Modeling Supply and Demand Dynamics of Vaccines against Epidemic-Prone Pathogens: Case Study of Ebola Virus Disease
Abstract
:1. Introduction
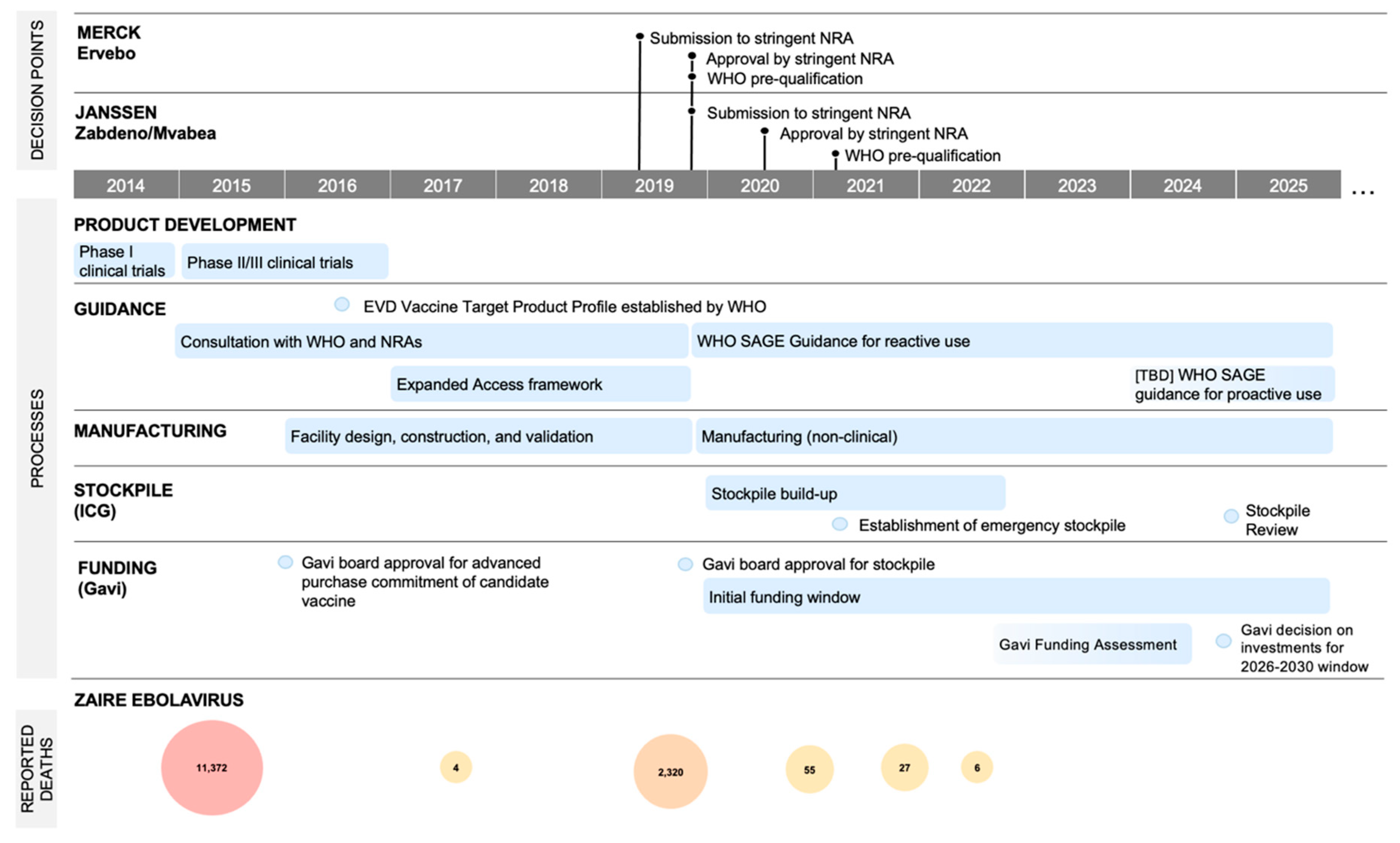
2. Case Study: EBOV
3. Methodology
3.1. Study Aim
3.2. Study Setting
3.3. Modeling Approach
3.4. Data Collection and Analysis
3.5. Model Validation
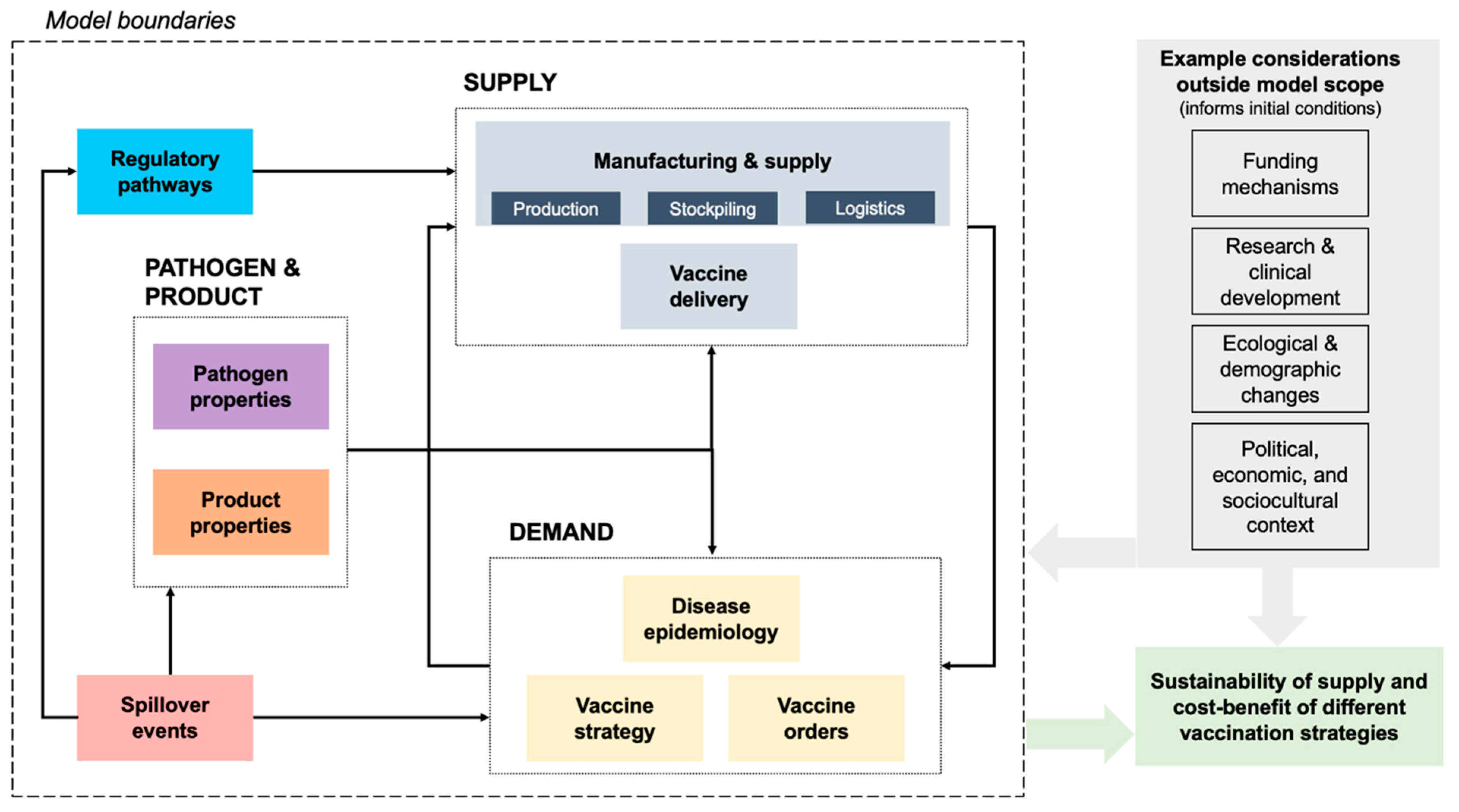
3.6. System Definition
3.7. Model Application
4. Model Subsystems
4.1. Pathogen Properties
4.2. Spillover Events
4.3. Disease Epidemiology
4.4. Vaccine Strategy and Country Orders
4.5. Product Properties
4.6. Regulatory Pathways
4.7. Supply System: Manufacturing, Stockpiling, Service Delivery
4.8. Costs and Benefits
5. Results and Analysis
