Polymer Chemistry Defines Adjuvant Properties and Determines the Immune Response against the Antigen or Vaccine
Abstract
:1. Introduction
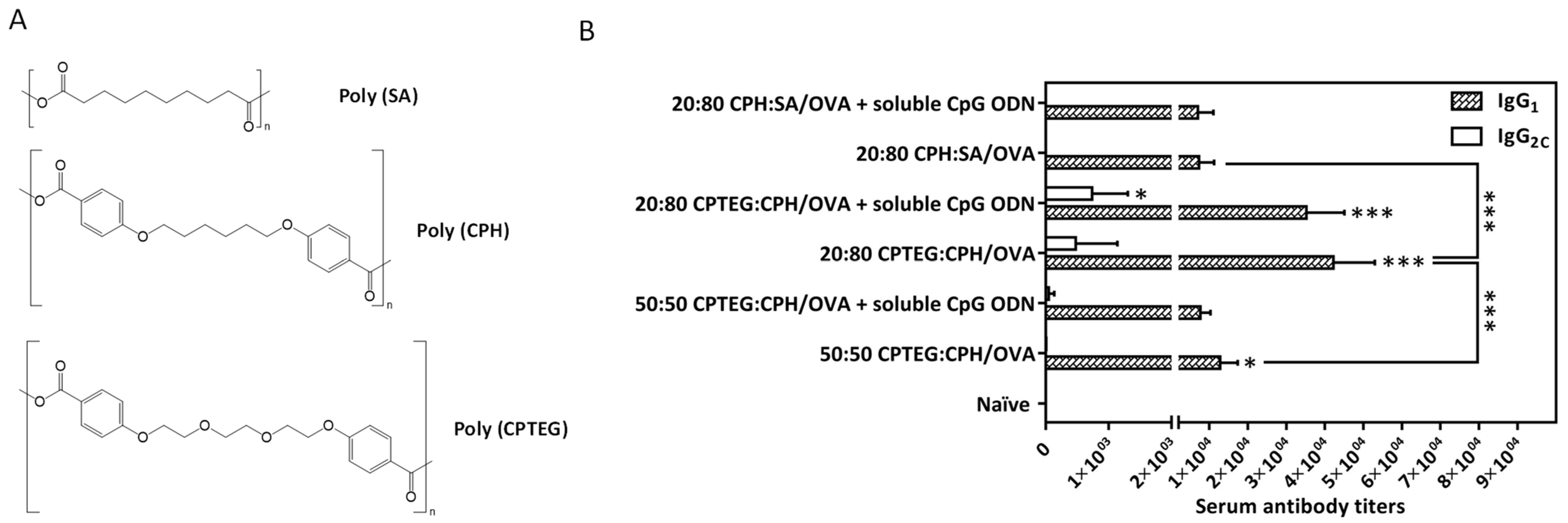
2. Anhydride Chemistry
3. Zwitterionic Chemistry
4. Amide Chemistry
5. Acid and Ester Chemistry
6. Alcohol Chemistry
7. Ionic Interactions
8. Coordination Interactions
9. Combination of Different Interactions
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shakya, A.K.; Nandakumar, K.S. Applications of polymeric adjuvants in studying autoimmune responses and vaccination against infectious diseases. J. R. Soc. Interface 2013, 10, 20120536. [Google Scholar] [CrossRef] [PubMed]
- Shakya, A.K.; Nandakumar, K.S. Polymers as immunological adjuvants: An update on recent developments. J. BioScience Biotechnol. 2012, 1, 199–210. [Google Scholar]
- Ragupathy, L.; Millar, D.G.; Tirelli, N.; Cellesi, F. An orthogonal click-chemistry approach to design poly(glycerol monomethacrylate)-based nanomaterials for controlled immunostimulation. Macromol. Biosci. 2014, 14, 1528–1538. [Google Scholar] [CrossRef] [PubMed]
- Shakya, A.K.; Sami, H.; Srivastava, A.; Kumar, A. Stability of responsive polymer-protein bioconjugates. Prog. Polym. Sci. 2010, 35, 459–486. [Google Scholar] [CrossRef]
- Shakya, A.K.; Kumar, A.; Holmdahl, R.; Nandakumar, K.S. Macrophage-derived reactive oxygen species protects against autoimmune priming with a defined polymeric adjuvant. Immunology 2016, 147, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Shakya, A.K.; Kumar, A.; Klaczkowska, D.; Hultqvist, M.; Hagenow, K.; Holmdahl, R.; Nandakumar, K.S. Collagen type II and a thermo-responsive polymer of N-isopropylacrylamide induce arthritis independent of Toll-like receptors: A strong influence by major histocompatibility complex class II and Ncf1 genes. Am. J. Pathol. 2011, 179, 2490–2500. [Google Scholar] [CrossRef]
- Shakya, A.K.; Kumar, A.; Nandakumar, K.S. Adjuvant properties of a biocompatible thermo-responsive polymer of N-isopropylacrylamide in autoimmunity and arthritis. J. R. Soc. Interface 2011, 8, 1748–1759. [Google Scholar] [CrossRef]
- Shakya, A.K.; Nandakumar, K.S. Synthetic polymer as an adjuvant in collagen-induced arthritis. Curr. Protoc. Mouse Biol. 2014, 4, 11–24. [Google Scholar] [CrossRef]
- Weiss, A.M.; Hossainy, S.; Rowan, S.J.; Hubbell, J.A.; Esser-Kahn, A.P. Immunostimulatory Polymers as Adjuvants, Immunotherapies, and Delivery Systems. Macromolecules 2022, 55, 6913–6937. [Google Scholar] [CrossRef]
- Love, R.J.; Jones, K.S. The recognition of biomaterials: Pattern recognition of medical polymers and their adsorbed biomolecules. J. Biomed. Mater. Res. A 2013, 101, 2740–2752. [Google Scholar] [CrossRef]
- Sevast’ianov, V.I.; Tseytlina, E.A. The activation of the complement system by polymer materials and their blood compatibility. J. Biomed. Mater. Res. 1984, 18, 969–978. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.A.; Gama, F.M.; Vilanova, M. Polymeric nanogels as vaccine delivery systems. Nanomedicine 2012, 9, 159–173. [Google Scholar] [CrossRef] [PubMed]
