Understanding the Impact of Vaccination and Self-Defense Measures on Epidemic Dynamics Using an Embedded Optimization and Evolutionary Game Theory Methodology
Abstract
:1. Introduction
2. Model and Methods
Derivation of Basic and Effective Reproduction Number
3. Statement of the Optimal Control Problem
4. Evolutionary Dynamics
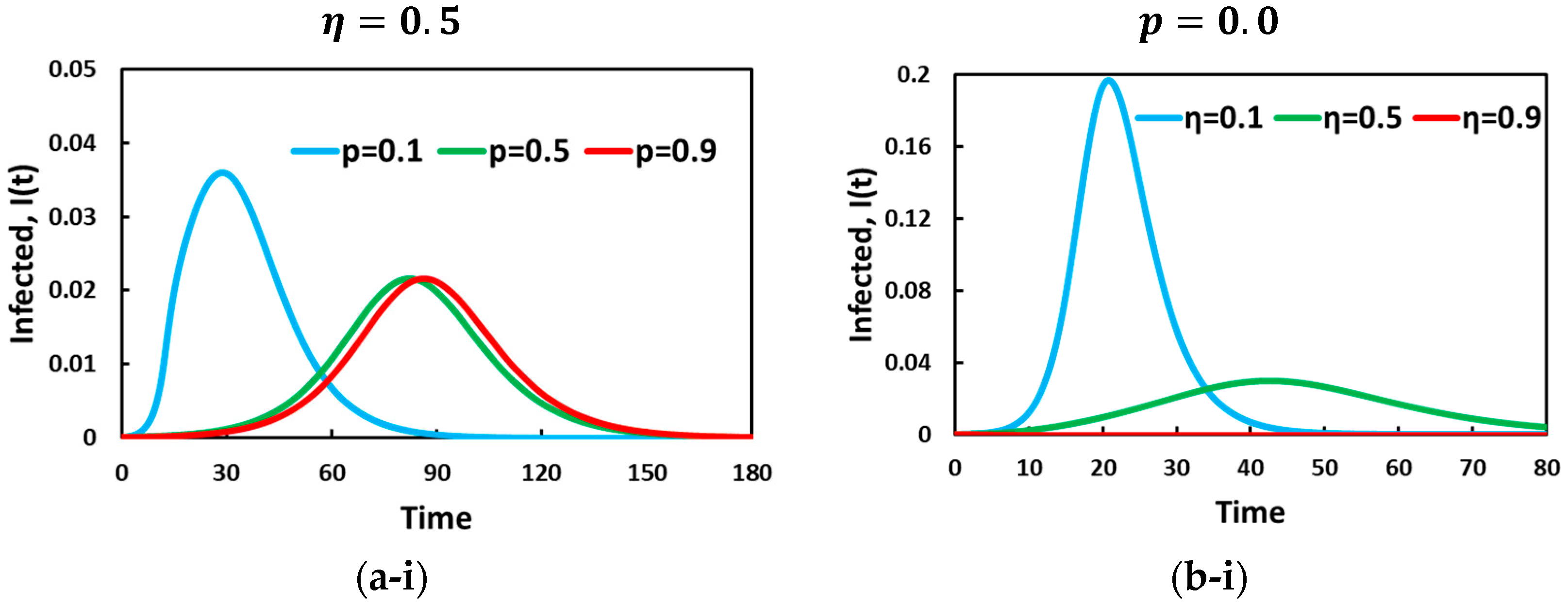
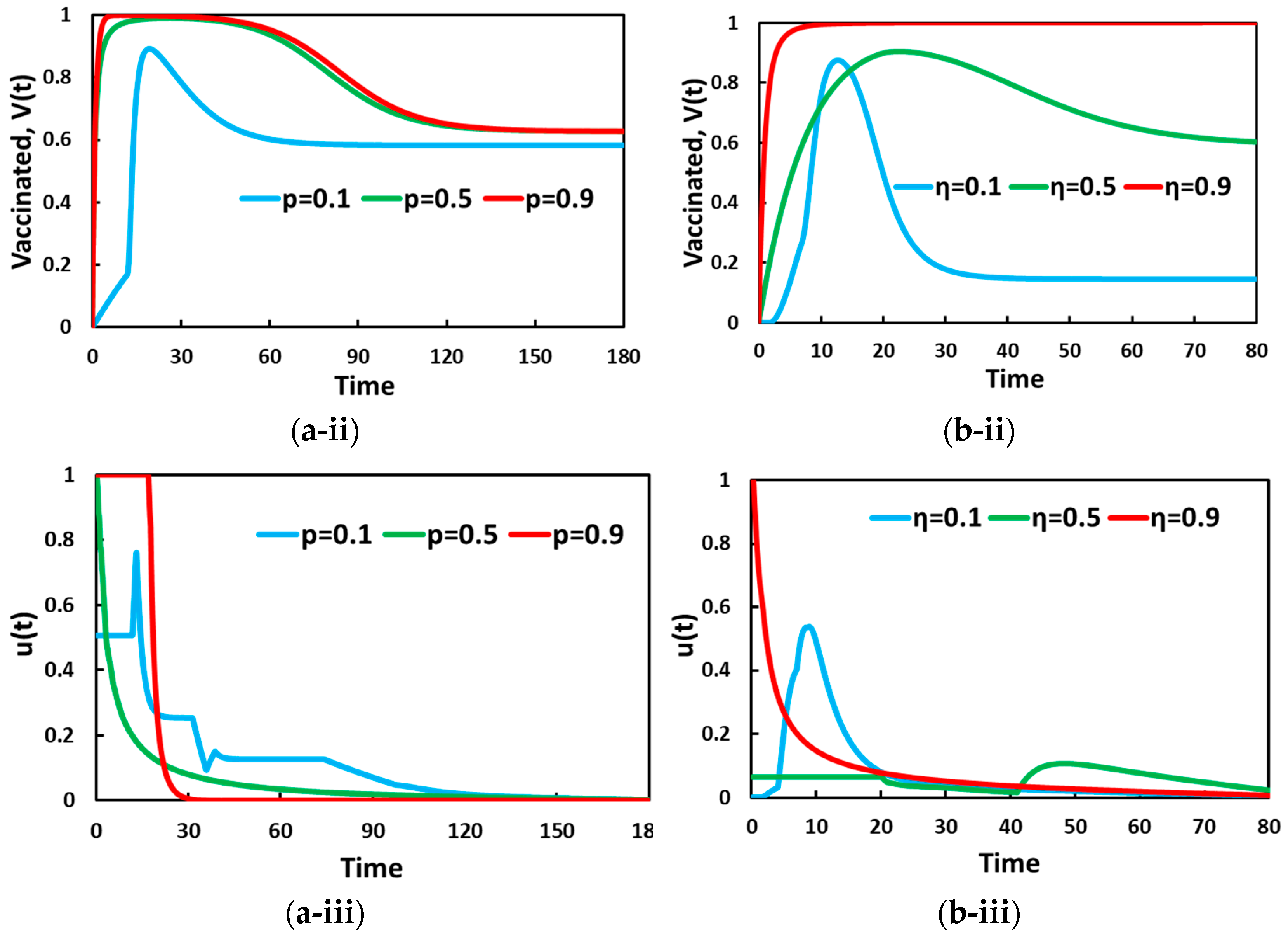
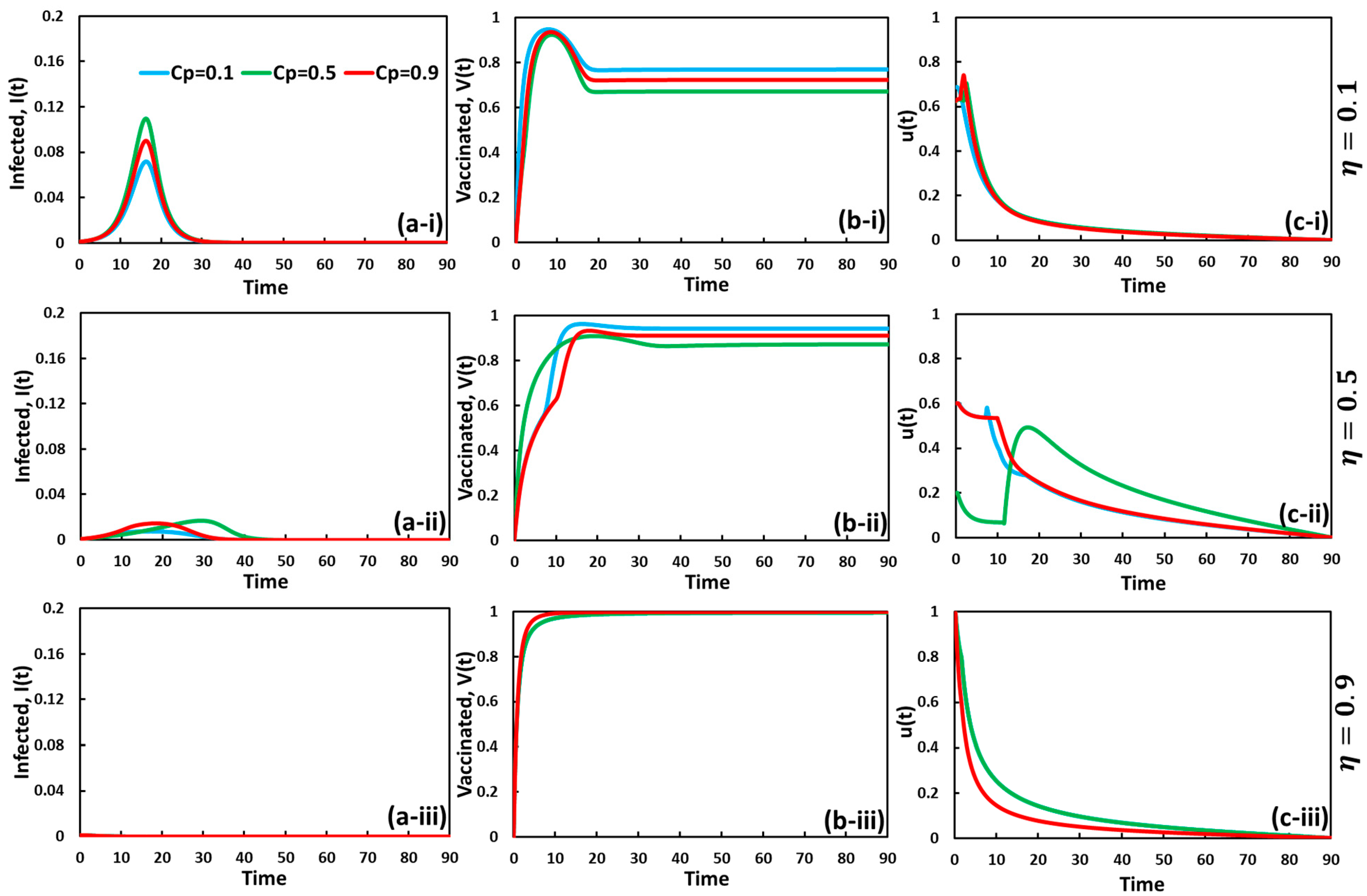
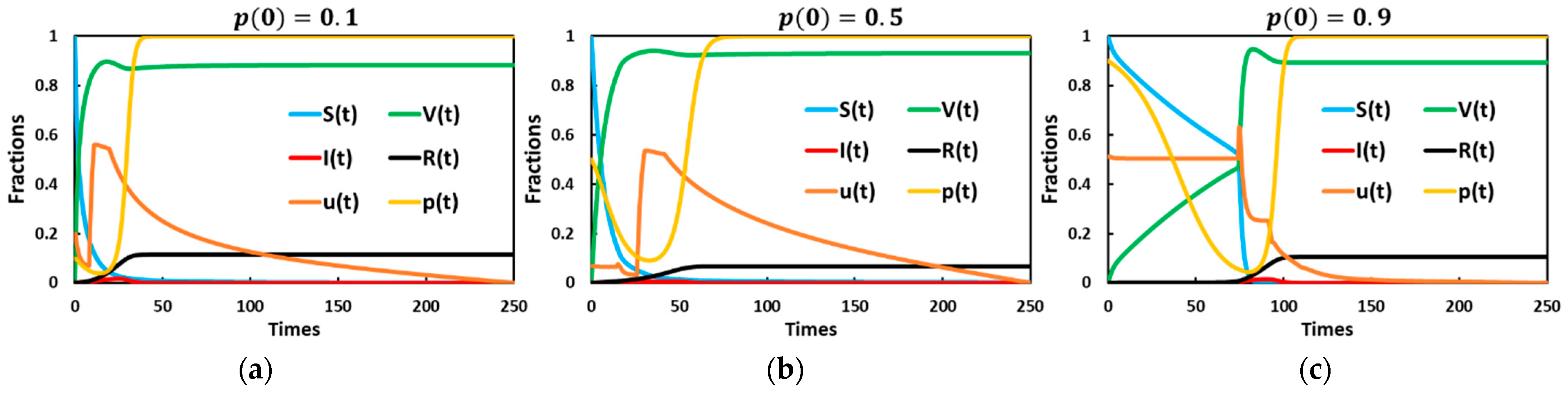
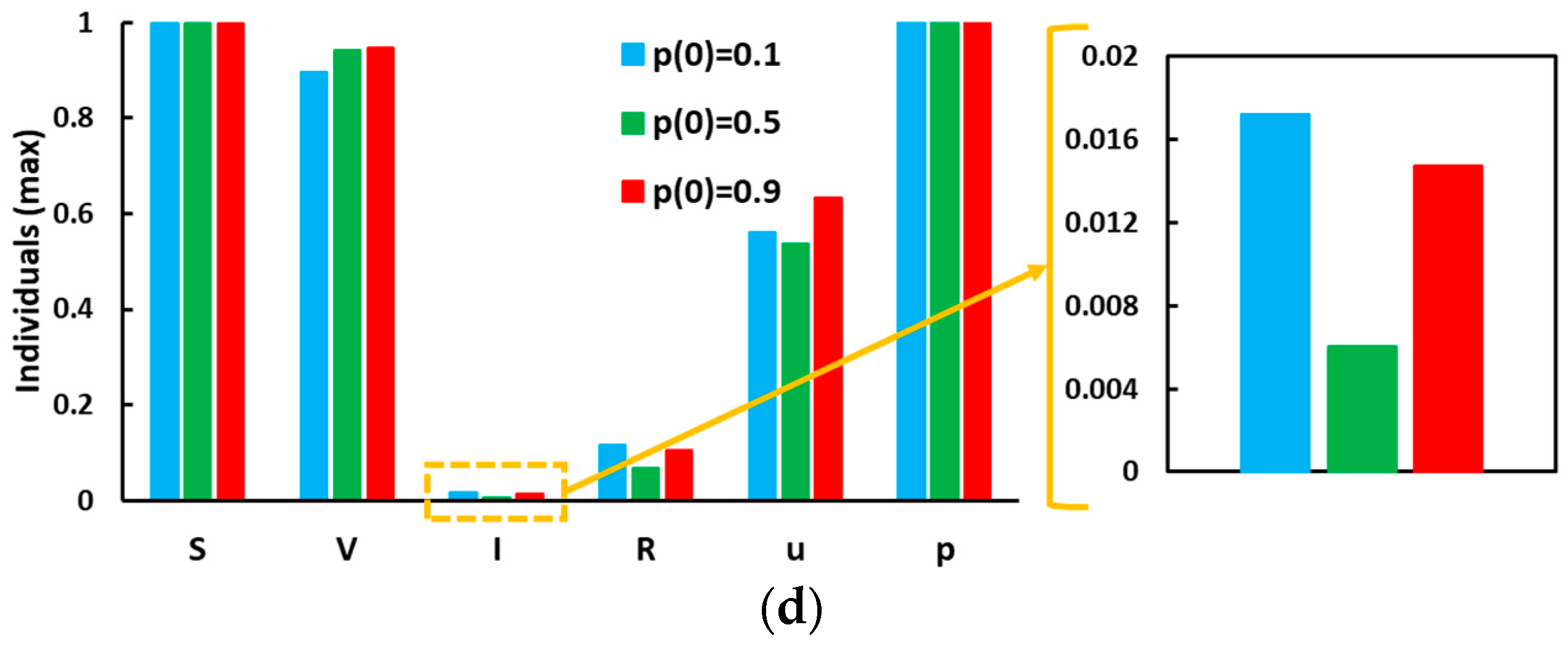
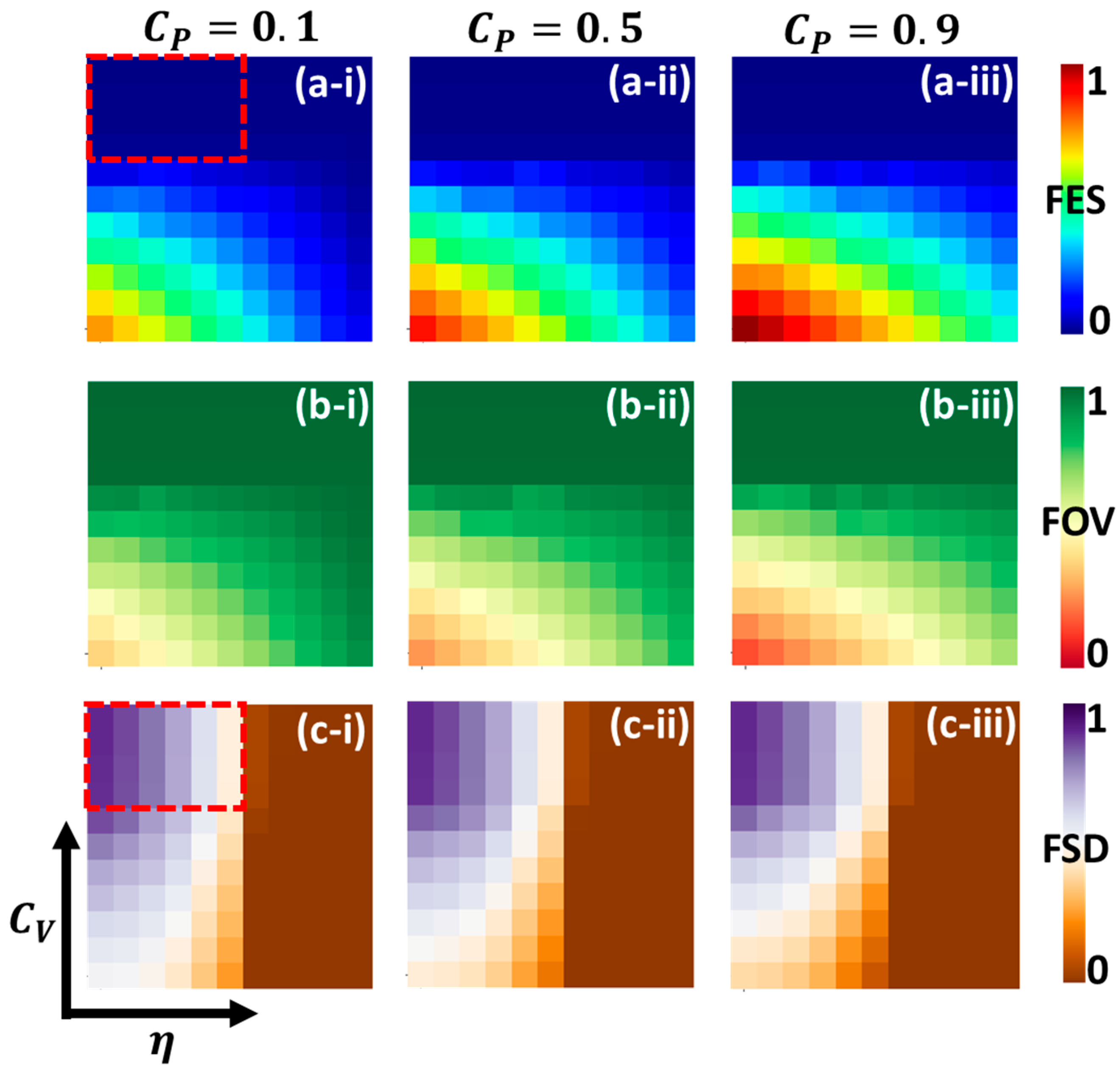
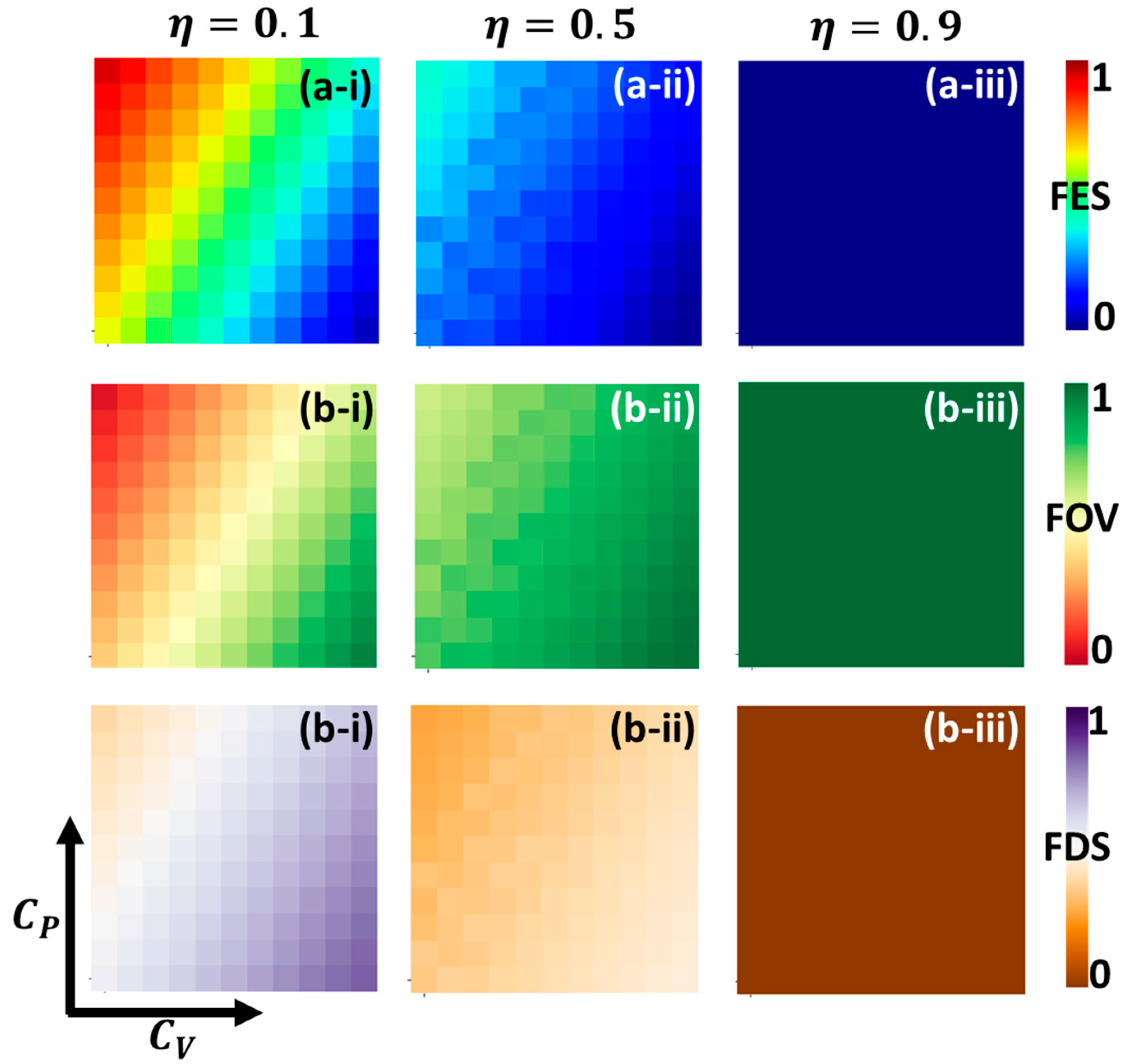
5. Result and Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Madhav, N.; Oppenheim, B.; Gallivan, M.; Mulembakani, P.; Rubin, E.; Wolfe, N. Pandemics: Risks, Impacts, and Mitigation. In Disease Control Priorities: Improving Health and Reducing Poverty, 3rd ed.; Jamison, D.T., Gelband, H., Horton, S., Eds.; The International Bank for Reconstruction and Development/The World Bank: Washington, DC, USA, 2017. [Google Scholar]
- Wang, X.; An, Q.; He, Z.; Fang, W. A Literature Review of Social Network Analysis in Epidemic Prevention and Control. Complexity 2021, 2021, 3816221. [Google Scholar] [CrossRef]
- Thomas, K.; Costas, P.D.; Nikolaos, N.; Rachaniotis, P. Epidemics control and logistics operations: A review. Int. J. Prod. Econ. 2012, 139, 393–410. [Google Scholar]