5.1. Parameter Sensitivity: Stockpile Dynamics
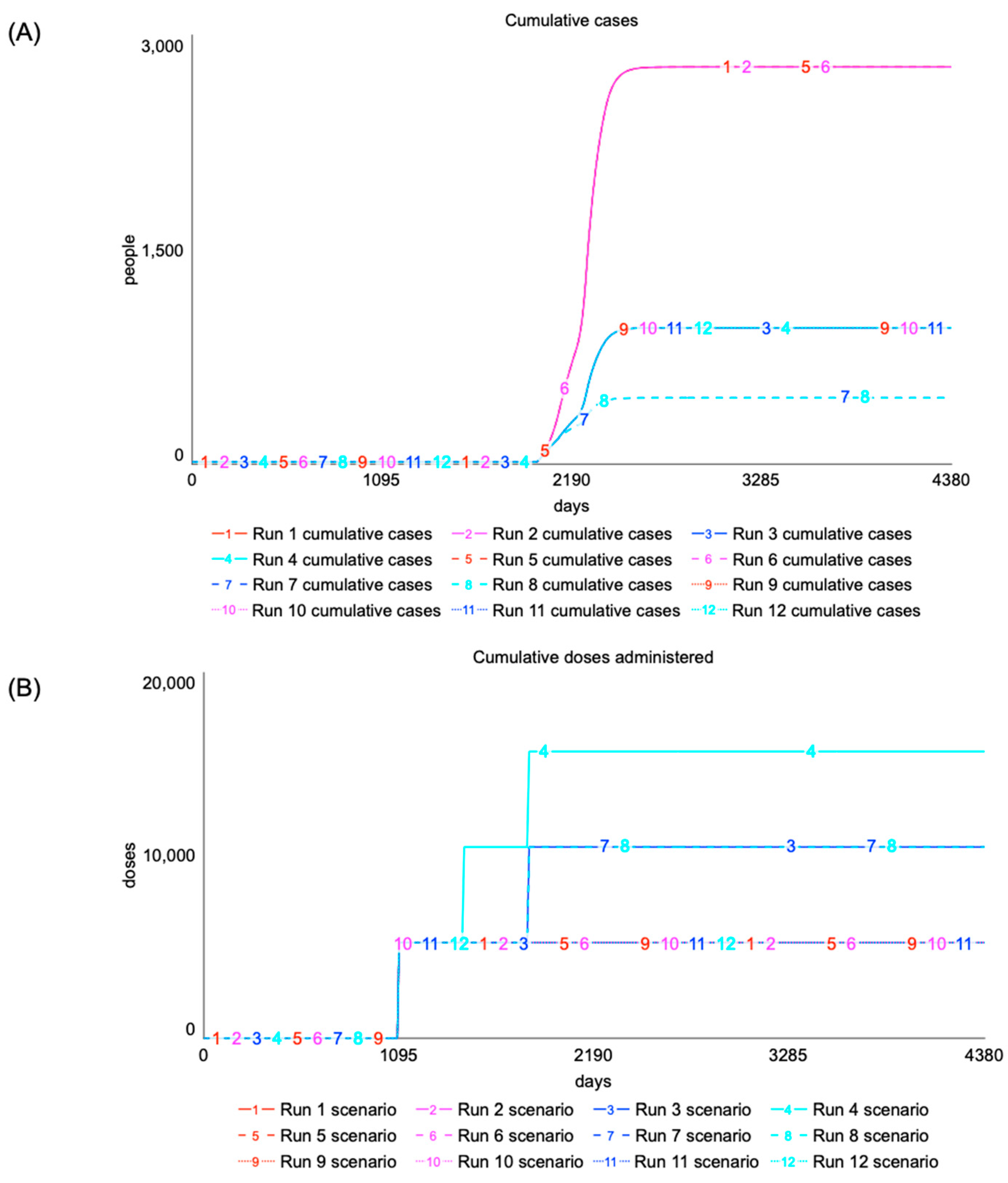
5.2. Cascading Delays: Timing of Proactive Vaccination Campaign
5.3. Policy Goal: Proactive Vaccine Strategies
6. Discussion
6.1. Limitations
6.2. Future Research
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bloom, D.E.; Cadarette, D. Infectious Disease Threats in the Twenty-First Century: Strengthening the Global Response. Front. Immunol. 2019, 10, 549. [Google Scholar] [CrossRef] [PubMed]
- G20 High Level Independent Panel on Financing the Global Commons for Pandemic Preparedness and Response. A Global Deal for Our Pandemic Age. 2021. Available online: https://pandemic-financing.org/report/foreword/ (accessed on 10 November 2023).
