Blood CD8+ Naïve T-Cells Identify MS Patients with High Probability of Optimal Cellular Response to SARS-CoV-2 Vaccine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patient Characteristics
2.3. Sample Collection
2.4. Cell Cultures and Interferon-γ Quantification
2.5. Monoclonal Antibodies
2.6. Labeling of Surface Antigens
2.7. Flow Cytometry
2.8. Flow Cytometry Analyses
2.9. Serum Anti-Spike Antibodies
2.10. Statistical Analysis
3. Results
3.1. Patients
3.2. Percentages of the Different Peripheral Blood Immune Cell Subsets
3.2.1. T-Cells
3.2.2. B-Cells
3.2.3. Innate Immune Cells
3.3. Absolute Cell Counts
3.4. Cutoff Values
3.5. Correlation of T-cells and IFN-γ Production in Response to Spike Antigen
3.6. Humoral Responses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cree, B.A.C.; Oksenberg, J.R.; Hauser, S.L. Multiple sclerosis: Two decades of progress. Lancet Neurol. 2022, 21, 211–214. [Google Scholar] [CrossRef]
- Sbragia, E.; Olobardi, D.; Novi, G.; Lapucci, C.; Cellerino, M.; Boffa, G.; Laroni, A.; Mikulska, M.; Sticchi, L.; Inglese, M. Vaccinations in patients with multiple sclerosis: A real-world, single-center experience. Hum. Vaccin. Immunother. 2022, 18, 2099171. [Google Scholar] [CrossRef]
- Loebermann, M.; Winkelmann, A.; Hartung, H.P.; Hengel, H.; Reisinger, E.C.; Zettl, U.K. Vaccination against infection in patients with multiple sclerosis. Nat. Rev. Neurol. 2012, 8, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Zingaropoli, M.A.; Pasculli, P.; Iannetta, M.; Perri, V.; Tartaglia, M.; Crisafulli, S.G.; Merluzzo, C.; Baione, V.; Mazzochi, L.; Taglietti, A.; et al. Infectious risk in multiple sclerosis patients treated with disease-modifying therapies: A three-year observational cohort study. Mult. Scler. J. Exp. Transl. Clin. 2022, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Barzegar, M.; Houshi, S.; Sadeghi, E.; Hashemi, M.S.; Pishgahi, G.; Bagherieh, S.; Afshari-Safavi, A.; Mirmosayyeb, O.; Shaygannejad, V.; Zabeti, A. Association of Disease-Modifying Therapies with COVID-19 Susceptibility and Severity in Patients with Multiple Sclerosis: A Systematic Review and Network Meta-Analysis. Mult. Scler. Int. 2022, 2022, 9388813. [Google Scholar] [CrossRef]
- Kelly, H.; Sokola, B.; Abboud, H. Safety and efficacy of COVID-19 vaccines in multiple sclerosis patients. J. Neuroimmunol. 2021, 356, 577599. [Google Scholar] [CrossRef] [PubMed]