- Vroman, L.; Adams, A.L.; Fischer, G.C.; Munoz, P.C. Interaction of high molecular weight kininogen, factor XII, and fibrinogen in plasma at interfaces. Blood 1980, 55, 156–159. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foreign body reaction to biomaterials. Semin. Immunol. 2008, 20, 86–100. [Google Scholar] [CrossRef]
- Wilson, C.J.; Clegg, R.E.; Leavesley, D.I.; Pearcy, M.J. Mediation of biomaterial-cell interactions by adsorbed proteins: A review. Tissue Eng. 2005, 11, 1–18. [Google Scholar] [CrossRef]
- Tang, L.; Eaton, J.W. Fibrin(ogen) mediates acute inflammatory responses to biomaterials. J. Exp. Med. 1993, 178, 2147–2156. [Google Scholar] [CrossRef]
- Tang, L.; Lucas, A.H.; Eaton, J.W. Inflammatory responses to implanted polymeric biomaterials: Role of surface-adsorbed immunoglobulin G. J. Lab. Clin. Med. 1993, 122, 292–300. [Google Scholar]
- Volle, J.M.; Tolleshaug, H.; Berg, T. Phagocytosis and chemiluminescence response of granulocytes to monodisperse latex particles of varying sizes and surface coats. Inflammation 2000, 24, 571–582. [Google Scholar] [CrossRef]
- Grego, E.A.; Siddoway, A.C.; Uz, M.; Liu, L.; Christiansen, J.C.; Ross, K.A.; Kelly, S.M.; Mallapragada, S.K.; Wannemuehler, M.J.; Narasimhan, B. Polymeric Nanoparticle-Based Vaccine Adjuvants and Delivery Vehicles. Curr. Top. Microbiol. Immunol. 2021, 433, 29–76. [Google Scholar] [CrossRef]
- Andorko, J.I.; Pineault, K.G.; Jewell, C.M. Impact of molecular weight on the intrinsic immunogenic activity of poly(beta amino esters). J. Biomed. Mater. Res. Part A. 2017, 105A, 1219–1229. [Google Scholar] [CrossRef]
- Ben-Akiva, E.; Est Witte, S.; Meyer, R.A.; Rhodes, K.R.; Green, J.J. Polymeric micro- and nanoparticles for immune modulation. Biomater. Sci. 2018, 7, 14–30. [Google Scholar] [CrossRef] [PubMed]
- Wafa, E.I.; Geary, S.M.; Goodman, J.T.; Narasimhan, B.; Salem, A.K. The effect of polyanhydride chemistry in particle-based cancer vaccines on the magnitude of the anti-tumor immune response. Acta Biomater. 2017, 50, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Erfani, A.; Hanna, A.; Zarrintaj, P.; Manouchehri, S.; Weigandt, K.; Aichele, C.P.; Ramsey, J.D. Biodegradable zwitterionic poly(carboxybetaine) microgel for sustained delivery of antibodies with extended stability and preserved function. Soft Matter 2021, 17, 5349–5361. [Google Scholar] [CrossRef] [PubMed]
- Koerner, J.; Horvath, D.; Herrmann, V.L.; MacKerracher, A.; Gander, B.; Yagita, H.; Rohayem, J.; Groettrup, M. PLGA-particle vaccine carrying TLR3/RIG-I ligand Riboxxim synergizes with immune checkpoint blockade for effective anti-cancer immunotherapy. Nat. Commun. 2021, 12, 2935. [Google Scholar] [CrossRef] [PubMed]
- Gu, P.; Wusiman, A.; Zhang, Y.; Liu, Z.; Bo, R.; Hu, Y.; Liu, J.; Wang, D. Rational Design of PLGA Nanoparticle Vaccine Delivery Systems To Improve Immune Responses. Mol. Pharm. 2019, 16, 5000–5012. [Google Scholar] [CrossRef]