- Nyaruaba, R.; Okoye, C.O.; Akan, O.D.; Mwaliko, C.; Ebido, C.C.; Ayoola, A.; Ayeni, E.A.; Odoh, C.K.; Abi, M.E.; Adebanjo, O.; et al. Socio-Economic Impacts of Emerging Infectious Diseases in Africa. Infect. Dis. 2022, 54, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Bambra, C. Pandemic Inequalities: Emerging Infectious Diseases and Health Equity. Int. J. Equity Health 2022, 21, 6. [Google Scholar] [CrossRef] [PubMed]
- Neiderud, C.J. How Urbanization Affects the Epidemiology of Emerging Infectious Diseases. Afr. J. Disabil. 2015, 5, 27060. [Google Scholar] [CrossRef] [PubMed]
- Castelli, F.; Sulis, G. Migration and Infectious Diseases. Clin. Microbiol. Infect. 2017, 23, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.E.; Mahmud, A.S.; Miller, I.F.; Rajeev, M.; Rasambainarivo, F.; Rice, B.L.; Takahashi, S.; Tatem, A.J.; Wagner, C.E.; Wang, L.F.; et al. Infectious Disease in an Era of Global Change. Nat. Rev. Microbiol. 2022, 20, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Mora, C.; McKenzie, T.; Gaw, I.M.; Dean, J.M.; von Hammerstein, H.; Knudson, T.A.; Setter, R.O.; Smith, C.Z.; Webster, K.M.; Patz, J.A.; et al. Over Half of Known Human Pathogenic Diseases Can Be Aggravated by Climate Change. Nat. Clim. Chang. 2022, 12, 869–875. [Google Scholar] [CrossRef]
- Haldane, V.; Jung, A.S.; De Foo, C.; Bonk, M.; Jamieson, M.; Wu, S.; Verma, M.; Abdalla, S.M.; Singh, S.; Nordström, A.; et al. Strengthening the Basics: Public Health Responses to Prevent the next Pandemic. BMJ 2021, 375, e067510. [Google Scholar] [CrossRef]
- Bown, C.P.; Bollyky, T.J. Working Paper: How COVID-19 Vaccine Supply Chains Emerged in the Midst of a Pandemic; CEPR: Washington, DC, USA, 2021. [Google Scholar]
- Torreele, E.; McNab, C.; Adeyi, O.; Bonnell, R.; Dhaliwal, M.; Hassan, F.; Kazatchkine, M.; Kim, H.; Kim, J.; Legido-Quigley, H.; et al. It Is Time for Ambitious, Transformational Change to the Epidemic Countermeasures Ecosystem. Lancet 2023, 401, 978–982. [Google Scholar] [CrossRef]
- Sim, S.Y.; Watts, E.; Constenla, D.; Brenzel, L.; Patenaud, B.N. Return on Investment from Immunization against 10 Pathogens in 94 Low-and Middle-Income Countries, 2011–2030. Health Aff. 2020, 39, 1343–1353. [Google Scholar] [CrossRef]
- Rodrigues, C.M.C.; Plotkin, S.A. Impact of Vaccines; Health, Economic and Social Perspectives. Front. Microbiol. 2020, 11, 1526. [Google Scholar] [CrossRef] [PubMed]
- Watson, O.J.; Barnsley, G.; Toor, J.; Hogan, A.B.; Winskill, P.; Ghani, A.C. Global Impact of the First Year of COVID-19 Vaccination: A Mathematical Modelling Study. Lancet Infect. Dis. 2022, 22, 1293–1302. [Google Scholar] [CrossRef] [PubMed]
- Segarra-Blasco, A.; Teruel, M.; Cattaruzzo, S. The Economic Reaction to Non-Pharmaceutical Interventions during Covid-19. Econ. Anal. Policy 2021, 72, 592–608. [Google Scholar] [CrossRef] [PubMed]
- Stokes, J.; Turner, A.J.; Anselmi, L.; Morciano, M.; Hone, T. The Relative Effects of Non-Pharmaceutical Interventions on Wave One Covid-19 Mortality: Natural Experiment in 130 Countries. BMC Public Health 2022, 22, 1113. [Google Scholar] [CrossRef] [PubMed]
- Excler, J.L.; Saville, M.; Berkley, S.; Kim, J.H. Vaccine Development for Emerging Infectious Diseases. Nat. Med. 2021, 27, 591–600. [Google Scholar] [CrossRef]
- WHO. An R&D Blueprint for Action to Prevent Epidemics; WHO: Geneva, Switzerland, 2016. [Google Scholar]
- Plotkin, S.A. Vaccines for Epidemic Infections and the Role of CEPI. Hum. Vaccin. Immunother. 2017, 13, 2755–2762. [Google Scholar] [CrossRef]
- Rappuoli, R.; Hanon, E. Sustainable Vaccine Development: A Vaccine Manufacturer’s Perspective. Curr. Opin. Immunol. 2018, 53, 111–118. [Google Scholar] [CrossRef]
- Gristwood, A. Fresh Approaches to Vaccine Development. EMBO Rep. 2018, 19, e46675. [Google Scholar] [CrossRef]
- Gouglas, D.; Marsh, K. Prioritizing Investments in Rapid Response Vaccine Technologies for Emerging Infections: A Portfolio Decision Analysis. PLoS ONE 2021, 16, e0246235. [Google Scholar] [CrossRef]
- Grais, R.F.; Juan-Ginera, A. Vaccination in Humanitarian Crises: Satisficing Should No Longer Suffice. Int. Health 2014, 6, 160–161. [Google Scholar] [CrossRef]
- Sigfrid, L.; Maskell, K.; Bannister, P.G.; Ismail, S.A.; Collinson, S.; Regmi, S.; Blackmore, C.; Harriss, E.; Longuere, K.S.; Gobat, N.; et al. Addressing Challenges for Clinical Research Responses to Emerging Epidemics and Pandemics: A Scoping Review. BMC Med. 2020, 18, 190. [Google Scholar] [CrossRef] [PubMed]
- Park, S.N.; Kim, H.H.; Lee, K.B. Inherently High Uncertainty in Predicting the Time Evolution of Epidemics. Epidemiol. Health 2021, 43, e2021014. [Google Scholar] [CrossRef] [PubMed]
- Govindan, K.; Sethi, S.P.; Cheng, T.C.E.; Lu, S.F. Designing Supply Chain Strategies against Epidemic Outbreaks Such as COVID-19: Review and Future Research Directions. Decis. Sci. 2023, 54, 365–374. [Google Scholar] [CrossRef]
- Yen, C.; Hyde, T.B.; Costa, A.J.; Fernandez, K.; Tam, J.S.; Hugonnet, S.; Huvos, A.M.; Duclos, P.; Dietz, V.J.; Burkholder, B.T. The Development of Global Vaccine Stockpiles. Lancet Infect. Dis. 2015, 15, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Jarrett, S.; Pagliusi, S.; Park, R.; Wilmansyah, T.; Jadhav, S.; Santana, P.C.; Krishnamurthy, K.R.; Yang, L. The Importance of Vaccine Stockpiling to Respond to Epidemics and Remediate Global Supply Shortages Affecting Immunization: Strategic Challenges and Risks Identified by Manufacturers. Vaccine X 2021, 9, 100119. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.T.; Crozier, I.; Fischer, W.A.; Hewlett, A.; Kraft, C.S.; de La Vega, M.A.; Soka, M.J.; Wahl, V.; Griffiths, A.; Bollinger, L.; et al. Ebola Virus Disease. Nat. Rev. Dis. Primers 2020, 6, 13. [Google Scholar] [CrossRef]
- Sridhar, S. Clinical Development of Ebola Vaccines. Ther. Adv. Vaccines 2015, 3, 125–138. [Google Scholar] [CrossRef]
- Hussein, H.A. Brief Review on Ebola Virus Disease and One Health Approach. Heliyon 2023, 9, e19036. [Google Scholar] [CrossRef]
- Huber, C.; Finelli, L.; Stevens, W. The Economic and Social Burden of the 2014 Ebola Outbreak in West Africa. J. Infect. Dis. 2018, 218, S698–S704. [Google Scholar] [CrossRef]
- Gavi Vaccine Alliance 500,000 Doses of Ebola Vaccine to Be Made Available to Countries for Outbreak Response. Available online: https://www.gavi.org/news/media-room/500000-doses-ebola-vaccine-be-made-available-countries-outbreak-response (accessed on 10 November 2023).
