Characteristics of Vaccine- and Infection-Induced Systemic IgA Anti-SARS-CoV-2 Spike Responses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Selection of Subjects and Blood Sample Processing
2.2. Assessment of Circulating IgG and IgA Anti-SARS-CoV-2 S Levels Using ELISA
2.3. Statistical Analysis
3. Results
3.1. Study Cohort
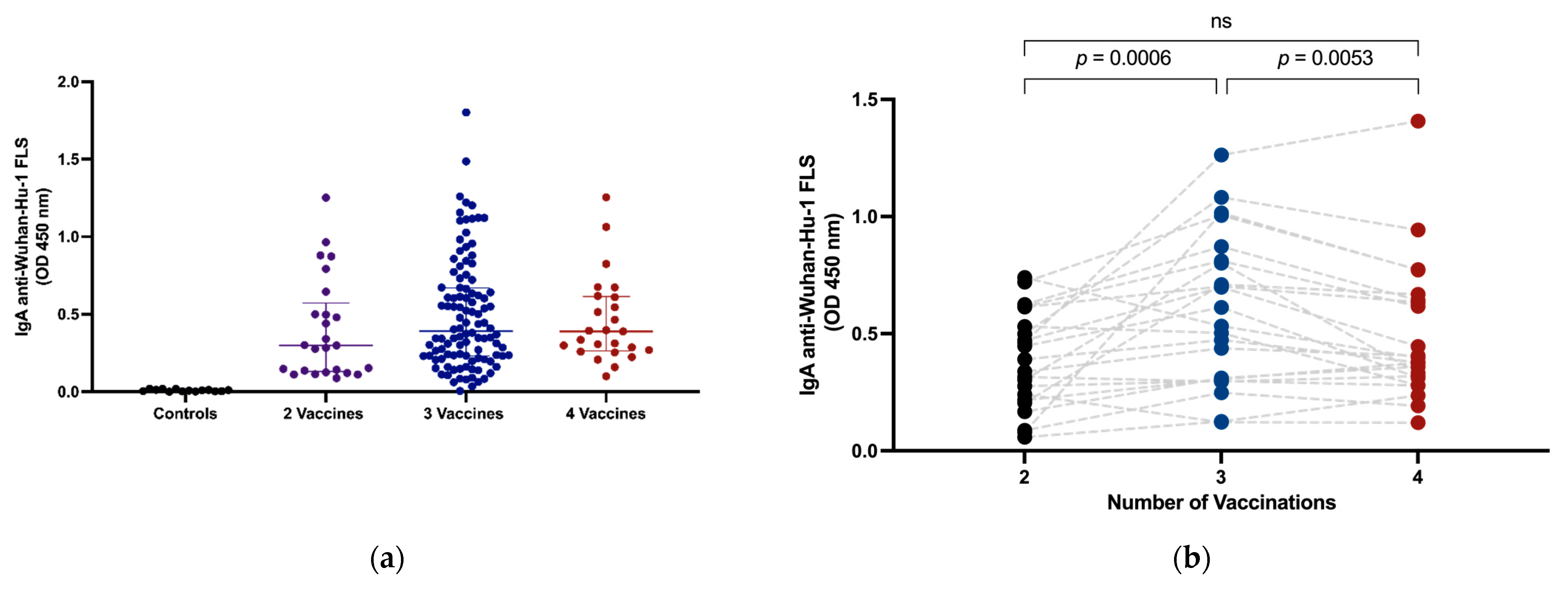
3.2. Intramuscular Vaccination Induces Circulating IgA