- Zhang, H.; Yang, Z.; Kasia, A.; Pawelek Liu, S. Optimal control strategies for a two-group epidemic model with vaccination-resource constraints. Appl. Math. Comput. 2020, 371, 124956. [Google Scholar] [CrossRef]
- Xue-Zhi, L.; Wang, J.; Ghosh, M. Stability and bifurcation of an SIVS epidemic model with treatment and age of vaccination. Appl. Math. Model. 2010, 34, 437–450. [Google Scholar]
- Feng, Z.; Towers, S.; Yang, Y. Modeling the effects of vaccination and treatment on pandemic influenza. AAPS J. 2011, 13, 427–437. [Google Scholar] [CrossRef]
- Aronna, M.S.; Guglielmi, R.; Moschen, L.M. A model for COVID-19 with isolation, quarantine and testing as control measures. Epidemics 2021, 34, 100437. [Google Scholar] [CrossRef]
- Amaral, M.A.; de Oliveira, M.M.; Javarone, M.A. An epidemiological model with voluntary quarantine strategies governed by evolutionary game dynamics. Chaos Solitons Fractals 2021, 143, 110616. [Google Scholar] [CrossRef]
- Chowdhury, A.R.; Kabir, K.M.A.; Tanimoto, J. How quarantine and social distancing policy can suppress the outbreak of novel coronavirus in developing or under poverty level countries: A mathematical and statistical analysis. Biom. Biostat. Int. J. 2021, 10, 145–152. [Google Scholar]
- Kabir, K.M.A.; Risa, T.; Tanimoto, J. Prosocial behavior of wearing a mask during an epidemic: An evolutionary explanation. Sci. Rep. 2021, 11, 12621. [Google Scholar] [CrossRef]