- Parish, L.A.; Stavale, E.J.; Houchens, C.R.; Wolfe, D.N. Developing Vaccines to Improve Preparedness for Filovirus Outbreaks: The Perspective of the USA Biomedical Advanced Research and Development Authority (BARDA). Vaccines 2023, 11, 1120. [Google Scholar] [CrossRef]
- Meyer, M.; Malherbe, D.C.; Bukreyev, A. Can Ebola Virus Vaccines Have Universal Immune Correlates of Protection? Trends Microbiol. 2019, 27, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Crozier, I.; Britson, K.A.; Wolfe, D.N.; Klena, J.D.; Hensley, L.E.; Lee, J.S.; Wolfraim, L.A.; Taylor, K.L.; Higgs, E.S.; Montgomery, J.M.; et al. The Evolution of Medical Countermeasures for Ebola Virus Disease: Lessons Learned and Next Steps. Vaccines 2022, 10, 1213. [Google Scholar] [CrossRef] [PubMed]
- WHO. Meeting of the Strategic Advisory Group of Experts on Immunization, 22–24 March 2021: Conclusions and Recommendations; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- UNICEF Market & Supply Update: Ebola Vaccine. Available online: https://www.unicef.org/supply/media/18971/file/VIC-MarketUpdate-Poster-Ebola-2023.pdf (accessed on 10 November 2023).
- Bausch, D.G. The Need for a New Strategy for Ebola Vaccination. Nat. Med. 2021, 27, 580–581. [Google Scholar] [CrossRef] [PubMed]
- Kucharski, A.J.; Eggo, R.M.; Watson, C.H.; Camacho, A.; Funk, S.; Edmunds, W.J. Effectiveness of Ring Vaccination as Control Strategy for Ebola Virus Disease. Emerg. Infect. Dis. 2016, 22, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Heymann, D.L. Health Workers Bury a Nun in Kikwit in January 1995, during an Ebola Outbreak in Zaire That Killed 254 People. Nature 2014, 514, 299–300. [Google Scholar] [CrossRef] [PubMed]
- Senga, M.; Koi, A.; Moses, L.; Wauquier, N.; Barboza, P.; Fernandez-Garcia, M.D.; Engedashet, E.; Kuti-George, F.; Mitiku, A.D.; Vandi, M.; et al. Contact Tracing Performance during the Ebola Virus Disease Outbreak in Kenema District, Sierra Leone. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160300. [Google Scholar] [CrossRef] [PubMed]
- Lévy, Y.; Lane, C.; Piot, P.; Beavogui, A.H.; Kieh, M.; Leigh, B.; Doumbia, S.; D’Ortenzio, E.; Lévy-Marchal, C.; Pierson, J.; et al. Prevention of Ebola Virus Disease through Vaccination: Where We Are in 2018. Lancet 2018, 392, 787–790. [Google Scholar] [CrossRef] [PubMed]
- Africa Centres for Disease Control and Prevention (Africa CDC). Risk Ranking and Prioritization of Epidemic-Prone Diseases; Africa CDC: Addis Ababa, Ethiopia, 2023. [Google Scholar]
- US CDC Ebola Disease Outbreaks by Species and Size, Since 1976. Available online: https://www.cdc.gov/vhf/ebola/history/distribution-map.html (accessed on 10 November 2023).
- Pruyt, E. Small System Dynamics Models for Big Issues: Triple Jump towards Real-World Dynamic Complexity; TU Delft Library: Delft, The Netherlands, 2013; ISBN 978-94-6186-195-5. [Google Scholar]
- Isee Systems How Model Equations Are Solved. Available online: https://www.iseesystems.com/resources/help/v10/Content/Reference/Integration%20methods/How_model_equations_are_solved.htm (accessed on 7 December 2023).
- Richardson, G.P.; Pugh, A. Introduction to System Dynamics Modeling with DYNAMO. J. Oper. Res. Soc. 1997, 48, 1146. [Google Scholar] [CrossRef]
- Bradley, D.T.; Mansouri, M.A.; Kee, F.; Garcia, L.M.T. A Systems Approach to Preventing and Responding to COVID-19. EClinicalMedicine 2020, 21, 100325. [Google Scholar] [CrossRef]
- Jia, S.; Li, Y.; Fang, T. System Dynamics Analysis of COVID-19 Prevention and Control Strategies. Environ. Sci. Pollut. Res. 2022, 29, 3944–3957. [Google Scholar] [CrossRef]
- Rahmandad, H.; Lim, T.Y.; Sterman, J. Behavioral Dynamics of COVID-19: Estimating Underreporting, Multiple Waves, and Adherence Fatigue across 92 Nations. Syst. Dyn. Rev. 2021, 37, 5–31. [Google Scholar] [CrossRef] [PubMed]
- Sansone, M.; Holmstrom, P.; Hallberg, S.; Nordén, R.; Andersson, L.M.; Westin, J. System Dynamic Modelling of Healthcare Associated Influenza -a Tool for Infection Control. BMC Health Serv. Res. 2022, 22, 709. [Google Scholar] [CrossRef] [PubMed]
- Maraz, O.M. Integrating Complex System Dynamics of Pandemic Influenza with a Multi-Criteria Decision Making Model for Evaluating Public Health Strategies. J. Syst. Sci. Syst. Eng. 2013, 22, 319–339. [Google Scholar] [CrossRef]
- Thompson, K.M.; Duintjer Tebbens, R.J. Lessons from the Polio Endgame: Overcoming the Failure to Vaccinate and the Role of Subpopulations in Maintaining Transmission. J. Infect. Dis. 2017, 216, S176–S182. [Google Scholar] [CrossRef] [PubMed]
- Pruyt, E.; Auping, W.L.; Kwakkel, J.H. Ebola in West Africa: Model-Based Exploration of Social Psychological Effects and Interventions. Syst. Res. Behav. Sci. 2015, 32, 2–14. [Google Scholar] [CrossRef]
- Sharareh, N.; Sabounchi, N.S.; Sayama, H. The Ebola Crisis and the Corresponding Public Behavior: A System Dynamics Approach The Ebola Crisis and the Corresponding Public Behavior. PLoS Curr. 2016, 8. [Google Scholar] [CrossRef]
- Auping, W.L.; Pruyt, E.; Kwakkel, J.H. Simulating Endogenous Dynamics of Intervention-Capacity Deployment: Ebola Outbreak in Liberia. Int. J. Syst. Sci. Oper. Logist. 2017, 4, 53–67. [Google Scholar] [CrossRef]
- Duintjer Tebbens, R.J.; Pallansch, M.A.; Wassilak, S.G.F.; Cochi, S.L.; Thompson, K.M. Characterization of Outbreak Response Strategies and Potential Vaccine Stockpile Needs for the Polio Endgame. BMC Infect. Dis. 2016, 16, 137. [Google Scholar] [CrossRef]
- Duintjer Tebbens, R.J.; Thompson, K.M. Poliovirus Vaccination during the Endgame: Insights from Integrated Modeling. Expert. Rev. Vaccines 2017, 16, 577–586. [Google Scholar] [CrossRef]
- Kochakkashani, F.; Kayvanfar, V.; Haji, A. Supply Chain Planning of Vaccine and Pharmaceutical Clusters under Uncertainty: The Case of COVID-19. Socioecon. Plann Sci. 2023, 87, 101602. [Google Scholar] [CrossRef]
- Shiri, M.; Ahmadizar, F. An Equitable and Accessible Vaccine Supply Chain Network in the Epidemic Outbreak of COVID-19 under Uncertainty. J. Ambient. Intell. Humaniz. Comput. 2022. [Google Scholar] [CrossRef] [PubMed]
- Sayarshad, H.R. Interventions in Demand and Supply Sides for Vaccine Supply Chain: An Analysis on Monkeypox Vaccine. Oper. Res. Perspect. 2023, 11, 100285. [Google Scholar] [CrossRef]
- Jalali, M.S.; Rahmandad, H.; Bullock, S.L.; Lee-Kwan, S.H.; Gittelsohn, J.; Ammerman, A. Dynamics of Intervention Adoption, Implementation, and Maintenance inside Organizations: The Case of an Obesity Prevention Initiative. Soc. Sci. Med. 2019, 224, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Richardson, G.P. Reflections on the Foundations of System Dynamics. Syst. Dyn. Rev. 2011, 27, 219–243. [Google Scholar] [CrossRef]
- Sterman, J.D. All Models Are Wrong: Reflections on Becoming a Systems Scientist. Syst. Dyn. Rev. 2002, 18, 501–531. [Google Scholar] [CrossRef]
- Decouttere, C.; Vandaele, N.; Lemmens, S.; Bernuzzi, M. The Vaccine Supply Chain Multathlon: The Reconciliation of Technology, Economy and Access to Medicines. In International Series in Operations Research and Management Science; Springer: Berlin/Heidelberg, Germany, 2016; pp. 205–227. [Google Scholar]
- Sterman, J. Business Dynamics: Systems Thinking and Modeling for a Complex World, 6th ed.; Irwin/McGraw-Hill: Boston, MA, USA, 2000. [Google Scholar]
- Barlas, Y. Model Validation in System Dynamics. In Proceedings of the 1994 International System Dynamics Conference, Stirling, UK, 11–15 July 1994. [Google Scholar]
- Kuzma, J.; Grieger, K.; Cummings, C.; Brown, Z. Pandemics Call for Systems Approaches to Research and Funding. Available online: https://issues.org/pandemics-call-for-systems-approaches/ (accessed on 10 November 2023).