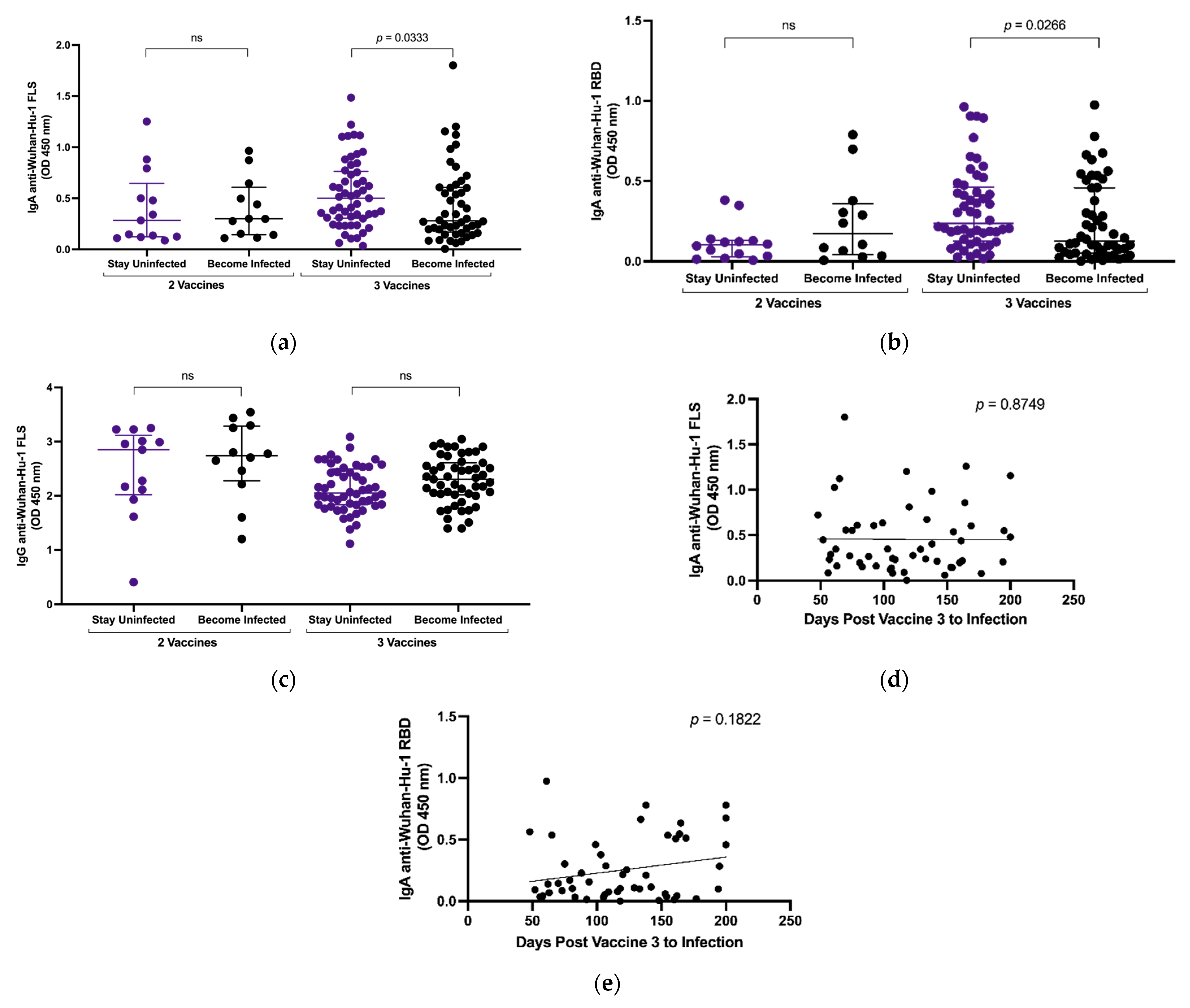
3.3. Plasma IgA Anti-SARS-CoV-2 S Levels and Breakthrough Infection
3.4. Effect of Omicron Breakthrough Infection on Plasma IgA
3.5. Selective Recognition of Wuhan-Hu-1 S Is Preserved despite Omicron Breakthrough Infection
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- John Hopkins Coronavirus Research Centre. Available online: https://coronavirus.jhu.edu/map.html (accessed on 16 July 2023).