- Alam, M.; Kabir, K.M.A.; Tanimoto, J. Based on mathematical epidemiology and evolutionary game theory, which is more effective: Quarantine or isolation policy? J. Stat. Mech. 2020, 2020, 033502. [Google Scholar] [CrossRef]
- Kermack, W.O.; McKendrick, A.G. A contribution to the mathematical theory of epidemics. Proc. Roy Soc. Lond. Ser. A 1927, 115, 700–721. [Google Scholar]
- Joshi, H.R. Optimal Control of an HIV Immunology Model. Optim. Control Appl. Methods 2002, 23, 199–213. [Google Scholar] [CrossRef]
- Fleming, W.H.; Rishel, R.W. Deterministic and Stochastic Optimal Control; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1975; Volume 1. [Google Scholar]
- Lenhart, S.; Wortman, J. Optimal Control Applied to Biological Models; Taylor & Francis: Boca Raton, FL, USA, 2007. [Google Scholar]
- Zaman, G.; Kang, Y.; Jung, I. Stability analysis and optimal vaccination of an sir epidemic model. BioSystems 2008, 93, 240–249. [Google Scholar] [CrossRef]
- Kirschner, D.; Lenhart, S.; Serbin, S. Optimal control of the chemotherapy of HIV. J. Math. Biol. 1997, 35, 775–792. [Google Scholar] [CrossRef] [PubMed]
- Fister, K.R.; Donnelly, J.H. Immunotherapy: An optimal control theory approach. Math. Biosci. Eng. 2005, 2, 499–510. [Google Scholar] [CrossRef]
- Gaff, H.; Schaefer, E. Optimal control applied to vaccination and treatment strategies for various epidemiological models. Math. Biosci. Eng. 2009, 6, 469–492. [Google Scholar] [PubMed]
