COVID-19 Bivalent Booster in Pregnancy: Maternal and Neonatal Antibody Response to Omicron BA.5, BQ.1, BF.7 and XBB.1.5 SARS-CoV-2
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. The Study Scheme for Collection of Samples and Clinical Data
2.3. Neutralizing Antibody (Nab) Inhibition Test of SARS-CoV-2 Omicron BA.5, BF.7, BQ.1, and XBB.1.5 Variants
2.4. Statistics
3. Results
3.1. Participants Characteristics
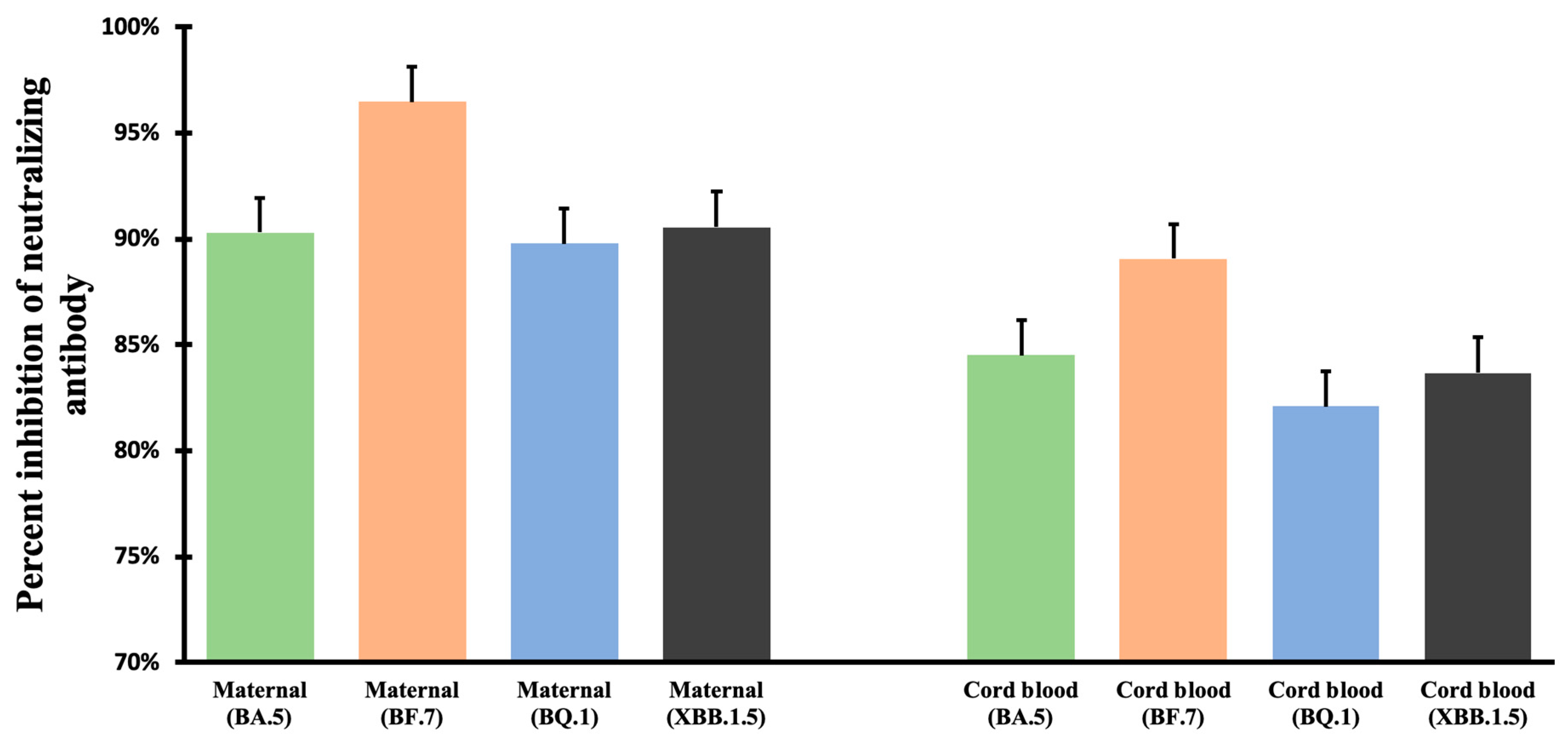
3.2. Neutralizing Antibody Inhibition Rates
3.3. Impact of Interval between Bivalent COVID-19 Vaccine and Childbirth to Neutralizing Antibody Inhibition Rates
3.4. Impact of Interval between the Third Dose COVID-19 Vaccine and Spikevax Bivalent Vaccine on Neutralizing Antibody Inhibition Rates
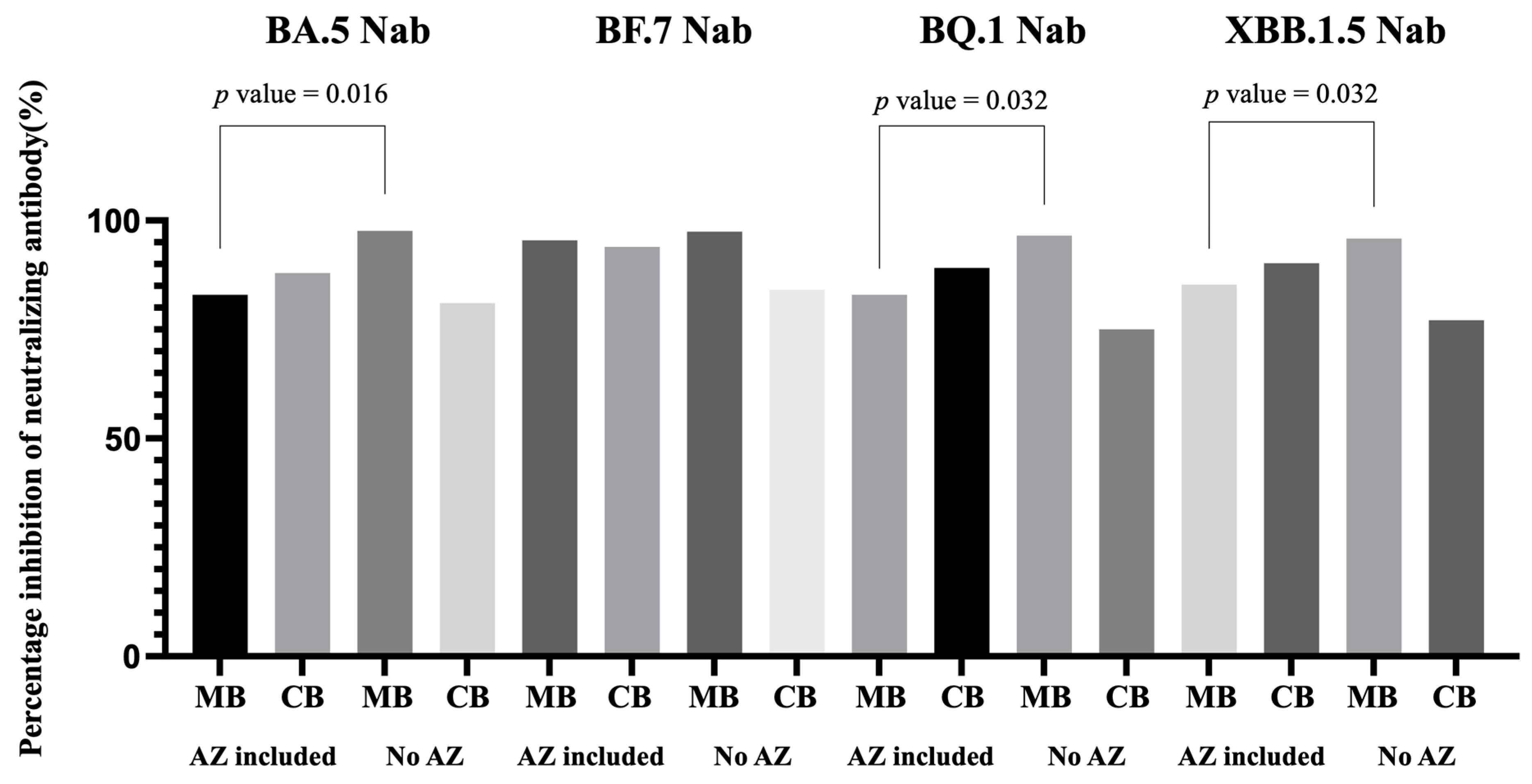
3.5. Impact of Previous COVID-19 Vaccine Combinations to Neutralizing Antibody Inhibition Rates
3.6. Maternal and Neonatal Clinical Factors and Neutralizing Antibody Inhibition Rates
4. Discussion
4.1. The Effectiveness of Bivalent COVID-19 Vaccination
4.2. The Bivalent COVID-19 Vaccination for Pregnant Women and the Neonates
4.3. Different Factors Related to Neutralizing Antibody Inhibition after Bivalent COVID-19 Vaccination in Pregnant Women
4.4. Clinical Significance of Our Current Study
4.5. Limitation of Our Current Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, R.; Zhao, X.; Li, J.; Niu, P.; Yang, B.; Wu, H.; Wang, W.; Song, H.; Huang, B.; Zhu, N.; et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet 2020, 395, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Butt, A.A.; Omer, S.B.; Yan, P.; Shaikh, O.S.; Mayr, F.B. SARS-CoV-2 Vaccine Effectiveness in a High-Risk National Population in a Real-World Setting. Ann. Intern. Med. 2021, 174, 1404–1408. [Google Scholar] [CrossRef]
- Paris, C.; Perrin, S.; Hamonic, S.; Bourget, B.; Roué, C.; Brassard, O.; Tadié, E.; Gicquel, V.; Bénézit, F.; Thibault, V.; et al. Effectiveness of mRNA-BNT162b2, mRNA-1273, and ChAdOx1 nCoV-19 vaccines against COVID-19 in healthcare workers: An observational study using surveillance data. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2021, 27, 1699.e5–1699.e8. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.Q.; Bilodeau-Bertrand, M.; Liu, S.; Auger, N. The impact of COVID-19 on pregnancy outcomes: A systematic review and meta-analysis. CMAJ Can. Med. Assoc. J. 2021, 193, E540–E548. [Google Scholar] [CrossRef]