- Zabalza, A.; Cárdenas-Robledo, S.; Tagliani, P.; Arrambide, G.; Otero-Romero, S.; Carbonell-Mirabent, P.; Rodriguez-Barranco, M.; Rodríguez-Acevedo, B.; Vera, J.L.R.; Resina-Salles, M.; et al. COVID-19 in multiple sclerosis patients: Susceptibility, severity risk factors and serological response. Eur. J. Neurol. 2021, 28, 3384–3395. [Google Scholar] [CrossRef] [PubMed]
- Achiron, A.; Mandel, M.; Dreyer-Alster, S.; Harari, G.; Magalashvili, D.; Sonis, P.; Dolev, M.; Menascu, S.; Flechter, S.; Falb, R.; et al. Humoral immune response to COVID-19 mRNA vaccine in patients with multiple sclerosis treated with high-efficacy disease-modifying therapies. Ther. Adv. Neurol. Disord. 2021, 14, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zabalza, A.; Arrambide, G.; Otero-Romero, S.; Pappolla, A.; Tagliani, P.; López-Maza, S.; Cárdenas-Robledo, S.; Esperalba, J.; Fernández-Naval, C.; Martínez-Gallo, M.; et al. Is humoral and cellular response to SARS-CoV-2 vaccine modified by DMT in patients with multiple sclerosis and other autoimmune diseases? Mult. Scler. 2022, 28, 1138–1145. [Google Scholar] [CrossRef]
- Reder, A.T.; Stuve, O.; Tankou, S.K.; Leist, T.P. T cell responses to COVID-19 infection and vaccination in patients with multiple sclerosis receiving disease-modifying therapy. Mult. Scler. 2023, 29, 648–656. [Google Scholar] [CrossRef]
- Apostolidis, S.A.; Kakara, M.; Painter, M.M.; Goel, R.R.; Mathew, D.; Lenzi, K.; Rezk, A.; Patterson, K.R.; Espinoza, D.A.; Kadri, J.C.; et al. Cellular and humoral immune responses following SARS-CoV-2 mRNA vaccination in patients with multiple sclerosis on anti-CD20 therapy. Nat. Med. 2021, 27, 1990–2001. [Google Scholar] [CrossRef]
- Wherry, E.J.; Barouch, D.H. T cell immunity to COVID-19 vaccines. Science 2022, 377, 821–822. [Google Scholar] [CrossRef] [PubMed]
- De la Maza, S.S.; Walo-Delgado, P.E.; Rodríguez-Domínguez, M.; Monreal, E.; Rodero-Romero, A.; Chico-García, J.L.; Pariente, R.; Rodríguez-Jorge, F.; Ballester-González, R.; Villarrubia, N.; et al. Short- and Long-Term Humoral and Cellular Immune Responses to SARS-CoV-2 Vaccination in Patients with Multiple Sclerosis Treated with Disease-Modifying Therapies. Vaccines 2023, 11, 786. [Google Scholar] [CrossRef]
- Owens, G.P.; Gilden, D.; Burgoon, M.P.; Yu, X.; Bennett, J.L. Viruses and multiple sclerosis. Neuroscientist 2011, 17, 659–676. [Google Scholar] [CrossRef]