- Abta, A.; Laarabi, H.; Alaoui, H.T. The hopf bifurcation analysis and optimal control of a delayed SIR epidemic model. Int. J. Anal. 2014, 2014, 940819. [Google Scholar] [CrossRef]
- Torres, D.F.M.; Silva, C.J. Optimal control strategies for tuberculosis treatment: A case study in angola. Numer. Algebra Control Optim. 2012, 2, 601–617. [Google Scholar] [CrossRef]
- Hattaf, K.; Yousfi, N. Optimal control of a delayed HIV infection model with immune response using an efficient numerical method. Int. Sch. Res. Netw. 2012, 2012, 215124. [Google Scholar] [CrossRef]
- Boklund, A.; Toft, N.; Alban, L.; Uttenthal, A. Comparing the epidemiological and economic effects of control strategies against classical swine fever in Denmark. Prev. Vet. Medi. 2009, 90, 180–193. [Google Scholar] [CrossRef]
- Aldila, D.; Götz, T.; Soewono, E. An optimal control problem arising from a dengue disease transmission model. Math. Biosci. 2013, 242, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Small, M.; Chen, G. Epidemic Control and Awareness. In Propagation Dynamics on Complex Networks: Models, Methods and Stability Analysis; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014. [Google Scholar]
- Fukuda, E.; Tanimoto, J.; Akimoto, M. Influence of breaking the symmetry between disease transmission and information propagation network on stepwise decisions concerning vaccination. Chaos Solitons Fractals 2015, 80, 47–55. [Google Scholar] [CrossRef]
- Kabir, K.A.; Kuga, K.; Tanimoto, J. Analysis of SIR epidemic model with information spreading of awareness. Chaos Solitons Fractals 2019, 119, 118–125. [Google Scholar] [CrossRef]
- Kuga, K.; Tanimoto, J. Which is more effective for suppressing an infectious disease: Imperfect vaccination or defense against contagion? J. Stat. Mech. 2018, 2, 023407. [Google Scholar] [CrossRef]
- Tanimoto, J. Evolutionary Games with Sociophysics: Analysis of Traffic Flow and Epidemics; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Tanimoto, J. Fundamentals of Evolutionary Game Theory and Its Applications; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Tanimoto, J.; Futamata, M.; Tanaka, M. Automated vehicle control systems need to solve social dilemmas to be disseminated. Chaos Solitons Fractals 2020, 138, 109861. [Google Scholar] [CrossRef]
- Bauch, C.T.; Bhattacharyya, S. Evolutionary game theory and social learning can determine how vaccine scares unfold. PLoS Comput. Biol. 2012, 8, e1002452. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Bauch, C.T.; Breban, R. Role of word-of-mouth for programs of voluntary vaccination: A game-theoretic approach. Math. Biosci. 2015, 269, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Caley, P.; Philp, D.J.; McCracken, K. Quantifying social distancing arising from pandemic influenza. J. R. Soc. Interface 2008, 5, 631–639. [Google Scholar] [CrossRef]