- Zimmermann, P.; Curtis, N. COVID-19 in Children, Pregnancy and Neonates: A Review of Epidemiologic and Clinical Features. Pediatr. Infect. Dis. J. 2020, 39, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Gurol-Urganci, I.; Jardine, J.E.; Carroll, F.; Draycott, T.; Dunn, G.; Fremeaux, A.; Harris, T.; Hawdon, J.; Morris, E.; Muller, P.; et al. Maternal and perinatal outcomes of pregnant women with SARS-CoV-2 infection at the time of birth in England: National cohort study. Am. J. Obstet. Gynecol. 2021, 225, 522.e1–522.e11. [Google Scholar] [CrossRef]
- Trostle, M.E.; Aguero-Rosenfeld, M.E.; Roman, A.S.; Lighter, J.L. High antibody levels in cord blood from pregnant women vaccinated against COVID-19. Am. J. Obstet. Gynecol. MFM 2021, 3, 100481. [Google Scholar] [CrossRef] [PubMed]
- Nir, O.; Schwartz, A.; Toussia-Cohen, S.; Leibovitch, L.; Strauss, T.; Asraf, K.; Doolman, R.; Sharabi, S.; Cohen, C.; Lustig, Y.; et al. Maternal-neonatal transfer of SARS-CoV-2 immunoglobulin G antibodies among parturient women treated with BNT162b2 messenger RNA vaccine during pregnancy. Am. J. Obstet. Gynecol. MFM 2022, 4, 100492. [Google Scholar] [CrossRef]
- Yang, Y.J.; Murphy, E.A.; Singh, S.; Sukhu, A.C.; Wolfe, I.; Adurty, S.; Eng, D.; Yee, J.; Mohammed, I.; Zhao, Z.; et al. Association of Gestational Age at Coronavirus Disease 2019 (COVID-19) Vaccination, History of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection, and a Vaccine Booster Dose with Maternal and Umbilical Cord Antibody Levels at Delivery. Obstet. Gynecol. 2022, 139, 373–380. [Google Scholar] [CrossRef]
- Romero-Ibarguengoitia, M.E.; Flores-Salazar, Z.L.; Arroyo-García, K.D.; Soto-Gámez, R.; Leal-Meléndez, J.A.; René Garza-Herrera, M.; Bennett-Vidales, G.; Cabrera, M.H.; González-Habib, R.; Jiménez, L.P.; et al. Evaluation of Transplacental Antibody Transfer in Pregnant Women Immunized with Different SARS-CoV-2 Homologous or Heterologous Schemes. Vaccines 2023, 11, 415. [Google Scholar] [CrossRef]
- Zilver, S.J.M.; de Groot, C.J.M.; Grobben, M.; Remmelzwaal, S.; Burgers, E.; Velasco, D.N.; Juncker, H.G.; van Keulen, B.J.; van Goudoever, J.B.; de Leeuw, R.A.; et al. Vaccination from the early second trimester onwards gives a robust SARS-CoV-2 antibody response throughout pregnancy and provides antibodies for the neonate. Int. J. Infect. Dis. 2023, 130, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.J.; Fu, Y.C.; Lin, Y.P.; Shen, C.F.; Sun, D.J.; Chen, H.Y.; Cheng, C.M. Evaluation of Transplacental Antibody Transfer in SARS-CoV-2-Immunized Pregnant Women. Vaccines 2022, 10, 101. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.C.; Lin, Y.P.; Cheng, C.M.; Shen, C.F.; Ching, A.; Chang, T.C.; Shen, C.J. Antibodies against SARS-CoV-2 Alpha, Beta, and Gamma Variants in Pregnant Women and Their Neonates under Antenatal Vaccination with Moderna (mRNA-1273) Vaccine. Vaccines 2022, 10, 1415. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Guo, Y.; Iketani, S.; Nair, M.S.; Li, Z.; Mohri, H.; Wang, M.; Yu, J.; Bowen, A.D.; Chang, J.Y.; et al. Antibody evasion by SARS-CoV-2 Omicron subvariants BA.2.12.1, BA.4 and BA.5. Nature 2022, 608, 603–608. [Google Scholar] [CrossRef]
- Choi, A.; Koch, M.; Wu, K.; Chu, L.; Ma, L.; Hill, A.; Nunna, N.; Huang, W.; Oestreicher, J.; Colpitts, T.; et al. Safety and immunogenicity of SARS-CoV-2 variant mRNA vaccine boosters in healthy adults: An interim analysis. Nat. Med. 2021, 27, 2025–2031. [Google Scholar] [CrossRef]
- Tseng, H.F.; Ackerson, B.K.; Bruxvoort, K.J.; Sy, L.S.; Tubert, J.E.; Lee, G.S.; Ku, J.H.; Florea, A.; Luo, Y.; Qiu, S.; et al. Effectiveness of mRNA-1273 vaccination against SARS-CoV-2 omicron subvariants BA.1, BA.2, BA.2.12.1, BA.4, and BA.5. Nat. Commun. 2023, 14, 189. [Google Scholar] [CrossRef]
- Chalkias, S.; Eder, F.; Essink, B.; Khetan, S.; Nestorova, B.; Feng, J.; Chen, X.; Chang, Y.; Zhou, H.; Montefiori, D.; et al. Safety, immunogenicity and antibody persistence of a bivalent Beta-containing booster vaccine against COVID-19: A phase 2/3 trial. Nat. Med. 2022, 28, 2388–2397. [Google Scholar] [CrossRef]
- Chalkias, S.; Harper, C.; Vrbicky, K.; Walsh, S.R.; Essink, B.; Brosz, A.; McGhee, N.; Tomassini, J.E.; Chen, X.; Chang, Y.; et al. A Bivalent Omicron-Containing Booster Vaccine against Covid-19. N. Engl. J. Med. 2022, 387, 1279–1291. [Google Scholar] [CrossRef]
- Winokur, P.; Gayed, J.; Fitz-Patrick, D.; Thomas, S.J.; Diya, O.; Lockhart, S.; Xu, X.; Zhang, Y.; Bangad, V.; Schwartz, H.I.; et al. Bivalent Omicron BA.1-Adapted BNT162b2 Booster in Adults Older than 55 Years. N. Engl. J. Med. 2023, 388, 214–227. [Google Scholar] [CrossRef]
- Munoz, F.M.; Posavad, C.M.; Richardson, B.A.; Badell, M.L.; Bunge, K.; Mulligan, M.J.; Parameswaran, L.; Kelly, C.; Olsen-Chen, C.; Novak, R.M.; et al. COVID-19 booster vaccination during pregnancy enhances maternal binding and neutralizing antibody responses and transplacental antibody transfer to the newborn (DMID 21-0004). medRxiv 2022. [Google Scholar] [CrossRef]
- Wu, J.; Nie, J.; Zhang, L.; Song, H.; An, Y.; Liang, Z.; Yang, J.; Ding, R.; Liu, S.; Li, Q.; et al. The antigenicity of SARS-CoV-2 Delta variants aggregated 10 high-frequency mutations in RBD has not changed sufficiently to replace the current vaccine strain. Signal Transduct. Target. Ther. 2022, 7, 18. [Google Scholar] [CrossRef] [PubMed]