- Castaldello, A.; Brocca-Cofano, E.; Voltan, R.; Triulzi, C.; Altavilla, G.; Laus, M.; Sparnacci, K.; Ballestri, M.; Tondelli, L.; Fortini, C.; et al. DNA prime and protein boost immunization with innovative polymeric cationic core-shell nanoparticles elicits broad immune responses and strongly enhance cellular responses of HIV-1 tat DNA vaccination. Vaccine 2006, 24, 5655–5669. [Google Scholar] [CrossRef]
- Semple, S.C.; Harasym, T.O.; Clow, K.A.; Ansell, S.M.; Klimuk, S.K.; Hope, M.J. Immunogenicity and rapid blood clearance of liposomes containing polyethylene glycol-lipid conjugates and nucleic Acid. J. Pharmacol. Exp. Ther. 2005, 312, 1020–1026. [Google Scholar] [CrossRef]
- Fan, Q.; Miao, C.; Huang, Y.; Yue, H.; Wu, A.; Wu, J.; Wu, J.; Ma, G. Hydroxypropyltrimethyl ammonium chloride chitosan-based hydrogel as the split H5N1 mucosal adjuvant: Structure-activity relationship. Carbohydr. Polym. 2021, 266, 118139. [Google Scholar] [CrossRef]
- Grant, E.V.; Thomas, M.; Fortune, J.; Klibanov, A.M.; Letvin, N.L. Enhancement of plasmid DNA immunogenicity with linear polyethylenimine. Eur. J. Immunol. 2012, 42, 2937–2948. [Google Scholar] [CrossRef]
- Jin, Z.; Dong, Y.T.; Liu, S.; Liu, J.; Qiu, X.R.; Zhang, Y.; Zong, H.; Hou, W.T.; Guo, S.Y.; Sun, Y.F.; et al. Potential of Polyethyleneimine as an Adjuvant To Prepare Long-Term and Potent Antifungal Nanovaccine. Front. Immunol. 2022, 13, 843684. [Google Scholar] [CrossRef]
- Tian, L.; Zhu, Y.; Xu, J.; Shao, M.; Zhu, W.; Xiao, Z.; Chen, Q.; Liu, Z. Coordination Polymers Integrating Metalloimmunology with Immune Modulation to Elicit Robust Cancer Chemoimmunotherapy. CCS Chem. 2021, 3, 2629–2642. [Google Scholar] [CrossRef]
- Gopferich, A.; Tessmar, J. Polyanhydride degradation and erosion. Adv. Drug Deliv. Rev. 2002, 54, 911–931. [Google Scholar] [CrossRef]
- Ahmed, K.K.; Geary, S.M.; Salem, A.K. Applying biodegradable particles to enhance cancer vaccine efficacy. Immunol. Res. 2014, 59, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Tamayo, I.; Irache, J.M.; Mansilla, C.; Ochoa-Reparaz, J.; Lasarte, J.J.; Gamazo, C. Poly(anhydride) nanoparticles act as active Th1 adjuvants through Toll-like receptor exploitation. Clin. Vaccine Immunol. 2010, 17, 1356–1362. [Google Scholar] [CrossRef] [PubMed]
- Schlenoff, J.B. Zwitteration: Coating surfaces with zwitterionic functionality to reduce nonspecific adsorption. Langmuir 2014, 30, 9625–9636. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yu, P.; Xie, J.; Li, J. Recent advances of zwitterionic-based topological polymers for biomedical applications. J. Mater. Chem. B 2022, 10, 2338–2356. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Yuan, Z.; Jain, P.; Hung, H.C.; He, Y.; Lin, X.; McMullen, P.; Jiang, S. De novo design of functional zwitterionic biomimetic material for immunomodulation. Sci. Adv. 2020, 6, eaba0754. [Google Scholar] [CrossRef]
- Vaisocherova, H.; Zhang, Z.; Yang, W.; Cao, Z.; Cheng, G.; Taylor, A.D.; Piliarik, M.; Homola, J.; Jiang, S. Functionalizable surface platform with reduced nonspecific protein adsorption from full blood plasma--material selection and protein immobilization optimization. Biosens. Bioelectron. 2009, 24, 1924–1930. [Google Scholar] [CrossRef]