- Meadows, D. Leverage Points: Places to Intervene in a System. Available online: https://donellameadows.org/archives/leverage-points-places-to-intervene-in-a-system/ (accessed on 10 November 2023).
- Carroll, D.; Watson, B.; Togami, E.; Daszak, P.; Mazet, J.A.K.; Chrisman, C.J.; Rubin, E.M.; Wolfe, N.; Morel, C.M.; Gao, G.F.; et al. Building a Global Atlas of Zoonotic Viruses. Bull. World Health Organ. 2018, 96, 292–294. [Google Scholar] [CrossRef] [PubMed]
- Doelger, J.; Chakraborty, A.K.; Kardar, M. A Simple Model for How the Risk of Pandemics from Different Virus Families Depends on Viral and Human Traits. Math. Biosci. 2022, 343, 108732. [Google Scholar] [CrossRef] [PubMed]
- Carroll, S.A.; Towner, J.S.; Sealy, T.K.; McMullan, L.K.; Khristova, M.L.; Burt, F.J.; Swanepoel, R.; Rollin, P.E.; Nichol, S.T. Molecular Evolution of Viruses of the Family Filoviridae Based on 97 Whole-Genome Sequences. J. Virol. 2013, 87, 2608–2616. [Google Scholar] [CrossRef]
- Sanchez, A.; Trappier, S.G.; Mahy, B.W.J.; Peters, C.J.; Nichol, S.T. The Virion Glycoproteins of Ebola Viruses Are Encoded in Two Reading Frames and Are Expressed through Transcriptional Editing. Proc. Natl. Acad. Sci. USA 1996, 93, 3602–3607. [Google Scholar] [CrossRef]
- Deng, L.; Liu, M.; Hua, S.; Peng, Y.; Wu, A.; Qin, F.X.F.; Cheng, G.; Jiang, T. Network of Co-Mutations in Ebola Virus Genome Predicts the Disease Lethality. Cell Res. 2015, 25, 753–756. [Google Scholar] [CrossRef]
- Wong, G.; He, S.; Leung, A.; Cao, W.; Bi, Y.; Zhang, Z.; Zhu, W.; Wang, L.; Zhao, Y.; Cheng, K.; et al. Naturally Occurring Single Mutations in Ebola Virus Observably Impact Infectivity. J. Virlogy 2018, 93, e02185-18. [Google Scholar] [CrossRef] [PubMed]
- Kugelman, J.R.; Sanchez-Lockhart, M.; Andersen, K.G.; Gire, S.; Park, D.J.; Sealfon, R.; Lin, A.E.; Wohl, S.; Sabeti, P.C.; Kuhn, J.H.; et al. Evaluation of the Potential Impact of Ebola Virus Genomic Drift on the Efficacy of Sequence-Based Candidate Therapeutics. mBio 2015, 6, e02227-14. [Google Scholar] [CrossRef] [PubMed]
- Holmes, E.C.; Dudas, G.; Rambaut, A.; Andersen, K.G. The Evolution of Ebola Virus: Insights from the 2013-2016 Epidemic. Nature 2016, 538, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Judson, S.D.; Fischer, R.; Judson, A.; Munster, V.J. Ecological Contexts of Index Cases and Spillover Events of Different Ebolaviruses. PLoS Pathog. 2016, 12, e1005780. [Google Scholar] [CrossRef] [PubMed]
- Pigott, D.M.; Golding, N.; Mylne, A.; Huang, Z.; Henry, A.J.; Weiss, D.J.; Brady, O.J.; Kraemer, M.U.G.; Smith, D.L.; Moyes, C.L.; et al. Mapping the Zoonotic Niche of Ebola Virus Disease in Africa. elife 2014, 3, e04395. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.P.; Park, A.W.; Kramer, A.M.; Han, B.A.; Alexander, L.W.; Drake, J.M. Spatiotemporal Fluctuations and Triggers of Ebola Virus Spillover. Emerg. Infect. Dis. 2017, 23, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Redding, D.W.; Atkinson, P.M.; Cunningham, A.A.; Lo Iacono, G.; Moses, L.M.; Wood, J.L.N.; Jones, K.E. Impacts of Environmental and Socio-Economic Factors on Emergence and Epidemic Potential of Ebola in Africa. Nat. Commun. 2019, 10, 4531. [Google Scholar] [CrossRef]
- Lee-Cruz, L.; Lenormand, M.; Cappelle, J.; Caron, A.; De Nys, H.; Peeters, M.; Bourgarel, M.; Roger, F.; Tran, A. Mapping of Ebola Virus Spillover: Suitability and Seasonal Variability at the Landscape Scale. PLoS Negl. Trop. Dis. 2021, 15, e0009683. [Google Scholar] [CrossRef]
- Telford Mph, C.T.; Amman Phd, B.R.; Towner, J.S.; Bowden Phd, S.; Montgomery, J.M.; Lessler, J.; Shoemaker Phd, T. A Predictive Model of Ebolavirus Spillover Incorporating Change in Forests and Human Populations across Spatial and Temporal Scales. medRxiv 2023. [Google Scholar] [CrossRef]
- Craig, C. How Does Government Listen to Scientists? Springer International Publishing: Berlin/Heidelberg, Germany, 2018; ISBN 9783319960869. [Google Scholar]
- Swallow, B.; Birrell, P.; Blake, J.; Burgman, M.; Challenor, P.; Coffeng, L.E.; Dawid, P.; De Angelis, D.; Goldstein, M.; Hemming, V.; et al. Challenges in Estimation, Uncertainty Quantification and Elicitation for Pandemic Modelling. Epidemics 2022, 38, 100547. [Google Scholar] [CrossRef]
- McCabe, R.; Kont, M.D.; Schmit, N.; Whittaker, C.; Løchen, A.; Walker, P.G.T.; Ghani, A.C.; Ferguson, N.M.; White, P.J.; Donnelly, C.A.; et al. Communicating Uncertainty in Epidemic Models. Epidemics 2021, 37, 100520. [Google Scholar] [CrossRef] [PubMed]
- Wells, C.R.; Pandey, A.; Parpia, A.S.; Fitzpatrick, M.C.; Meyers, L.A.; Singer, B.H.; Galvani, A.P. Ebola Vaccination in the Democratic Republic of the Congo. Proc. Natl. Acad. Sci. USA 2019, 116, 10178–10183. [Google Scholar] [CrossRef] [PubMed]