- Viana, R.; Moyo, S.; Amoako, D.G.; Tegally, H.; Scheepers, C.; Althaus, C.L.; Anyaneji, U.J.; Bester, P.A.; Boni, M.F.; Chand, M.; et al. Rapid epidemic expansion of the SARS-CoV-2 Omicron variant in southern Africa. Nature 2022, 603, 679–686. [Google Scholar] [CrossRef]
- Berkhout, B.; Herrera-Carrillo, E. SARS-CoV-2 Evolution: On the Sudden Appearance of the Omicron Variant. J. Virol. 2022, 96, e0009022. [Google Scholar] [CrossRef]
- Liu, C.; Ginn, H.M.; Dejnirattisai, W.; Supasa, P.; Wang, B.; Tuekprakhon, A.; Nutalai, R.; Zhou, D.; Mentzer, A.J.; Zhao, Y.; et al. Reduced neutralization of SARS-CoV-2 B.1.617 by vaccine and convalescent serum. Cell 2021, 184, 4220–4236.e13. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, R.; Gilby, N.B.; Wei, G.W. Omicron Variant (B.1.1.529): Infectivity, Vaccine Breakthrough, and Antibody Resistance. J. Chem. Inf. Model. 2022, 62, 412–422. [Google Scholar] [CrossRef]
- Dhama, K.; Tuglo, L.S.; Chakraborty, C.; Saikumar, G. BF. 7 Omicron subvariant (BA.5.2.1.7) posing fears of a rise in COVID-19 cases again: A critical appraisal and salient counteracting strategies. Int. J. Surg. 2023, 109, 1058–1059. [Google Scholar] [CrossRef] [PubMed]
- Sabbatucci, M.; Vitiello, A.; Clemente, S.; Zovi, A.; Boccellino, M.; Ferrara, F.; Cimmino, C.; Langella, R.; Ponzo, A.; Stefanelli, P.; et al. Omicron variant evolution on vaccines and monoclonal antibodies. Inflammopharmacology 2023, 31, 1779–1788. [Google Scholar] [CrossRef]
- Lin, D.-Y.; Xu, Y.; Gu, Y.; Zeng, D.; Wheeler, B.; Young, H.; Sunny, S.K.; Moore, Z. Effectiveness of Bivalent Boosters against Severe Omicron Infection. N. Engl. J. Med. 2023, 388, 764–766. [Google Scholar] [CrossRef]
- Joseph, N.T.; Dude, C.M.; Verkerke, H.P.; Irby, L.S.; Dunlop, A.L.; Patel, R.M.; Easley, K.A.; Smith, A.K.; Stowell, S.R.; Jamieson, D.J.; et al. Maternal Antibody Response, Neutralizing Potency, and Placental Antibody Transfer After Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection. Obstet. Gynecol. 2021, 138, 189–197. [Google Scholar] [CrossRef]
- Hannawi, S.; Saifeldin, L.; Abuquta, A.; Alamadi, A.; Mahmoud, S.A.; Hassan, A.; Liu, D.; Yan, L.; Xie, L. Safety and immunogenicity of a bivalent SARS-CoV-2 protein booster vaccine, SCTV01C, in adults previously vaccinated with mRNA vaccine: A randomized, double-blind, placebo-controlled phase 1/2 clinical trial. EBioMedicine 2023, 87, 104386. [Google Scholar] [CrossRef]