- Focosi, D.; Maggi, F.; Casadevall, A. Mucosal Vaccines, Sterilizing Immunity, and the Future of SARS-CoV-2 Virulence. Viruses 2022, 14, 187. [Google Scholar] [CrossRef]
- Isho, B.; Abe, K.T.; Zuo, M.; Jamal, A.J.; Rathod, B.; Wang, J.H.; Li, Z.; Chao, G.; Rojas, O.L.; Bang, Y.M.; et al. Persistence of serum and saliva antibody responses to SARS-CoV-2 spike antigens in COVID-19 patients. Sci. Immunol. 2020, 5, eabe5511. [Google Scholar] [CrossRef]
- Havervall, S.; Nayager, J.; Bamford, L.; Bekker, L.-G.; Zylstra, M.; Gray, G. Anti-Spike Mucosal IgA Protection against SARS-CoV-2 Omicron Infection. N. Engl. J. Med. 2022, 387, 1332–1333. [Google Scholar] [CrossRef] [PubMed]
- Sheikh-Mohamed, S.; Isho, B.; Chao, G.Y.C.; Zuo, M.; Cohen, C.; Lustig, Y.; Nahass, G.R.; Salomon-Shulman, R.E.; Blacker, G.; Fazel-Zarandi, M.; et al. Systemic and mucosal IgA responses are variably induced in response to SARS-CoV-2 mRNA vaccination and are associated with protection against subsequent infection. Mucosal Immunol. 2022, 15, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Nabi, R.; Moldoveanu, Z.; Wei, Q.; Golub, E.T.; Durkin, H.G.; Greenblatt, R.M.; Herold, B.C.; Nowicki, M.J.; Kassaye, S.; Cho, M.W.; et al. Differences in serum IgA responses to HIV-1 gp41 in elite controllers compared to viral suppressors on highly active antiretroviral therapy. PLoS ONE 2017, 12, e0180245. [Google Scholar] [CrossRef] [PubMed]
- Montoya, J.G.; Adams, A.E.; Bonetti, V.; Deng, S.; Link, N.A.; Pertsch, S.; Olson, K.; Li, M.; Dillon, E.C.; Frosch, D.L. Differences in IgG Antibody Responses following BNT162b2 and mRNA-1273 SARS-CoV-2 Vaccines. Microbiol. Spectr. 2021, 9, e0116221. [Google Scholar] [CrossRef]
- Mubarak, A.; Almutairi, S.; Al-Dhabbah, A.D.; Aldabas, S.Y.; Bhat, R.; Alqoufail, M.M.; A Abdel-Maksoud, M.; Almanaa, T.N.; A Farrag, M.; Alturaiki, W. Durability of SARS-CoV-2 Specific IgG Antibody Responses Following Two Doses of Match and Mixed COVID-19 Vaccines Regimens in Saudi Population. Infect. Drug Resist. 2022, 15, 3791–3800. [Google Scholar] [CrossRef]
- Castro-Dopico, X.; Ols, S.; Loré, K.; Hedestam, G.B.K. Immunity to SARS-CoV-2 induced by infection or vaccination. J. Intern. Med. 2022, 291, 32–50. [Google Scholar] [CrossRef]
- Rose, R.; Neumann, F.; Grobe, O.; Lorentz, T.; Fickenscher, H.; Krumbholz, A. Humoral immune response after different SARS-CoV-2 vaccination regimens. BMC Med. 2022, 20, 1–13. [Google Scholar] [CrossRef]