- Otero-Romero, S.; Rodríguez-García, J.; Vilella, A.; Ara, J.; Brieva, L.; Calles, C.; Carmona, O.; Casanova, V.; Costa-Frossard, L.; Eichau, S.; et al. Recommendations for vaccination in patients with multiple sclerosis who are eligible for immunosuppressive therapies: Spanish consensus statement. Recomendaciones para la vacunación en pacientes con esclerosis múltiple candidatos a terapias inmunosupresoras: Documento de consenso español. Neurologia 2021, 36, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Gold, R.; Fätkenheuer, G.; Hartung, H.-P.; Kleinschnitz, C.; Marks, R.; Maschke, M.; Bayas, A.; Löbermann, M.; Zettl, U.K.; Wiendl, H. Vaccination in multiple sclerosis patients treated with highly effective disease-modifying drugs: An overview with consideration of cladribine tablets. Ther. Adv. Neurol. Disord. 2021, 14, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Cabreira, V.; Abreu, P.; Soares-Dos-Reis, R.; Guimarães, J.; Sá, M.J. Multiple Sclerosis, Disease-Modifying Therapies and COVID-19: A Systematic Review on Immune Response and Vaccination Recommendations. Vaccines 2021, 9, 773. [Google Scholar] [CrossRef]
- Pušnik, J.; Monzon-Posadas, W.O.; Zorn, J.; Peters, K.; Baum, M.; Proksch, H.; Schlüter, C.B.; Alter, G.; Menting, T.; Streeck, H. SARS-CoV-2 humoral and cellular immunity following different combinations of vaccination and breakthrough infection. Nat. Commun. 2023, 14, 572. [Google Scholar] [CrossRef]
- Bar-Or, A.; Calkwood, J.C.; Chognot, C.; Evershed, J.; Fox, E.J.; Herman, A.; Manfrini, M.; McNamara, J.; Robertson, D.S.; Stokmaier, D.; et al. Effect of ocrelizumab on vaccine responses in patients with multiple sclerosis: The VELOCE study. Neurology 2020, 95, e1999–e2008. [Google Scholar] [CrossRef] [PubMed]
- Liebers, N.; Speer, C.; Benning, L.; Bruch, P.-M.; Kraemer, I.; Meissner, J.; Schnitzler, P.; Kräusslich, H.-G.; Dreger, P.; Mueller-Tidow, C.; et al. Humoral and cellular responses after COVID-19 vaccination in anti-CD20-treated lymphoma patients. Blood 2022, 139, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, V.; Gadi, N.; Spihlman, A.P.; Wu, S.C.; Choi, C.H.; Moulton, V.R. Aging, Immunity, and COVID-19: How Age Influences the Host Immune Response to Coronavirus Infections? Front. Physiol. 2021, 11, 571416. [Google Scholar] [CrossRef] [PubMed]
- Picón, C.; Tejeda-Velarde, A.; Fernández-Velasco, J.I.; Comabella, M.; Álvarez-Lafuente, R.; Quintana, E.; de la Maza, S.S.; Monreal, E.; Villarrubia, N.; Álvarez-Cermeño, J.C.; et al. Identification of the Immunological Changes Appearing in the CSF During the Early Immunosenescence Process Occurring in Multiple Sclerosis. Front. Immunol. 2021, 12, 685139. [Google Scholar] [CrossRef]