- Reluga, T.C. Game theory of social distancing in response to an epidemic. PLoS Comput. Biol. 2010, 6, e1000793. [Google Scholar] [CrossRef]
- Kabir, K.A.; Kuga, K.; Tanimoto, J. Effect of information spreading to suppress the disease contagion on the epidemic vaccination game. Chaos Solitons Fractals 2019, 119, 180–187. [Google Scholar] [CrossRef]
- ALAM, M.; Kuga, K.; Tanimoto, J. Three-strategy and four-strategy model of vaccination game introducing an intermediate protecting measure. Appl. Math. Comput. 2019, 346, 408–422. [Google Scholar] [CrossRef]
- Iwamura, Y.; Tanimoto, J.; Fukuda, E. Effect of intermediate defense measures in voluntary vaccination games. J. Stat. Mech. Theory Exp. 2016, 2016, 093501. [Google Scholar] [CrossRef]
- Arefin, M.R.; Tanaka, M.; Kabir, K.M.A.; Tanimoto, J. Interplay between cost and effectiveness in influenza vaccination uptake: Vaccination game approach. Proc. R. Soc. A 2019, 475, 20190608. [Google Scholar] [CrossRef] [PubMed]
- Kabir, K.M.A.; Tanimoto, J. Modelling and analyzing the coexistence of dual dilemmas in the proactive vaccination game and retroactive treatment game in epidemic viral dynamics. Proc. R. Soc. A 2019, 475, 20190484. [Google Scholar] [CrossRef] [PubMed]