- Tan, N.H.; Geers, D.; Sablerolles, R.S.G.; Rietdijk, W.J.R.; Goorhuis, A.; Postma, D.F.; Visser, L.G.; Bogers, S.; van Dijk, L.L.A.; Gommers, L.; et al. Immunogenicity of bivalent omicron (BA.1) booster vaccination after different priming regimens in health-care workers in the Netherlands (SWITCH ON): Results from the direct boost group of an open-label, multicentre, randomised controlled trial. Lancet Infect. Dis. 2023, 23, 901–913. [Google Scholar] [CrossRef]
- Lin, D.-Y.; Xu, Y.; Gu, Y.; Zeng, D.; Sunny, S.K.; Moore, Z. Durability of Bivalent Boosters against Omicron Subvariants. N. Engl. J. Med. 2023, 388, 1818–1820. [Google Scholar] [CrossRef]
- Juliá-Burchés, C.; Martínez-Varea, A. An Update on COVID-19 Vaccination and Pregnancy. J. Pers. Med. 2023, 13, 797. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Interim Clinical Considerations for Use of COVID-19 Vaccines in the United States. Available online: https://www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-considerations-us.html#:~:text=People%20ages%2018%20years%20and,doses)%2C%20or%20at%20least%202 (accessed on 2 July 2023).
- Borobia, A.M.; Carcas, A.J.; Pérez-Olmeda, M.; Castaño, L.; Bertran, M.J.; García-Pérez, J.; Campins, M.; Portolés, A.; González-Pérez, M.; García Morales, M.T.; et al. Immunogenicity and reactogenicity of BNT162b2 booster in ChAdOx1-S-primed participants (CombiVacS): A multicentre, open-label, randomised, controlled, phase 2 trial. Lancet 2021, 398, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Barros-Martins, J.; Hammerschmidt, S.I.; Cossmann, A.; Odak, I.; Stankov, M.V.; Morillas Ramos, G.; Dopfer-Jablonka, A.; Heidemann, A.; Ritter, C.; Friedrichsen, M.; et al. Immune responses against SARS-CoV-2 variants after heterologous and homologous ChAdOx1 nCoV-19/BNT162b2 vaccination. Nat. Med. 2021, 27, 1525–1529. [Google Scholar] [CrossRef]
- Sikalidis, A.K. Amino acids and immune response: A role for cysteine, glutamine, phenylalanine, tryptophan and arginine in T-cell function and cancer? Pathol. Oncol. Res. 2015, 21, 9–17. [Google Scholar] [CrossRef]

| N (Total N = 11) | Percentage (%) | |
|---|---|---|
| Median age, years (range) | 33 (27–43) | |
| Previous parity | ||
| 0 | 2 | 18.18 |
| 1 | 4 | 36.36 |
| 2 | 4 | 36.36 |
| 3 | 1 | 9.09 |
| Median BMI (range) | 28.12 (21.64–29.94) | |
| Delivery weeks | ||
| 38 weeks | 3 | 27.27 |
| 39 weeks | 4 | 36.36 |
| 40 weeks | 4 | 36.36 |
| Interval to delivery | ||
| 0–4 weeks | 4 | 36.36 |
| 5–8 weeks | 4 | 36.36 |
| 9–12 weeks | 1 | 9.09 |
| >12 weeks | 2 | 18.18 |
| Interval between 3rd and 4th COVID vaccine | ||
| 36–40 weeks | 2 | 18.18 |
| 41–44 weeks | 5 | 45.45 |
| 45–48 weeks | 2 | 18.18 |