- Chowell, G.; Kiskowski, M. Mathematical and Statistical Modeling for Emerging and Re-Emerging Infectious Diseases: Modeling Ring-Vaccination Strategies to Control Ebola Virus Disease Epidemics; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Area, I.; Ndairou, F.; Nieto, J.J.; Silva, C.J.; Torres, D.F.M. Ebola Model and Optimal Control with Vaccination Constraints. J. Ind. Manag. Optim. 2017, 14, 427–446. [Google Scholar] [CrossRef]
- Robert, A.; Camacho, A.; Edmunds, W.J.; Baguelin, M.; Muyembe Tamfum, J.J.; Rosello, A.; Kéïta, S.; Eggo, R.M. Control of Ebola Virus Disease Outbreaks: Comparison of Health Care Worker-Targeted and Community Vaccination Strategies. Epidemics 2019, 27, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Fan, W.; Guo, X. A Hybrid Simulation Model to Study the Impact of Combined Interventions on Ebola Epidemic. PLoS ONE 2021, 16, e0254044. [Google Scholar] [CrossRef]
- Legrand, J.; Grais, R.F.; Boelle, P.Y.; Valleron, A.J.; Flahault, A. Understanding the Dynamics of Ebola Epidemics. Epidemiol. Infect. 2007, 135, 610–621. [Google Scholar] [CrossRef]
- Potluri, R.; Kumar, A.; Oriol-mathieu, V.; Van Effelterre, T.; Metz, L.; Bhandari, H. Model-Based Evaluation of the Impact of Prophylactic Vaccination Applied to Ebola Epidemics in Sierra Leone and Democratic Republic of Congo. BMC Infect. Dis. 2022, 22, 769. [Google Scholar] [CrossRef]
- Gillespie, D.T. A General Method for Numerically Simulating the Stochastic Time Evolution of Coupled Chemical Reactions. J. Comput. Phys. 1976, 22, 403–434. [Google Scholar] [CrossRef]
- Miller, M.; Barrett, S.; Henderson, D.A. Chapter 62: Control and Eradication. In Disease Control Priorities in Developing Countries; The World Bank: Washington, DC, USA, 2006. [Google Scholar]
- Jones, S.A.; Gopalakrishnan, S.; Ameh, C.A.; White, S.; Van Den Broek, N.R. ‘Women and Babies Are Dying but Not of Ebola’: The Effect of the Ebola Virus Epidemic on the Availability, Uptake and Outcomes of Maternal and Newborn Health Services in Sierra Leone. BMJ Glob. Health 2016, 1, e000065. [Google Scholar] [CrossRef]
- Ngo, T.M.P.; Rogers, B.; Patnaik, R.; Jambai, A.; Sharkey, A.B. The Effect of Ebola Virus Disease on Maternal and Child Health Services and Child Mortality in Sierra Leone, 2014–2015: Implications for COVID-19. Am. J. Trop. Med. Hyg. 2021, 104, 1085–1092. [Google Scholar] [CrossRef]
- WHO. Health Worker Ebola Infections in Guinea, Liberia and Sierra Leone: A Preliminary Report; WHO: Geneva, Switzerland, 2015. [Google Scholar]
- Evans, D.K.; Goldstein, M.; Popova, A. Health-Care Worker Mortality and the Legacy of the Ebola Epidemic. Lancet Glob. Health 2015, 3, e439–e440. [Google Scholar] [CrossRef] [PubMed]
- Doshi, R.H.; Hoff, N.A.; Bratcher, A.; Mukadi, P.; Gadoth, A.; Nicholson, B.P.; Williams, R.; Mukadi, D.; Mossoko, M.; Wasiswa, J.; et al. Risk Factors for Ebola Exposure in Health Care Workers in Boende, Tshuapa Province, Democratic Republic of the Congo. J. Infect. Dis. 2022, 226, 608–615. [Google Scholar] [CrossRef] [PubMed]
- Mate, S.E.; Kugelman, J.R.; Nyenswah, T.G.; Ladner, J.T.; Wiley, M.R.; Cordier-Lassalle, T.; Christie, A.; Schroth, G.P.; Gross, S.M.; Davies-Wayne, G.J.; et al. Molecular Evidence of Sexual Transmission of Ebola Virus. N. Engl. J. Med. 2015, 373, 2448–2454. [Google Scholar] [CrossRef] [PubMed]
- Doshi, R.H.; Fleming, M.; Mukoka, A.K.; Carter, R.J.; Hyde, T.B.; Choi, M.; Nzaji, M.K.; Bateyi, S.H.; Christie, A.; Nichol, S.T.; et al. Vaccination of Contacts of Ebola Virus Disease Survivors to Prevent Further Transmission. Lancet Glob. Health 2020, 8, e1455–e1456. [Google Scholar] [CrossRef] [PubMed]
- Keita, A.K.; Koundouno, F.R.; Faye, M.; Düx, A.; Hinzmann, J.; Diallo, H.; Ayouba, A.; Le Marcis, F.; Soropogui, B.; Ifono, K.; et al. Resurgence of Ebola Virus in 2021 in Guinea Suggests a New Paradigm for Outbreaks. Nature 2021, 597, 539–543. [Google Scholar] [CrossRef]
- Den Boon, S.; Marston, B.J.; Nyenswah, T.G.; Jambai, A.; Barry, M.; Keita, S.; Durski, K.; Senesie, S.S.; Perkins, D.; Shah, A.; et al. Ebola Virus Infection Associated with Transmission from Survivors. Emerg. Infect. Dis. 2019, 25, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Rugarabamu, S.; George, J.; Mbanzulu, K.M.; Mwanyika, G.O.; Misinzo, G.; Mboera, L.E.G. Estimating Risk of Introduction of Ebola Virus Disease from the Democratic Republic of Congo to Tanzania: A Qualitative Assessment. Epidemiologia 2022, 3, 68–80. [Google Scholar] [CrossRef]
- Dada, S.; McKay, G.; Mateus, A.; Lees, S. Lessons Learned from Engaging Communities for Ebola Vaccine Trials in Sierra Leone: Reciprocity, Relatability, Relationships and Respect (the Four R’s). BMC Public. Health 2019, 19, 1665. [Google Scholar] [CrossRef]