- Kabir, K.M.A.; Jusup, M.; Tanimoto, J. Behavioral incentives in a vaccination-dilemma setting with optional treatment. Phys. Rev. E 2019, 100, 062402. [Google Scholar] [CrossRef]
- Chang, S.L.; Piraveenan, M.; Pattison, P.; Prokopenko, M. Game theoretic modelling of infectious disease dynamics and intervention methods: A review. J. Biol. Dyn. 2020, 14, 57–89. [Google Scholar] [CrossRef]
- Kabir, K.M.A. Impact of human cooperation on vaccination behaviors. Heliyon 2023, 9, e16748. [Google Scholar] [CrossRef]
- Perc, M.; Szolnoki, A. Coevolutionary games—A mini review. Biosystems 2010, 99, 109–125. [Google Scholar] [CrossRef]
- Wang, Z.; Bauch, C.T.; Bhattacharyya, S.; d’Onofrio, A.; Manfredi, P.; Perc, M.; Perra, N.; Salathé, M.; Zhao, D. Statistical physics of vaccination. Phys. Rep. 2016, 664, 1–13. [Google Scholar] [CrossRef]
- Kabir, K.M.A.; Tanimoto, J. Evolutionary game theory modelling to represent the behavioral dynamics of economic shutdowns and shield immunity in the COVID-19 pandemic. R. Soc. Open Sci. 2020, 7, 201095. [Google Scholar] [CrossRef]
- Li, T.; Guo, Y. Optimal control and cost-effectiveness analysis of a new COVID-19 model for Omicron strain. Phys. A Stat. Mech. Its Appl. 2022, 606, 128134. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, T.T.; Abidemi, A. Effective strategies towards eradicating the tuberculosis epidemic: An optimal control theory alternative. Healthc. Anal. 2022, 3, 100131. [Google Scholar] [CrossRef]