| >48 weeks | 2 | 18.18 |
| Tdap vaccine | ||
| Yes | 10 | 90.90 |
| No | 1 | 9.09 |
| Flu vaccine | ||
| Yes | 9 | 81.81 |
| No | 2 | 18.18 |
| Previous COVID vaccine | ||
| 2 AZ before | 5 | 45.45 |
| 1 AZ before | 1 | 9.09 |
| No AZ before | 5 | 45.45 |
| Median neonatal body weight, gm (range) | 3150 (2520–3555) | |
| Neonatal gender | ||
| Male | 8 | 72.73 |
| Female | 3 | 27.27 |
| Interval | N | BA.5 | BF.7 | BQ.1 | XBB.1.5 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MB (%) | CB (%) | Ratio | MB (%) | CB (%) | Ratio | MB (%) | CB (%) | Ratio | MB (%) | CB (%) | Ratio | ||
| <4 weeks | 4 a | 95.58 | 62.51 | 0.66 | 97.89 | 68.20 | 0.69 | 88.55 | 45.84 | 0.54 | 94.44 | 53.55 | 0.56 |
| ≥4 weeks | 7 b | 86.30 | 91.85 | 1.04 | 95.62 | 96.02 | 1.00 | 89.96 | 94.19 | 1.06 | 89.51 | 93.73 | 1.06 |
| p value | 0.558 | 0.286 | 0.643 | 0.500 | 0.143 | 0.071 | 0.500 | 0.143 | 0.143 | 0.889 | 0.143 | 0.071 | |
| <8 weeks | 8 c | 88.87 | 82.72 | 0.91 | 96.15 | 85.52 | 0.88 | 87.20 | 73.61 | 0.87 | 90.39 | 78.59 | 0.88 |
| ≥8 weeks | 3 | 87.34 | 87.52 | 1.00 | 96.08 | 94.97 | 0.980 | 94.54 | 96.25 | 1.02 | 91.02 | 92.18 | 1.01 |
| p value | 0.905 | 0.571 | 0.393 | 0.905 | 1.000 | 0.786 | 0.262 | 0.393 | 1.000 | 0.548 | 0.786 | 1.000 | |
| <12 weeks | 9 c | 90.04 | 85.20 | 0.93 | 96.39 | 87.48 | 0.90 | 88.72 | 77.29 | 0.89 | 91.19 | 81.42 | 0.90 |
| ≥12 weeks | 2 | 82.47 | 82.47 | 1.00 | 95.22 | 93.84 | 0.98 | 92.87 | 96.54 | 1.04 | 88.57 | 90.47 | 1.02 |
| p value | 1.000 | 0.857 | 0.286 | 1.000 | 1.000 | 0.857 | 0.667 | 0.429 | 0.643 | 0.889 | 0.857 | 0.857 | |
| Interval | N | BA.5 | BF.7 | BQ.1 | XBB.1.5 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MB (%) | CB (%) | Ratio | MB (%) | CB (%) | Ratio | MB (%) | CB (%) | Ratio | MB (%) | CB (%) | Ratio | ||
| ≤40 weeks | 2 | 85.78 | 95.35 | 1.11 | 95.84 | 96.76 | 1.01 | 80.57 | 89.33 | 1.13 | 83.12 | 94.47 | 1.15 |
| >40 weeks | 9 a | 89.09 | 80.91 | 0.89 | 96.21 | 86.50 | 0.90 | 92.24 | 79.69 | 0.86 | 92.75 | 80.09 | 0.86 |
| p value | 0.500 | 0.857 | 0.071 | 1.000 | 0.429 | 0.143 | 0.500 | 0.857 | 0.143 | 0.222 | 0.643 | 0.071 | |
| ≤44 weeks | 7 b | 86.67 | 77.15 | 0.91 | 95.62 | 84.94 | 0.89 | 87.81 | 75.97 | 0.89 | 87.12 | 77.38 | 0.91 |
| >44 weeks | 4 c | 90.47 | 96.79 | 1.00 | 96.76 | 95.95 | 0.98 | 91.94 | 92.32 | 0.99 | 94.96 | 94.19 | 0.98 |
| p value | 0.730 | 0.250 | 1.000 | 0.730 | 0.780 | 0.571 | 0.556 | 0.571 | 0.250 | 0.286 | 0.571 | 0.250 | |
| ≤48 weeks | 9 b | 89.32 | 82.65 | 0.94 | 96.27 | 87.90 | 0.91 | 88.61 | 80.16 | 0.92 | 89.81 | 81.98 | 0.93 |