- Qiao, C.; Liu, J.; Yang, J.; Li, Y.; Weng, J.; Shao, Y.; Zhang, X. Enhanced non-inflammasome mediated immune responses by mannosylated zwitterionic-based cationic liposomes for HIV DNA vaccines. Biomaterials 2016, 85, 1–17. [Google Scholar] [CrossRef]
- Tzianabos, A.; Wang, J.Y.; Kasper, D.L. Biological chemistry of immunomodulation by zwitterionic polysaccharides. Carbohydr. Res. 2003, 338, 2531–2538. [Google Scholar] [CrossRef]
- Pifferi, C.; Fuentes, R.; Fernandez-Tejada, A. Natural and synthetic carbohydrate-based vaccine adjuvants and their mechanisms of action. Nat. Rev. Chem. 2021, 5, 197–216. [Google Scholar] [CrossRef]
- Zhang, Q.; Overkleeft, H.S.; van der Marel, G.A.; Codee, J.D. Synthetic zwitterionic polysaccharides. Curr. Opin. Chem. Biol. 2017, 40, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Gallorini, S.; Berti, F.; Parente, P.; Baronio, R.; Aprea, S.; D’Oro, U.; Pizza, M.; Telford, J.L.; Wack, A. Introduction of zwitterionic motifs into bacterial polysaccharides generates TLR2 agonists able to activate APCs. J. Immunol. 2007, 179, 8208–8215. [Google Scholar] [CrossRef] [PubMed]
- Gallorini, S.; Berti, F.; Mancuso, G.; Cozzi, R.; Tortoli, M.; Volpini, G.; Telford, J.L.; Beninati, C.; Maione, D.; Wack, A. Toll-like receptor 2 dependent immunogenicity of glycoconjugate vaccines containing chemically derived zwitterionic polysaccharides. Proc. Natl. Acad. Sci. USA 2009, 106, 17481–17486. [Google Scholar] [CrossRef] [PubMed]
- Shakya, A.K.; Sharma, P.; Kumar, A. Synthesis and characterization of thermo-responsive poly(N-isopropylacrylamide)-bovine liver catalase bioconjugate. Enzym. Microb. Technol. 2010, 47, 277–282. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Silva, A.L.; Soema, P.C.; Slutter, B.; Ossendorp, F.; Jiskoot, W. PLGA particulate delivery systems for subunit vaccines: Linking particle properties to immunogenicity. Hum. Vaccin. Immunother. 2016, 12, 1056–1069. [Google Scholar] [CrossRef]
- Allen, R.P.; Bolandparvaz, A.; Ma, J.A.; Manickam, V.A.; Lewis, J.S. Latent, Immunosuppressive Nature of Poly(lactic-co-glycolic acid) Microparticles. ACS Biomater. Sci. Eng. 2018, 4, 900–918. [Google Scholar] [CrossRef]
- Allahyari, M.; Mohit, E. Peptide/protein vaccine delivery system based on PLGA particles. Hum. Vaccin. Immunother. 2016, 12, 806–828. [Google Scholar] [CrossRef]
- Thomas, C.; Rawat, A.; Hope-Weeks, L.; Ahsan, F. Aerosolized PLA and PLGA nanoparticles enhance humoral, mucosal and cytokine responses to hepatitis B vaccine. Mol. Pharm. 2011, 8, 405–415. [Google Scholar] [CrossRef]
- Lewis, J.S.; Dolgova, N.V.; Zhang, Y.; Xia, C.Q.; Wasserfall, C.H.; Atkinson, M.A.; Clare-Salzler, M.J.; Keselowsky, B.G. A combination dual-sized microparticle system modulates dendritic cells and prevents type 1 diabetes in prediabetic NOD mice. Clin. Immunol. 2015, 160, 90–102. [Google Scholar] [CrossRef]
- Nicolete, R.; dos Santos, D.F.; Faccioli, L.H. The uptake of PLGA micro or nanoparticles by macrophages provokes distinct in vitro inflammatory response. Int. Immunopharmacol. 2011, 11, 1557–1563. [Google Scholar] [CrossRef] [PubMed]