- Ojo, M.M.; Benson, T.O.; Peter, O.J.; Goufo, E.F.D. Nonlinear optimal control strategies for a mathematical model of COVID-19 and influenza co-infection. Phys. A Stat. Mech. Its Appl. 2022, 607, 128173. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Jian, S.; Gao, J. Dynamics analysis and optimal control of SIVR epidemic model with incomplete immunity. Adv. Contin. Discret. Models 2022, 2022, 51. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kabir, K.M.A.; Islam, M.S.; Sharif Ullah, M. Understanding the Impact of Vaccination and Self-Defense Measures on Epidemic Dynamics Using an Embedded Optimization and Evolutionary Game Theory Methodology. Vaccines 2023, 11, 1421. https://doi.org/10.3390/vaccines11091421
Kabir KMA, Islam MS, Sharif Ullah M. Understanding the Impact of Vaccination and Self-Defense Measures on Epidemic Dynamics Using an Embedded Optimization and Evolutionary Game Theory Methodology. Vaccines. 2023; 11(9):1421. https://doi.org/10.3390/vaccines11091421
Chicago/Turabian StyleKabir, K. M. Ariful, MD Shahidul Islam, and Mohammad Sharif Ullah. 2023. "Understanding the Impact of Vaccination and Self-Defense Measures on Epidemic Dynamics Using an Embedded Optimization and Evolutionary Game Theory Methodology" Vaccines 11, no. 9: 1421. https://doi.org/10.3390/vaccines11091421