| >48 weeks | 2 c | 84.99 | 97.61 | 1.01 | 95.62 | 97.25 | 0.99 | 93.28 | 95.67 | 0.98 | 93.41 | 95.59 | 1.00 |
| p value | 0.667 | 0.750 | 1.000 | 0.667 | 1.000 | 1.000 | 0.667 | 1.000 | 0.500 | 1.000 | 1.000 | 0.750 | |
| Vaccines | N | BA.5 | BF.7 | BQ.1 | XBB.1.5 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MB (%) | CB (%) | Ratio | MB (%) | CB (%) | Ratio | MB (%) | CB (%) | Ratio | MB (%) | CB (%) | Ratio | ||
| AZ included | 6 a | 80.94 | 87.97 | 1.06 | 95.03 | 93.98 | 0.98 | 84.11 | 89.14 | 1.09 | 86.39 | 90.22 | 1.07 |
| No AZ | 5 b | 97.63 | 81.06 | 0.83 | 97.50 | 84.16 | 0.86 | 96.57 | 75.06 | 0.77 | 95.88 | 77.15 | 0.79 |
| p value | 0.016 | 0.343 | 0.114 | 0.111 | 0.886 | 0.886 | 0.032 | 0.486 | 0.114 | 0.032 | 0.686 | 0.200 | |
| 2 AZ | 5 a | 78.95 | 85.11 | 1.05 | 94.28 | 92.62 | 0.98 | 81.22 | 86.34 | 1.11 | 84.66 | 87.67 | 1.08 |
| 1 AZ | 1 | 88.91 | 96.55 | 1.09 | 98.06 | 98.05 | 1.00 | 95.63 | 97.53 | 1.02 | 93.30 | 97.90 | 1.05 |
| No AZ | 5 b | 97.63 | 81.06 | 0.83 | 97.50 | 84.16 | 0.86 | 96.57 | 75.06 | 0.77 | 95.88 | 77.15 | 0.80 |
| p value | 0.046 | 0.400 | 0.200 | 0.088 | 0.201 | 0.806 | 0.046 | 0.392 | 0.223 | 0.083 | 0.311 | 0.297 | |
| 2 AZ | 5 a | 78.95 | 85.11 | 1.05 | 94.28 | 92.62 | 0.98 | 81.22 | 86.34 | 1.11 | 84.66 | 87.66 | 1.08 |
| 1 or no AZ | 6 b | 95.88 | 84.16 | 0.88 | 97.61 | 86.94 | 0.89 | 96.38 | 79.55 | 0.82 | 95.36 | 81.30 | 0.85 |
| p value | 0.032 | 0.250 | 0.393 | 0.032 | 0.250 | 1.000 | 0.016 | 0.250 | 0.250 | 0.063 | 0.250 | 0.571 | |
| Maternal Condition | N | BA.5 | BF.7 | BQ.1 | XBB.1.5 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MB (%) | CB (%) | Ratio | MB (%) | CB (%) | Ratio | MB (%) | CB (%) | Ratio | MB (%) | CB (%) | Ratio | ||
| Age < 34 | 6 a | 91.97 | 96.13 | 1.05 | 96.64 | 96.23 | 1.00 | 87.67 | 91.02 | 1.05 | 90.78 | 94.09 | 1.05 |
| Age ≥ 34 | 5 b | 83.85 | 65.15 | 0.77 | 95.49 | 77.14 | 0.81 | 92.11 | 67.23 | 0.73 | 90.39 | 66.34 | 0.75 |
| p value | 0.905 | 0.571 | 0.036 | 1.000 | 0.393 | 0.250 | 0.905 | 1.000 | 0786 | 0.905 | 0.571 | 0.571 | |
| BMI < 28 | 5 b | 89.12 | 97.34 | 1.03 | 96.33 | 97.67 | 1.00 | 94.61 | 96.97 | 1.00 | 94.13 | 96.80 | 1.02 |
| BMI ≥ 28 | 6 a | 87.75 | 76.82 | 0.89 | 95.97 | 83.91 | 0.88 | 85.67 | 73.18 | 0.88 | 87.79 | 75.81 | 0.88 |
| p value | 1.000 | 0.250 | 0.571 | 0.905 | 0.143 | 0.393 | 0.286 | 0.250 | 1.000 | 0.730 | 0.250 | 0.786 | |
| N | BA.5 | BF.7 | BQ.1 | XBB.1.5 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MB (%) | CB (%) | Ratio | MB (%) | CB (%) | Ratio | MB (%) | CB (%) | Ratio | MB (%) | CB (%) | Ratio | ||