- Meca-Lallana, V.; Esparcia-Pinedo, L.; Aguirre, C.; Díaz-Pérez, C.; Gutierrez-Cobos, A.; Sobrado, M.; Carabajal, E.; del Río, B.; Ropero, N.; Villagrasa, R.; et al. Analysis of humoral and cellular immunity after SARS-CoV-2 vaccination in patients with multiple sclerosis treated with immunomodulatory drugs. Clin. Immunol. Commun. 2023, 3, 6–13. [Google Scholar] [CrossRef]
- Conway, S.; Saxena, S.; Baecher-Allan, C.; Krishnan, R.; Houtchens, M.; Glanz, B.; Saraceno, T.J.; Polgar-Turcsanyi, M.; Bose, G.; Bakshi, R.; et al. Preserved T cell but attenuated antibody response in MS patients on fingolimod and ocrelizumab following 2nd and 3rd SARS-CoV-2 mRNA vaccine. Mult. Scler. J. Exp. Transl. Clin. 2023, 9, 1–11. [Google Scholar] [CrossRef]
- Chen, Y.; Klein, S.L.; Garibaldi, B.T.; Li, H.; Wu, C.; Osevala, N.M.; Li, T.; Margolick, J.B.; Pawelec, G.; Leng, S.X. Aging in COVID-19: Vulnerability, immunity and intervention. Ageing Res. Rev. 2021, 65, 101205. [Google Scholar] [CrossRef] [PubMed]
- Laidl Ghadiri, M.; Rezk, A.; Li, R.; Evans, A.; Giacomini, P.S.; Barnett, M.H.; Antel, J.; Bar-Or, A. Pre-treatment T-cell subsets associate with fingolimod treatment responsiveness in multiple sclerosis. Sci. Rep. 2020, 10, 356. [Google Scholar] [CrossRef]

| Characteristics | Total Population (n = 68) |
|---|---|
| Age, median [IQR (years)] | 41.1 [32.9–51.9] |
| Females, n (%) | 42.0 (61.8) |
| Time since first MS Symptoms, median [IQR (years)] | 9.5 [6.1–16.2] |
| MS phenotype, n (%) | |
| Relapsing-remitting | 53 (77.9) |
| Secondary progressive | 9 (13.2) |
| Primary progressive | 6 (8.8) |
| DMT, n (%) | |
| None | 4 (5.9) |
| Pulsed treatments | |
| Alemtuzumab | 13 (19.1) |
| Cladribine | 11 (16.2) |
| Anti-CD20 treatments | |
| Ocrelizumab | 13 (19.1) |
| Rituximab | 8 (11.8) |
| Continuous treatments | |
| Fingolimod | 7 (10.3) |
| Natalizumab | 5 (7.3) |
| Dimethylfumarate | 3 (4.4) |
| Platform (GA, IFN beta, teriflunomide) | 4 (5.9) |
| SARS-CoV-2 vaccine, n (%) | |
| BNT162b2 (Pfizer/BioNTech) | 13 (19.1) |
| mRNA-1273 (Moderna) | 49 (72.0) |
| AZD1222 (AstraZeneca) | 5 (7.4) |
| JNJ78436735 (Johnson & Johnson) | 1 (1.5) |
| DMT | Treatment Duration, Years [Median, IQR] | Time Since Last Infusion, Years [Median, IQR] |
|---|---|---|
| Pulsed Treatments Alemtuzumab Cladribine | 3.5 [2.4–4.0] 0.5 [0.3–1.8] | 1.9 [1.4–2.7] 0.3 [0.2–0.6] |