- Semete, B.; Booysen, L.I.; Kalombo, L.; Venter, J.D.; Katata, L.; Ramalapa, B.; Verschoor, J.A.; Swai, H. In vivo uptake and acute immune response to orally administered chitosan and PEG coated PLGA nanoparticles. Toxicol. Appl. Pharmacol. 2010, 249, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Chen, X.; Jia, J.; Zhang, W.; Yang, T.; Wang, L.; Ma, G. pH-Responsive Poly(D,L-lactic-co-glycolic acid) Nanoparticles with Rapid Antigen Release Behavior Promote Immune Response. ACS Nano 2015, 9, 4925–4938. [Google Scholar] [CrossRef] [PubMed]
- Joshi, V.B.; Geary, S.M.; Salem, A.K. Biodegradable particles as vaccine delivery systems: Size matters. AAPS J. 2013, 15, 85–94. [Google Scholar] [CrossRef]
- Sarti, F.; Perera, G.; Hintzen, F.; Kotti, K.; Karageorgiou, V.; Kammona, O.; Kiparissides, C.; Bernkop-Schnurch, A. In vivo evidence of oral vaccination with PLGA nanoparticles containing the immunostimulant monophosphoryl lipid A. Biomaterials 2011, 32, 4052–4057. [Google Scholar] [CrossRef]
- Bowles, E.J.; Schiffner, T.; Rosario, M.; Needham, G.A.; Ramaswamy, M.; McGouran, J.; Kessler, B.; LaBranche, C.; McMichael, A.J.; Montefiori, D.; et al. Comparison of neutralizing antibody responses elicited from highly diverse polyvalent heterotrimeric HIV-1 gp140 cocktail immunogens versus a monovalent counterpart in rhesus macaques. PLoS ONE 2014, 9, e114709. [Google Scholar] [CrossRef]
- Krashias, G.; Simon, A.K.; Wegmann, F.; Kok, W.L.; Ho, L.P.; Stevens, D.; Skehel, J.; Heeney, J.L.; Moghaddam, A.E.; Sattentau, Q.J. Potent adaptive immune responses induced against HIV-1 gp140 and influenza virus HA by a polyanionic carbomer. Vaccine 2010, 28, 2482–2489. [Google Scholar] [CrossRef]
- Gartlan, K.H.; Krashias, G.; Wegmann, F.; Hillson, W.R.; Scherer, E.M.; Greenberg, P.D.; Eisenbarth, S.C.; Moghaddam, A.E.; Sattentau, Q.J. Sterile inflammation induced by Carbopol elicits robust adaptive immune responses in the absence of pathogen-associated molecular patterns. Vaccine 2016, 34, 2188–2196. [Google Scholar] [CrossRef]
- Judge, A.; McClintock, K.; Phelps, J.R.; Maclachlan, I. Hypersensitivity and loss of disease site targeting caused by antibody responses to PEGylated liposomes. Mol. Ther. 2006, 13, 328–337. [Google Scholar] [CrossRef]
- Nevagi, R.J.; Khalil, Z.G.; Hussein, W.M.; Powell, J.; Batzloff, M.R.; Capon, R.J.; Good, M.F.; Skwarczynski, M.; Toth, I. Polyglutamic acid-trimethyl chitosan-based intranasal peptide nano-vaccine induces potent immune responses against group A streptococcus. Acta Biomater. 2018, 80, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Zhang, L.; Nandakumar, K.S.; Cheng, K. Imidazole Scaffold Based Compounds in the Development of Therapeutic Drugs. Curr. Top. Med. Chem. 2021, 21, 2514–2528. [Google Scholar] [CrossRef] [PubMed]
- Le, H.V.; Babak, M.V.; Ehsan, M.A.; Altaf, M.; Reichert, L.; Gushchin, A.L.; Ang, W.H.; Isab, A.A. Highly cytotoxic gold(i)-phosphane dithiocarbamate complexes trigger an ER stress-dependent immune response in ovarian cancer cells. Dalton Trans. 2020, 49, 7355–7363. [Google Scholar] [CrossRef]