| NBW < 3000 g | 3 | 97.64 | 75.46 | 0.77 | 97.99 | 79.65 | 0.81 | 96.67 | 67.51 | 0.69 | 95.71 | 70.56 | 0.73 |
| NBW ≥ 3000 g | 8 a | 83.72 | 89.95 | 1.05 | 95.20 | 94.72 | 0.99 | 86.13 | 90.86 | 1.07 | 88.06 | 91.56 | 1.06 |
| p value | 0.037 | 0.590 | 0.354 | 0.036 | 0.490 | 0.433 | 0.165 | 0.512 | 0.330 | 0.112 | 0.508 | 0.350 | |
| NBW < 3100 g | 5 b | 97.64 | 75.46 | 0.77 | 97.99 | 79.65 | 0.81 | 96.67 | 67.51 | 0.69 | 95.71 | 70.56 | 0.73 |
| NBW ≥ 3100 g | 6 c | 83.72 | 89.95 | 1.05 | 95.20 | 94.72 | 0.99 | 86.13 | 90.86 | 1.07 | 88.06 | 91.56 | 1.06 |
| p value | 0.037 | 0.590 | 0.354 | 0.036 | 0.490 | 0.433 | 0.165 | 0.512 | 0.330 | 0.112 | 0.508 | 0.350 | |
| NBW < 3200 g | 6 b | 89.90 | 73.27 | 0.83 | 96.58 | 82.17 | 0.85 | 94.41 | 74.34 | 0.79 | 91.65 | 73.65 | 0.81 |
| NBW ≥ 3200 g | 5 c | 87.13 | 95.77 | 1.06 | 95.77 | 95.97 | 1.00 | 85.83 | 89.86 | 1.07 | 89.77 | 93.71 | 1.06 |
| p value | 0.752 | 0.255 | 0.238 | 0.641 | 0.365 | 0.368 | 0.244 | 0.514 | 0.288 | 0.768 | 0.366 | 0.292 | |
| Male baby | 8 d | 89.09 | 80.91 | 0.89 | 96.21 | 86.50 | 0.90 | 92.24 | 79.69 | 0.86 | 92.75 | 80.09 | 0.86 |
| Female baby | 3 e | 85.78 | 95.35 | 1.11 | 95.84 | 96.76 | 1.01 | 80.57 | 89.33 | 1.13 | 83.12 | 94.47 | 1.15 |
| p value | 0.751 | 0.508 | 0.328 | 0.858 | 0.541 | 0.517 | 0.579 | 0.729 | 0.387 | 0.175 | 0.556 | 0.297 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.-C.; Hu, S.-Y.; Shen, C.-F.; Chuang, H.-Y.; Ker, C.-R.; Shen, C.-J.; Cheng, C.-M. COVID-19 Bivalent Booster in Pregnancy: Maternal and Neonatal Antibody Response to Omicron BA.5, BQ.1, BF.7 and XBB.1.5 SARS-CoV-2. Vaccines 2023, 11, 1425. https://doi.org/10.3390/vaccines11091425
Chen W-C, Hu S-Y, Shen C-F, Chuang H-Y, Ker C-R, Shen C-J, Cheng C-M. COVID-19 Bivalent Booster in Pregnancy: Maternal and Neonatal Antibody Response to Omicron BA.5, BQ.1, BF.7 and XBB.1.5 SARS-CoV-2. Vaccines. 2023; 11(9):1425. https://doi.org/10.3390/vaccines11091425
Chicago/Turabian StyleChen, Wei-Chun, Shu-Yu Hu, Ching-Fen Shen, Hui-Yu Chuang, Chin-Ru Ker, Ching-Ju Shen, and Chao-Min Cheng. 2023. "COVID-19 Bivalent Booster in Pregnancy: Maternal and Neonatal Antibody Response to Omicron BA.5, BQ.1, BF.7 and XBB.1.5 SARS-CoV-2" Vaccines 11, no. 9: 1425. https://doi.org/10.3390/vaccines11091425
APA StyleChen, W.-C., Hu, S.-Y., Shen, C.-F., Chuang, H.-Y., Ker, C.-R., Shen, C.-J., & Cheng, C.-M. (2023). COVID-19 Bivalent Booster in Pregnancy: Maternal and Neonatal Antibody Response to Omicron BA.5, BQ.1, BF.7 and XBB.1.5 SARS-CoV-2. Vaccines, 11(9), 1425. https://doi.org/10.3390/vaccines11091425