| Anti-CD20 treatments Ocrelizumab Rituximab | 1.8 [0.8–2.9] 1.8 [0.6–2.6] | 0.3 [0.2–0.4] 0.5 [0.4–0.6] |
| Continuous Treatments Fingolimod Natalizumab Dimethylfumarate Platform (GA, IFN beta, TF) | 6.4 [4.5–7.3] 2.2 [0.2–8.6] 4.9 [3.3–5.3] 6.4 [4.5–7.3] | NA NA NA NA |
| Responders (n = 61) | Non-Responders (n = 7) | p Value | |
|---|---|---|---|
| Age at vaccionation onset (years), median (IQR) | 39.6 (32.3–51.4) | 50.4 (40.4–60.0) | 0.049 |
| Sex (male/female) | 25/36 | 1/6 | NS |
| Disease duration (years), median (IQR) | 12.2 (8.8–21.0) | 9.2 (5.4–15.2) | NS |
| MS phenotype, n (%) Relapsing-remitting Secondary progressive Primary progressive | 48 (78.7) 8 (13.1) 5 (8.2) | 5 (71.4) 1 (14.3) 1 (14.3) | NS |
| DMT (n) | 0.0013 | ||
| Pulsed treatments | |||
| Alemtuzumab | 13 | 0 | |
| Cladribine | 10 | 1 | |
| Anti-CD20 treatments | |||
| Ocrelizumab | 12 | 1 | |
| Rituximab | 7 | 1 | |
| Continuous treatments | |||
| Fingolimod | 3 | 4 | |
| Natalizumab | 5 | 0 | |
| Dimethylfumarate | 3 | 0 | |
| Platform (GA, IFN beta, TF) | 4 | 0 | |
| None | 4 | 0 |
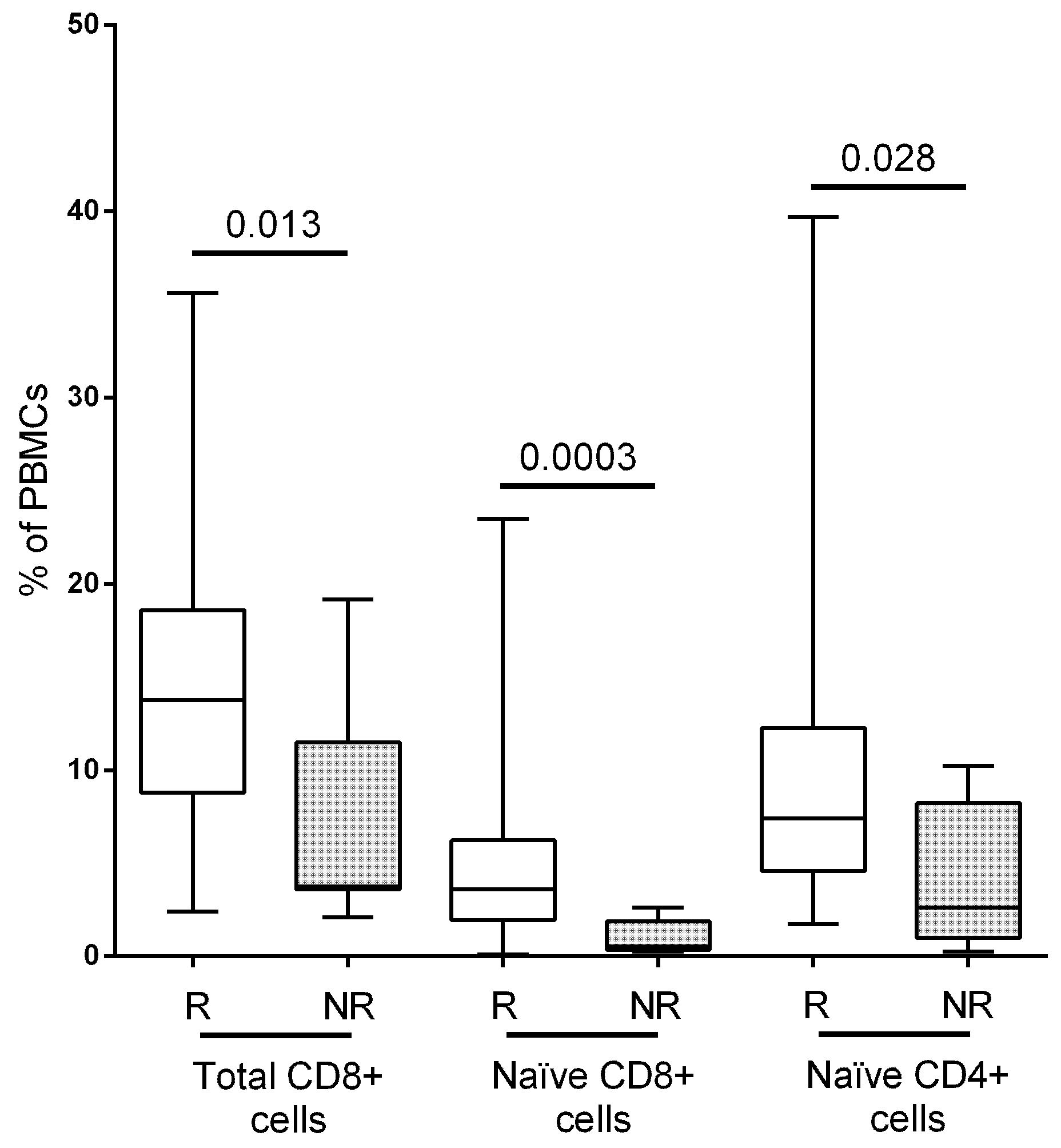
| Cell Subset | Responders (n = 67) (%, Median, IQR) | Non-Responders (n = 7) (%, Median, IQR) | p Value | Responders (n = 67) (AN/µL, Median, IQR) | Non-Responders (n = 7) (AN/µL, Median, IQR) | p Value |
|---|---|---|---|---|---|---|
| Monocytes | 14.5 (9.6 18.1) | 31.6 (15.7–41.2) | 0.0036 | 453 (102–540) | 1250 (525–1950) | 0.023 |
| Lymphocytes | 85.9 (81.9–90.2) | 68.3 (58.8–84.3) | 0.0043 | 1680 (955–2145) | 640 (500–1540) | 0.008 |
| CD4+ T-cells | 40.2 (30.3–50.9) | 10.1 (6.5–51.2) | 0.138 | |||
| Naïve | 7.4 (4.6–12.3) | 2.6 (1–8.2) | 0.028 | 124 (71.5–292.5) | 28.3 (9.3–188.7) | 0.019 |
| CM | 12.7 (5.5–26.7) | 1.9 (1.2–40.2) | 0.191 | |||
| EM | 5.9 (3.7–7.8) | 3.2 (1.2–4.8) | 0.056 | |||
| TD | 5.8 (3.3–8.7) | 4.7 (2.4–11.2) | 0.834 | |||
| Treg | 0.6 (0.3–2.5) | 1.4 (0.4–2.6) | 0.465 | |||
| TIGIT+ | 0.6 (0.4–0.9) | 0.3 (0.1–1.1) | 0.237 | |||
| Senescent | 1.2 (0.7–1.9) | 1.1 (0.2–2.1) | 0.369 | |||
| TIM3+ | 0.7 (0.4–1.3) | 1.0 (0.7–1.4) | 0.503 | |||
| CD8+ T-cells | 13.8 (8.8–18.6) | 3.8 (3.6–11.5) | 0.0135 | 231 (158–449) | 77.5 (34.6–132.9) | 0.0019 |
| Naïve | 3.6 (1.9–6.2) | 0.6 (0.4–1.9) | 0.0003 | 73.6 (32.5–133.1) | 7.7 (3.8–15.9) | 0.0003 |
| CM | 0.4 (0.2–0.7) | 0.3 (0.1–0.5) | 0.210 | |||
| EM | 1.8 (0.8–3.3) | 0.7 (0.4–2.5) | 0.156 | |||
| TD | 6 (4.1–8.8) | 2 (1.2–7.9) | 0.075 | |||
| TIGIT+ | 0.3 (0.2–0.7) | 0.2 (0.1–0.5) | 0.325 | |||