- Vinck, P.; Pham, P.N.; Bindu, K.K.; Bedford, J.; Nilles, E.J. Institutional Trust and Misinformation in the Response to the 2018–19 Ebola Outbreak in North Kivu, DR Congo: A Population-Based Survey. Lancet Infect. Dis. 2019, 19, 529–536. [Google Scholar] [CrossRef]
- Zola Matuvanga, T.; Larivière, Y.; Lemey, G.; De Bie, J.; Milolo, S.; Meta, R.; Esanga, E.; Vermeiren, P.P.; Thys, S.; Van Geertruyden, J.P.; et al. Setting-up an Ebola Vaccine Trial in a Remote Area of the Democratic Republic of the Congo: Challenges, Mitigations, and Lessons Learned. Vaccine 2022, 40, 3470–3480. [Google Scholar] [CrossRef]
- WHO. Ebola Virus Disease (EVD) Vaccine Target Product Profile; WHO: Geneva, Switzerland, 2016. [Google Scholar]
- Zaffran, M.; Vandelaer, J.; Kristensen, D.; Melgaard, B.; Yadav, P.; Antwi-Agyei, K.O.; Lasher, H. The Imperative for Stronger Vaccine Supply and Logistics Systems. Vaccine 2013, 31, B73–B80. [Google Scholar] [CrossRef] [PubMed]
- Doshi, R.H.; Garbern, S.C.; Kulkarni, S.; Perera, S.M.; Fleming, M.K.; Muhayangabo, R.F.; Ombeni, A.B.; Tchoualeu, D.D.; Kallay, R.; Song, E.; et al. Ebola Vaccine Uptake and Attitudes among Healthcare Workers in North Kivu, Democratic Republic of the Congo, 2021. Front. Public Health 2023, 11, 1080700. [Google Scholar] [CrossRef] [PubMed]
- Henao-Restrepo, A.M.; Camacho, A.; Longini, I.M.; Watson, C.H.; Edmunds, W.J.; Egger, M.; Carroll, M.W.; Dean, N.E.; Diatta, I.; Doumbia, M.; et al. Efficacy and Effectiveness of an RVSV-Vectored Vaccine in Preventing Ebola Virus Disease: Final Results from the Guinea Ring Vaccination, Open-Label, Cluster-Randomised Trial (Ebola Ça Suffit!). Lancet 2017, 389, 505–518. [Google Scholar] [CrossRef] [PubMed]
- Madewell, Z.J.; Dean, N.E.; Berlin, J.A.; Coplan, P.M.; Davis, K.J.; Struchiner, C.J.; Halloran, M.E. Challenges of Evaluating and Modelling Vaccination in Emerging Infectious Diseases. Epidemics 2021, 37, 100506. [Google Scholar] [CrossRef]
- US FDA Code of Federal Regulations Title 21. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=314&showFR=1&subpartNode=21:5.0.1.1.4.9 (accessed on 10 November 2023).
- Roozendaal, R.; Hendriks, J.; van Effelterre, T.; Spiessens, B.; Dekking, L.; Solforosi, L.; Czapska-Casey, D.; Bockstal, V.; Stoop, J.; Splinter, D.; et al. Nonhuman Primate to Human Immunobridging to Infer the Protective Effect of an Ebola Virus Vaccine Candidate. NPJ Vaccines 2020, 5, 112. [Google Scholar] [CrossRef]
- Bockstal, V.; Leyssen, M.; Heerwegh, D.; Spiessens, B.; Robinson, C.; Stoop, J.N.; Roozendaal, R.; Van Effelterre, T.; Gaddah, A.; Van Roey, G.A.; et al. Non-Human Primate to Human Immunobridging Demonstrates a Protective Effect of Ad26.ZEBOV, MVA-BN-Filo Vaccine against Ebola. NPJ Vaccines 2022, 7, 156. [Google Scholar] [CrossRef]
- Sullivan, N.J.; Martin, J.E.; Graham, B.S.; Nabel, G.J. Correlates of Protective Immunity for Ebola Vaccines: Implications for Regulatory Approval by the Animal Rule. Nat. Rev. Microbiol. 2009, 7, 393–400. [Google Scholar] [CrossRef]
- Plotkin, S.A. Correlates of Vaccine-Induced Immunity. Clin. Infect. Dis. 2008, 47, 401–409. [Google Scholar] [CrossRef]
- WHO. Emergency Use of Unproven Clinical Interventions Outside Clinical Trials: Ethical Considerations; WHO: Geneva, Switzerland, 2022. [Google Scholar]
- WHO. Emergency Use Listing Procedure; WHO: Geneva, Switzerland, 2019. [Google Scholar]
- WHO. Meeting of the Strategic Group of Experts on Immunization, April 2017—Conclusions and Recommendations; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Dellepiane, N.; Pagliusi, S.; Akut, P.; Comellas, S.; De Clercq, N.; Ghadge, S.; Gastineau, T.; McGoldrick, M.; Nurnaeni, I.; Scheppler, L. Alignment in Post-Approval Changes (PAC) Guidelines in Emerging Countries May Increase Timely Access to Vaccines: An Illustrative Assessment by Manufacturers. Vaccine X 2020, 6, 100075. [Google Scholar] [CrossRef]
- Sadique, Z.M.; Edmunds, J.W.; Smith, R.D.; Meerding, W.J.; de Zwart, O.; Brug, J.; Beutels, P. Precautionary Behavior in Response to Perceived Threat of Pandemic Influenza. Emerg. Infect. Dis. 2007, 13, 1307–1313. [Google Scholar] [CrossRef]
- Wilder-Smith, A.; Longini, I.; Zuber, P.L.; Bärnighausen, T.; Edmunds, W.J.; Dean, N.; Spicher, V.M.; Benissa, M.R.; Gessner, B.D. The Public Health Value of Vaccines beyond Efficacy: Methods, Measures and Outcomes. BMC Med. 2017, 15, 138. [Google Scholar] [CrossRef] [PubMed]
- Phelps, C.; Madhavan, G.; Rappuoli, R.; Colwell, R.; Fineberg, H. Beyond Cost-Effectiveness: Using Systems Analysis for Infectious Disease Preparedness. Vaccine 2017, 35, A46–A49. [Google Scholar] [CrossRef] [PubMed]
- isee systems Statistical Builtins. Available online: https://www.iseesystems.com/resources/help/v1-8/Content/08-Reference/07-Builtins/Statistical_builtins.htm (accessed on 7 December 2023).