- Romero-Ibarguengoitia, M.E.; Rivera-Salinas, D.; Hernández-Ruíz, Y.G.; Armendariz-Vázquez, A.G.; González-Cantú, A.; Barco-Flores, I.A.; González-Facio, R.; Montelongo-Cruz, L.P.; Del Rio-Parra, G.F.; Garza-Herrera, M.R.; et al. Effect of the third dose of BNT162b2 vaccine on quantitative SARS-CoV-2 spike 1-2 IgG antibody titers in healthcare personnel. PLoS ONE 2022, 17, e0263942. [Google Scholar] [CrossRef]
- Holder, K.A.; Ings, D.P.; Harnum, D.O.A.; Russell, R.S.; Grant, M.D. Moderate to severe SARS-CoV-2 infection primes vaccine-induced immunity more effectively than asymptomatic or mild infection. npj Vaccines 2022, 7, 1–13. [Google Scholar] [CrossRef]
- Andrews, N.; Tessier, E.; Stowe, J.; Gower, C.; Kirsebom, F.; Simmons, R.; Gallagher, E.; Thelwall, S.; Groves, N.; Dabrera, G.; et al. Duration of Protection against Mild and Severe Disease by COVID-19 Vaccines. N. Engl. J. Med. 2022, 386, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.P.; Zeng, C.; Carlinn, C.; Lozanski, G.; Saif, L.J.; Oltz, E.M.; Gumina, R.J.; Liu, S.L. Neutralizing Antibody Responses Elicited by SARS-CoV-2 mRNA Vaccination Wane Over Time and Are Boosted by Breakthrough Infection. Sci. Transl. Med. 2022, 14, eabn8057. [Google Scholar] [CrossRef] [PubMed]
- Grant, M.D.; Bentley, K.; Fielding, C.A.; Hatfield, K.M.; Ings, D.P.; Harnum, D.; Wang, E.C.; Stanton, R.J.; Holder, K.A. Anti-S1 and S2 antibodies from hybrid immunity elicit potent cross-variant ADCC against SARS-CoV-2. JCI Insight 2023, 8, e170681. [Google Scholar] [CrossRef]
- Francis, T. On the Doctrine of Original Antigenic Sin. Proc. Am. Philos. Soc. 1960, 104, 572–578. [Google Scholar]
- Ings, D.P.; Hatfield, K.M.; Fifield, K.E.; Harnum, D.O.A.; Holder, K.A.; Russell, R.S.; Grant, M.D. Few SARS-CoV-2 infections detected in Newfoundland and Labrador in the absence of Public Health Laboratory-based confirmation. PLoS ONE 2022, 17, e0262957. [Google Scholar] [CrossRef]
- Paas-Lang, C. Canada’s First Cases of the Omicron Coronavirus Variant Confirmed in Ottawa. CBC. Available online: https://www.cbc.ca/news/politics/omicron-variant-canada-travellers-1.6265927#:~:text=Politics-,Canada%27s%20first%20cases%20of%20the%20omicron%20coronavirus%20variant%20confirmed%20in,the%20first%20confirmed%20in%20Canada (accessed on 21 May 2023).
- Omicron Arrives in N.L. as Provincial Government Prepares for Rapid Border Testing. Available online: https://www.cbc.ca/news/canada/newfoundland-labrador/nl-covid-december-15-2021-1.6286434 (accessed on 16 May 2023).