| Senescent | 1.9 (0.8–4) | 1.9 (0.3–5.8) | 0.814 | |||
| TIM3+ | 2.1 (0.1–3.6) | 1.9 (1.1–4.7) | 0.526 | |||
| CD19+ cells | 3.9 (0.1–9.6) | 1.7 (1.4–5.1) | 0.296 | |||
| Naïve | 3.3 (0.1–8.3) | 1.3 (0.9–4.2) | 0.251 | |||
| Memory | 0.4 (0.1–1) | 0.2 (0.1–0.3) | 0.300 | |||
| PBs | 0.01 (0–0.02) | 0.01 (0.01–0.03) | 0.390 | |||
| TR | 0.08 (0.01–0.2) | 0.03 (0.22–0.65) | 0.150 | |||
| NK cells | ||||||
| NKT cells | 1.7 (0.7–2.9) | 1.5 (0.8–8.7) | 0.616 | |||
| Bright | 1.1 (0.5–1.7) | 0.8 (0.7–1.3) | 0.558 | |||
| Dim | 12 (8.1–19) | 18.8 (10.9–25.2) | 0.081 | |||
| DCs | ||||||
| PC | 0.16 (0.1–0.3) | 0.9 (0.2–1) | 0.0132 | 3.1 (1.4–5.2) | 7.2 (4.1–8.8) | 0.108 |
| Myeloid | 0.3 (0.1–1.7) | 3.3 (0.2–4.3) | 0.0075 | 5.5 (2.5–31.1) | 4.9 (3.4–13.5) | 0.701 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodero-Romero, A.; Sainz de la Maza, S.; Fernández-Velasco, J.I.; Monreal, E.; Walo-Delgado, P.E.; Chico-García, J.L.; Villarrubia, N.; Rodríguez-Jorge, F.; Rodríguez-Ramos, R.; Masjuan, J.; et al. Blood CD8+ Naïve T-Cells Identify MS Patients with High Probability of Optimal Cellular Response to SARS-CoV-2 Vaccine. Vaccines 2023, 11, 1399. https://doi.org/10.3390/vaccines11091399
Rodero-Romero A, Sainz de la Maza S, Fernández-Velasco JI, Monreal E, Walo-Delgado PE, Chico-García JL, Villarrubia N, Rodríguez-Jorge F, Rodríguez-Ramos R, Masjuan J, et al. Blood CD8+ Naïve T-Cells Identify MS Patients with High Probability of Optimal Cellular Response to SARS-CoV-2 Vaccine. Vaccines. 2023; 11(9):1399. https://doi.org/10.3390/vaccines11091399
Chicago/Turabian StyleRodero-Romero, Alexander, Susana Sainz de la Maza, José Ignacio Fernández-Velasco, Enric Monreal, Paulette Esperanza Walo-Delgado, Juan Luis Chico-García, Noelia Villarrubia, Fernando Rodríguez-Jorge, Rafael Rodríguez-Ramos, Jaime Masjuan, and et al. 2023. "Blood CD8+ Naïve T-Cells Identify MS Patients with High Probability of Optimal Cellular Response to SARS-CoV-2 Vaccine" Vaccines 11, no. 9: 1399. https://doi.org/10.3390/vaccines11091399
APA StyleRodero-Romero, A., Sainz de la Maza, S., Fernández-Velasco, J. I., Monreal, E., Walo-Delgado, P. E., Chico-García, J. L., Villarrubia, N., Rodríguez-Jorge, F., Rodríguez-Ramos, R., Masjuan, J., Costa-Frossard, L., & Villar, L. M. (2023). Blood CD8+ Naïve T-Cells Identify MS Patients with High Probability of Optimal Cellular Response to SARS-CoV-2 Vaccine. Vaccines, 11(9), 1399. https://doi.org/10.3390/vaccines11091399






