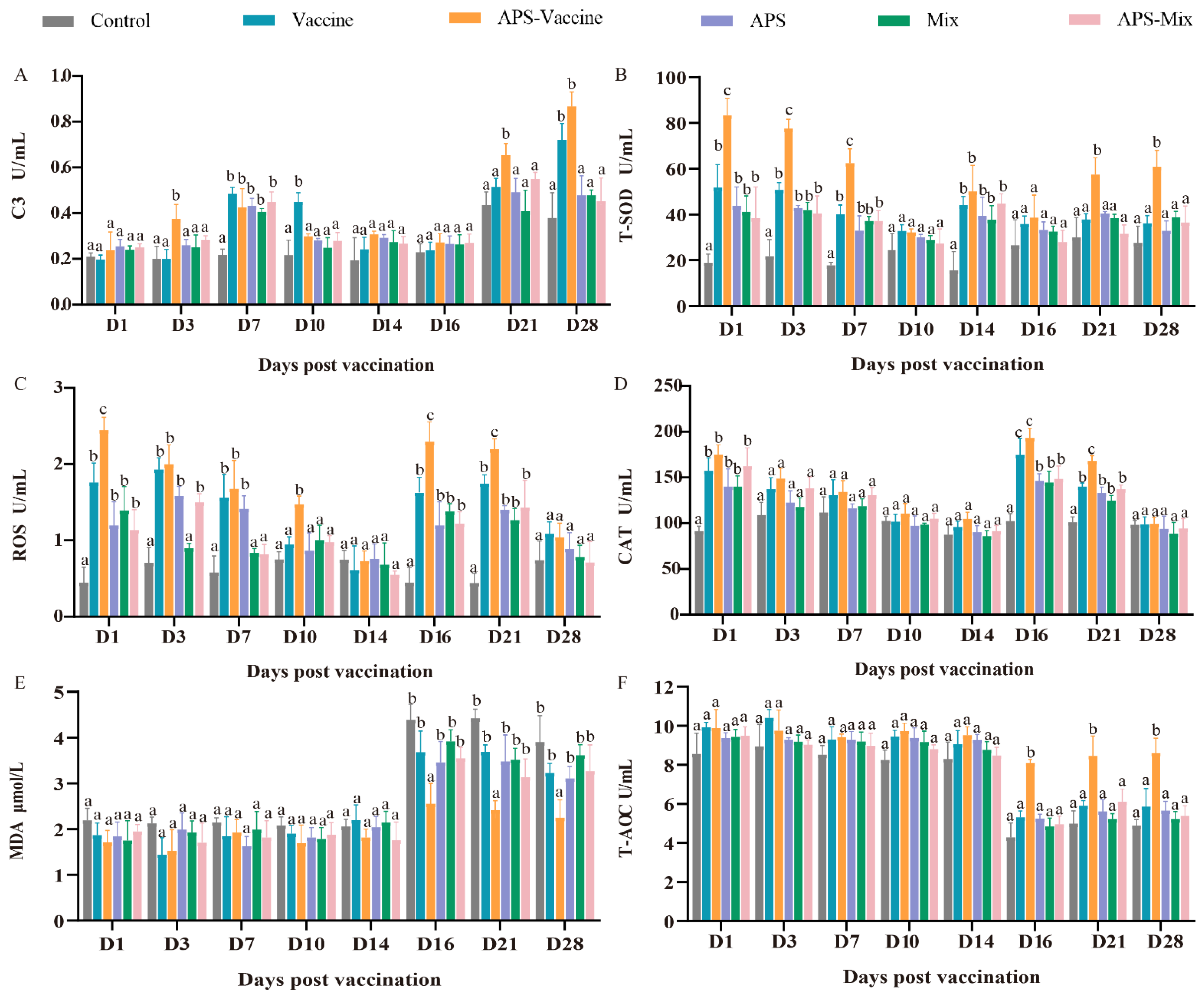
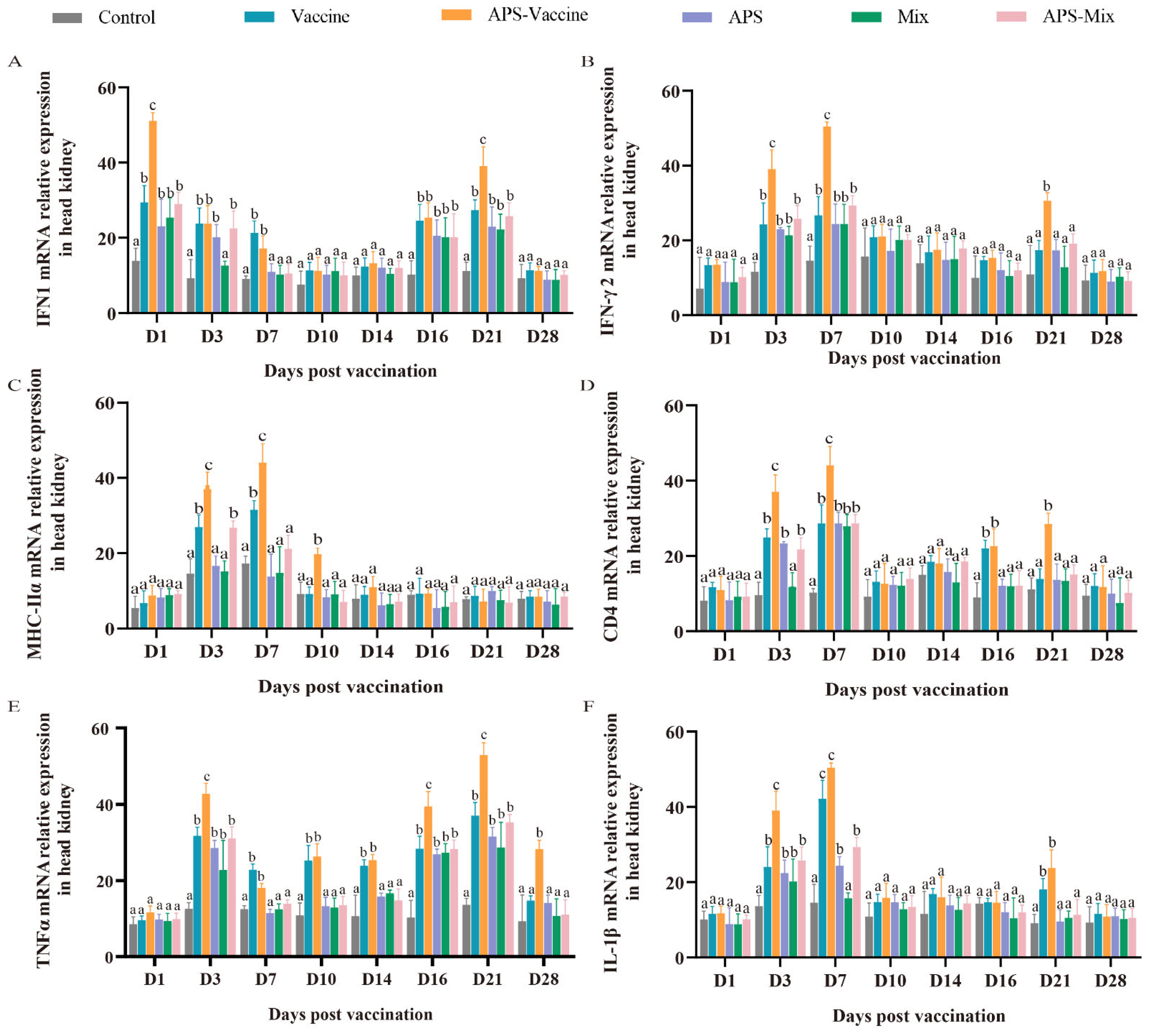

| Gene Name | Primer Sequence (5′−3′) | Amplicon Length (nt) and Primer Information |
|---|---|---|
| VP4 | F: CGCGGATCCACTATCATGGGAAACGTC | 1968 |
| R: CCG CTCGAGGTACACGACGTAAGACGG | Gene cloning | |
| VP56 | F: CGCGGATCC ATGGCCACTCGTGACAGCCGC | 1539 |
| R: CCGCTCGAGTTACTTACAGC | Gene cloning | |
| VP35 | F: GCGTCGACATGGAATCAGCAAAACC | 933 |
| R: CCGCTCGAGTTACTGTCCCTGG | Gene cloning | |
| IL-1β | F: AAGTTCCCGCTTTGGAGAGTA | 126 |
| R: GCCACATACCAGTCGTTCAGT | qRT-PCR | |
| MHC-IIα | F: TACTACCAGATTCACTCGG | 111 |
| R: CGGGTTCCAGTCAAAGAT | qRT-PCR | |
| IFN1 | F: GGTGAAGTTTCTTGCCCTGACCTTAG | 173 |
| R: CCTTATGTGATGGCTGGTATCGGG | qRT-PCR | |
| CD4 | F: GTGCAGAGCTGCACTGCGACA | 118 |
| R: GCACTATTTTGCCTCCTTCAGA | qRT-PCR | |