- Wisnewski, A.V.; Campillo Luna, J.; Redlich, C.A. Human IgG and IgA responses to COVID-19 mRNA vaccines. PLoS ONE 2021, 16, e0249499. [Google Scholar] [CrossRef]
- Levin, E.G.; Lustig, Y.; Cohen, C.; Fluss, R.; Indenbaum, V.; Amit, S.; Doolman, R.; Asraf, K.; Mendelson, E.; Ziv, A.; et al. Waning Immune Humoral Response to BNT162b2 COVID-19 Vaccine over 6 Months. N. Engl. J. Med. 2021, 385, e84. [Google Scholar] [CrossRef]
- Shrotri, M.; Krutikov, M.; Palmer, T.; Giddings, R.; Azmi, B.; Subbarao, S.; Fuller, C.; Irwin-Singer, A.; Davies, D.; Tut, G.; et al. Vaccine effectiveness of the first dose of ChAdOx1 nCoV-19 and BNT162b2 against SARS-CoV-2 infection in residents of long-term care facilities in England (VIVALDI): A prospective cohort study. Lancet Infect. Dis. 2021, 21, 1529–1538. [Google Scholar] [CrossRef]
- Takamatsu, Y.; Omata, K.; Shimizu, Y.; Kinoshita-Iwamoto, N.; Terada, M.; Suzuki, T.; Morioka, S.; Uemura, Y.; Ohmagari, N.; Maeda, K.; et al. SARS-CoV-2-Neutralizing Humoral IgA Response Occurs Earlier but Is Modest and Diminishes Faster than IgG Response. Microbiol. Spectr. 2022, 10, e0271622. [Google Scholar] [CrossRef]
- Sterlin, D.; Malaussena, A.; Gorochov, G. IgA Dominates the Early Neutralizing Antibody Response to SARS-CoV-2. Sci. Transl. Med. 2021, 13, eabd2223. [Google Scholar] [CrossRef] [PubMed]
- Pollard, A.J.; Bijker, E.M. A guide to vaccinology: From basic principles to new developments. Nat. Rev. Immunol. 2021, 21, 83–100. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Glass, R.I.; Jiang, B.; Santosham, M.; Lopman, B.; Parashar, U. A systematic review of anti-rotavirus serum IgA antibody titer as a potential correlate of rotavirus vaccine efficacy. J. Infect. Dis. 2013, 208, 284–294. [Google Scholar] [CrossRef]
- Rossi, A.; Michelini, Z.; Leone, P.; Borghi, M.; Blasi, M.; Bona, R.; Spada, M.; Grasso, F.; Gugliotta, A.; Klotman, M.E.; et al. Optimization of mucosal responses after intramuscular immunization with integrase defective lentiviral vector. PLoS ONE 2014, 9, e107377. [Google Scholar] [CrossRef] [PubMed]
- Zervou, F.N.; Louie, P.; Stachel, A.; Zacharioudakis, I.M.; Ortiz-Mendez, Y.; Thomas, K.; Aguero-Rosenfeld, M.E. SARS-CoV-2 antibodies: IgA correlates with severity of disease in early COVID-19 infection. J. Med. Virol. 2021, 93, 5409–5415. [Google Scholar] [CrossRef]
- Crescenzo-Chaigne, B.; Behillil, S.; Enouf, V.; Escriou, N.; Petres, S.; Ungeheuer, M.N.; Ghosn, J.; Tubiana, S.; Bouadma, L.; van der Werf, S.; et al. Nasopharyngeal and serological anti SARS-CoV-2 IgG/IgA responses in COVID-19 patients. J. Clin. Virol. Plus 2021, 1, 100041. [Google Scholar] [CrossRef]
- Ma, H.; Zeng, W.; He, H.; Zhao, D.; Jiang, D.; Zhou, P.; Cheng, L.; Li, Y.; Ma, X.; Jin, T. Serum IgA, IgM, and IgG responses in COVID-19. Cell. Mol. Immunol. 2020, 17, 773–775. [Google Scholar] [CrossRef]
- Carsetti, R.; Zaffina, S.; Mortari, E.P.; Terreri, S.; Corrente, F.; Capponi, C.; Palomba, P.; Mirabella, M.; Cascioli, S.; Palange, P.; et al. Different Innate and Adaptive Immune Responses to SARS-CoV-2 Infection of Asymptomatic, Mild, and Severe Cases. Front. Immunol. 2020, 11, 610300. [Google Scholar] [CrossRef]