- Fatrekar, A.P.; Morajkar, R.; Krishnan, S.; Dusane, A.; Madhyastha, H.; Vernekar, A.A. Delineating the Role of Tailored Gold Nanostructures at the Biointerface. ACS Appl. Bio Mater. 2021, 4, 8172–8191. [Google Scholar] [CrossRef] [PubMed]
- Moyano, D.F.; Goldsmith, M.; Solfiell, D.J.; Landesman-Milo, D.; Miranda, O.R.; Peer, D.; Rotello, V.M. Nanoparticle hydrophobicity dictates immune response. J. Am. Chem. Soc. 2012, 134, 3965–3967. [Google Scholar] [CrossRef] [PubMed]


| Functionality | Polymer | Antigen/Vaccine | Application | Reference |
|---|---|---|---|---|
Anhydride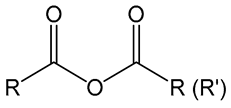 | Polyanhydride- copolymers (CPTEG+CPH §, CPTEG+SA §§) | Ovalbumin | Activation of immune system | [22] |
Zwitterionic  | Poly (carboxybetaine methacrylate) (pCBMA) | Immunoglobulin (Ig) | Therapeutic purpose | [23] |
Amide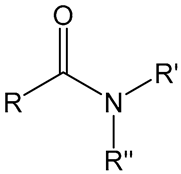 | Poly-N-isopropyl acrylamide (PNiPAAm), | CII † | Development of experimental autoimmune arthritis mouse model | [5,6,7] |
| Polyacrylamide (PAAm), | CII | |||
| Poly-N-isopropylacrylamide-co-Poly-N-tertbutylacrylamide (PNiPAAm-co-PNtBAAm) | CII | |||
| Poly-N-tertbutylacrylamide (PNtBAAm) | CII | |||
Acid and ester | Poly-lactide-co-glycolide (PLGA) | Cancer antigen | Anticancer immunotherapy | [24] |
| Polyethyleneimine PLGA NPs (PEI-PLGA | Ova | Immune system activation | [25] | |
| Polymethylmethacrylate (PMMA) | HIV tat antigen | Immune system activation | [26] | |
Alcohol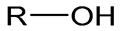 | Polyethylene glycol (PEG) | Nucleic acids | Therapy | [27] |
Electrostatic interactions | 2-hydroxypropyl trimethyl ammonium chloride chitosan (HTCC) | Influenza vaccine | Immune system activation | [28] |
| Polyethyleneimines (PEIs) | Nucleic acid antigen | Immune system activation | [29,30] | |
Coordination interactions | Zn-PI complex (A complex of Zn ions with 4-phenyl imidazole (PI)) | Doxorubicin | Anticancer immunotherapy | [31] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shakya, A.K.; Nandakumar, K.S. Polymer Chemistry Defines Adjuvant Properties and Determines the Immune Response against the Antigen or Vaccine. Vaccines 2023, 11, 1395. https://doi.org/10.3390/vaccines11091395
Shakya AK, Nandakumar KS. Polymer Chemistry Defines Adjuvant Properties and Determines the Immune Response against the Antigen or Vaccine. Vaccines. 2023; 11(9):1395. https://doi.org/10.3390/vaccines11091395
Chicago/Turabian StyleShakya, Akhilesh Kumar, and Kutty Selva Nandakumar. 2023. "Polymer Chemistry Defines Adjuvant Properties and Determines the Immune Response against the Antigen or Vaccine" Vaccines 11, no. 9: 1395. https://doi.org/10.3390/vaccines11091395
APA StyleShakya, A. K., & Nandakumar, K. S. (2023). Polymer Chemistry Defines Adjuvant Properties and Determines the Immune Response against the Antigen or Vaccine. Vaccines, 11(9), 1395. https://doi.org/10.3390/vaccines11091395