| IgM | F: TGGAGCAACGGCACAGTATT | 131 |
| R: TCTGGGGGTGCTAACAGGTA | qRT-PCR | |
| β-actin | F: TCCTCACCGAGAGAGGCTAC | 101 |
| R: CTGCTCGAAGTCAAGAGCCA | qRT-PCR | |
| TNFα | F: CCAGCTCTTCCCAAACCAGT | 126 |
| R: CCATCATCCTTCGCCCATGA | qRT-PCR | |
| IFN-γ2 | F: TCACAGTCAGGAAGCGAGTTC | 100 |
| R: AAGGTTTGCGGCCCATCTTT | qRT-PCR | |
| RIG-I | F: ACTACACTGAACACCTGCGGAA | 108 |
| R: GCATCTTTAGTGCGGGCG | qRT-PCR | |
| VP56 | F: AGCAGGCTATTCATCACCAGT | 107 |
| R: GTTCTAACGCTCACCGTCTTTTC | qRT-PCR | |
| 18S rRNA | F: ATTTCCGACACGGAGAGG | 90 |
| R: CATGGGTTTAGGATACGCTC | qRT-PCR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Q.; Huo, X.; Liu, Q.; Yang, C.; Zhang, Y.; Su, J. VP4/VP56/VP35 Virus-like Particles Effectively Protect Grass Carp (Ctenopharyngodon idella) against GCRV-II Infection. Vaccines 2023, 11, 1373. https://doi.org/10.3390/vaccines11081373
Tian Q, Huo X, Liu Q, Yang C, Zhang Y, Su J. VP4/VP56/VP35 Virus-like Particles Effectively Protect Grass Carp (Ctenopharyngodon idella) against GCRV-II Infection. Vaccines. 2023; 11(8):1373. https://doi.org/10.3390/vaccines11081373
Chicago/Turabian StyleTian, Qingqing, Xingchen Huo, Qian Liu, Chunrong Yang, Yongan Zhang, and Jianguo Su. 2023. "VP4/VP56/VP35 Virus-like Particles Effectively Protect Grass Carp (Ctenopharyngodon idella) against GCRV-II Infection" Vaccines 11, no. 8: 1373. https://doi.org/10.3390/vaccines11081373
APA StyleTian, Q., Huo, X., Liu, Q., Yang, C., Zhang, Y., & Su, J. (2023). VP4/VP56/VP35 Virus-like Particles Effectively Protect Grass Carp (Ctenopharyngodon idella) against GCRV-II Infection. Vaccines, 11(8), 1373. https://doi.org/10.3390/vaccines11081373