- Reyneveld, G.I.; Savelkoul, H.F.J.; Parmentier, H.K. Current Understanding of Natural Antibodies and Exploring the Possibilities of Modulation Using Veterinary Models. A Review. Front. Immunol. 2020, 11, 2139. [Google Scholar] [CrossRef]
- Davtyan, T.K.; Hovsepyan, M.P.; Mkhitaryan, L.M.; Hakobyan, G.S.; Brazil, A.; Barrett, L.; Hirsch, G.; Peltekian, K.M.; Grant, M.D. The 1F7 idiotype is selectively expressed on CD5+ B cells and elevated in chronic hepatitis C virus infection. Immunol. Cell Biol. 2009, 87, 457–463. [Google Scholar] [CrossRef]
- Guerra, E.N.S.; de Castro, V.T.; dos Santos, J.A.; Acevedo, A.C.; Chardin, H. Saliva is suitable for SARS-CoV-2 antibodies detection after vaccination: A rapid systematic review. Front. Immunol. 2022, 13, 1006040. [Google Scholar] [CrossRef] [PubMed]
- Klingler, J.; Lambert, G.S.; Itri, V.; Liu, S.; Bandres, J.C.; Enyindah-Asonye, G.; Liu, X.; Simon, V.; Gleason, C.R.; Kleiner, G.; et al. Detection of Antibody Responses Against SARS-CoV-2 in Plasma and Saliva from Vaccinated and Infected Individuals. Front. Immunol. 2021, 12, 759688. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.; German, G. Salivary antibodies are detected with a commercial anti-SARS-CoV-2 assay only after two doses of vaccine using serum thresholds. Clin. Biochem. 2022, 104, 66–69. [Google Scholar] [CrossRef] [PubMed]


| 2 Vaccines | 2 Vaccines + Omicron | 3 Vaccines | 3 Vaccines + Omicron | 4 Vaccines | |
|---|---|---|---|---|---|
| n | 13 | 12 | 49 | 47 | 22 |
| Sex (M/F) | 6/7 | 6/6 | 20/29 | 16/31 | 10/13 |
| Age in years (mean, range) | 41 (16–65) | 40 (15–65) | 55 (25–72) | 53 (22–75) | 63 (37–74) |
| a Days Post Vaccine (mean) | 61 | 79 | 57 | 66 | 59 |
| b Vaccine Types (mRNA-1273/BNT162b2/ChAdOx1) | 1/10/2 2/11/0 | 2/11/0 3/10/0 | 5/38/6 13/36/0 22/27/0 | 0/39/8 4/41/2 21/26/0 | 2/18/2 8/14/0 9/13/0 9/13/0 |
| c Days Post Infection (mean) | - | 40 | - | 52 | - |
| Number of Immunogenic Exposures | 2 | 3 | 3 | 4 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Norton, N.J.; Ings, D.P.; Fifield, K.E.; Barnes, D.A.; Barnable, K.A.; Harnum, D.O.A.; Holder, K.A.; Russell, R.S.; Grant, M.D. Characteristics of Vaccine- and Infection-Induced Systemic IgA Anti-SARS-CoV-2 Spike Responses. Vaccines 2023, 11, 1462. https://doi.org/10.3390/vaccines11091462
Norton NJ, Ings DP, Fifield KE, Barnes DA, Barnable KA, Harnum DOA, Holder KA, Russell RS, Grant MD. Characteristics of Vaccine- and Infection-Induced Systemic IgA Anti-SARS-CoV-2 Spike Responses. Vaccines. 2023; 11(9):1462. https://doi.org/10.3390/vaccines11091462
Chicago/Turabian StyleNorton, Natasha J., Danielle P. Ings, Kathleen E. Fifield, David A. Barnes, Keeley A. Barnable, Debbie O. A. Harnum, Kayla A. Holder, Rodney S. Russell, and Michael D. Grant. 2023. "Characteristics of Vaccine- and Infection-Induced Systemic IgA Anti-SARS-CoV-2 Spike Responses" Vaccines 11, no. 9: 1462. https://doi.org/10.3390/vaccines11091462