- Bratianu, C.; Bejinaru, R. COVID-19 Induced Emergent Knowledge Strategies. Knowl. Process Manag. 2021, 28, 11–17. [Google Scholar] [CrossRef]
- Dupuy, L.C.; Spiropoulou, C.F.; Towner, J.S.; Spengler, J.R.; Sullivan, N.J.; Montgomery, J.M. Filoviruses: Scientific Gaps and Prototype Pathogen Recommendation. J. Infect. Dis. 2023, 228, S446–S459. [Google Scholar] [CrossRef]
- Dzau, V.J.; Balatbat, C.A.; Offodile, A.C. Closing the Global Vaccine Equity Gap: Equitably Distributed Manufacturing. Lancet 2022, 399, 1924–1926. [Google Scholar] [CrossRef]
- Torreele, E.; Wolfe, D.; Kazatchkine, M.; Sall, A.; Ruxrungtham, K.; Fitchett, J.R.A.; Liu, J.; Kobinger, G.; Vaca-González, C.; Gómez, C.; et al. From Private Incentives to Public Health Need: Rethinking Research and Development for Pandemic Preparedness. Lancet Glob. Health 2023, 11, e1658–e1666. [Google Scholar] [CrossRef]
- Rimoin, A.W.; Lu, K.; Bramble, M.S.; Steffen, I.; Doshi, R.H.; Hoff, N.A.; Mukadi, P.; Nicholson, B.P.; Alfonso, V.H.; Olinger, G.; et al. Ebola Virus Neutralizing Antibodies Detectable in Survivors of TheYambuku, Zaire Outbreak 40 Years after Infection. Journal of Infectious Diseases 2018, 217, 223–231. [Google Scholar] [CrossRef]
- Meakin, S.R.; Tildesley, M.J.; Davis, E.; Keeling, M.J. A Metapopulation Model for the 2018 Ebola Virus Disease Outbreak in Equateur Province in the Democratic Republic of the Congo. bioRxiv 2019. [Google Scholar] [CrossRef]
- UN Population Fund World Population Dashboard Congo, the Democratic Republic of the. Available online: https://www.unfpa.org/data/world-population/CD (accessed on 10 November 2023).
- The World Bank Physicians (per 1,000 People). Available online: https://data.worldbank.org/indicator/SH.MED.PHYS.ZS (accessed on 10 November 2023).
- The World Bank Nurses and Midwives (per 1,000 People). Available online: https://data.worldbank.org/indicator/SH.MED.NUMW.P3 (accessed on 10 November 2023).
- Ripani, T. In DRC, a Young UN Volunteer Tries to Make a Difference in People’s Lives. Available online: https://www.unv.org/Success-stories/drc-young-un-volunteer-tries-make-difference-peoples-lives (accessed on 10 November 2023).
- Henao Restrepo, A.M. Ebola Vaccines Powering the World’s Pandemic Response–Now and in the Future. Available online: https://cdn.who.int/media/docs/default-source/blue-print/9.-ana-maria_session-2_ebola-vax-update_who-consult-afirm_30march2022.pdf?sfvrsn=920308d0_7 (accessed on 10 November 2023).
- WHO Ebola Virus Disease-African Region (AFRO), Democratic Republic of the Congo. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2020-DON284 (accessed on 10 November 2023).
- Chen, L.; Evans, T.; Anand, S.; Ivey Boufford, J.; Brown, H.; Chowdhury, M.; Cueto, M.; Dare, L.; Dussault, G.; Elzinga, G.; et al. Human Resources for Health: Overcoming the Crisis. Lancet 2004, 364, 1984–1990. [Google Scholar] [CrossRef]
- Financing Alliance for Health Investment in Community Health Workers: A Necessity, Not an Option. Available online: https://financingalliance.org/investment-in-community-health-workers-a-necessity-not-an-option/#:~:text=Over%20the%20years%2C%20the%20rate,at%2040%25%5B2%5D (accessed on 10 November 2023).
- Bedford, J. Key Considerations: The Context of North Kivu Province, DRC. Available online: www.socialscienceinaction.org (accessed on 10 November 2023).
- European Medicines Agency Summary of Product Characteristics: Zabdeno. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/zabdeno#product-information-section (accessed on 10 November 2023).
- European Medicines Agency Summary of Product Characteristics: Ervebo. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/ervebo#:~:text=Ervebo%20contains%20a%20virus%20known,or%20no%20effect%20on%20humans (accessed on 10 November 2023).
- CDC Expanded Access Investigational New Drug (IND) Protocol: Ervebo ® (Ebola Zaire Vaccine, Live) Booster Dose for Domestic Pre-Exposure Prophylaxis (PrEP) Vaccination of Adults (≥18 Years of Age) at Potential Occupational Risk for Exposure to Zaire Ebolavirus; 2022. Available online: https://www.cdc.gov/vhf/ebola/pdf/ebola-vaccine-protocol_508.pdf (accessed on 10 November 2023).
- Simon, J.K.; Kennedy, S.B.; Mahon, B.E.; Dubey, S.A.; Grant-Klein, R.J.; Liu, K.; Hartzel, J.; Coller, B.A.G.; Welebob, C.; Hanson, M.E.; et al. Immunogenicity of RVSVΔG-ZEBOV-GP Ebola Vaccine (ERVEBO®) in African Clinical Trial Participants by Age, Sex, and Baseline GP-ELISA Titer: A Post Hoc Analysis of Three Phase 2/3 Trials. Vaccine 2022, 40, 6599–6606. [Google Scholar] [CrossRef]
- Wadoum, R.E.G.; Sevalie, S.; Minutolo, A.; Clarke, A.; Russo, G.; Colizzi, V.; Mattei, M.; Montesano, C. The 2018–2020 Ebola Outbreak in the Democratic Republic of Congo: A Better Response Had Been Achieved Through Inter-State Coordination in Africa. Risk Manag. Healthc. Policy 2021, 14, 4923–4930. [Google Scholar] [CrossRef] [PubMed]
- McLean, C.; Dijkman, K.; Gaddah, A.; Keshinro, B.; Katwere, M.; Douoguih, M.; Robinson, C.; Solforosi, L.; Czapska-Casey, D.; Dekking, L.; et al. Persistence of Immunological Memory as a Potential Correlate of Long-Term, Vaccine-Induced Protection against Ebola Virus Disease in Humans. Front. Immunol. 2023, 14. [Google Scholar] [CrossRef] [PubMed]
- Kiggundu, T.; Ario, A.; Kadobera, D.; Kwesiga, B.; Migisha, R. Notes from the Field: Outbreak of Ebola Virus Disease Caused by Sudan Ebolavirus—Uganda, August–October 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 1457. [Google Scholar] [CrossRef] [PubMed]
- WHO Ebola Trial Candidate Vaccines in Uganda in Record 79 after Outbreak Declared. Available online: https://www.who.int/news/item/09-12-2022-ebola-trial-candidate-vaccines-arrive-in-uganda-in-record-79-days-after-outbreak-declared#:~:text=%C2%A9-,Ebola%20trial%20candidate%20vaccines%20arrive%20in%20Uganda,79%20days%20after%20outbreak%20declared&text=The%20first%20doses%20of%20one,Against%20Ebola%20or%20Tokomeza%20Ebola (accessed on 10 November 2023).
- Wolf, J.; Bruno, S.; Eichberg, M.; Jannat, R.; Rudo, S.; VanRheenen, S.; Coller, B.A. Applying Lessons from the Ebola Vaccine Experience for SARS-CoV-2 and Other Epidemic Pathogens. NPJ Vaccines 2020, 5, 51. [Google Scholar] [CrossRef]
- WHO Expert Committee on Biological Standardization Annex 4: Guidelines on Procedures and Data Requirements for Changes to Approved Vaccines; 2015. Available online: https://www.who.int/publications/m/item/procedures-and-data-requirements-changes-to-approved-vaccines-annex-4-trs-no-993 (accessed on 10 November 2023).
- WHO Ebola Vaccine Dashboard. Available online: https://www.who.int/groups/icg/ebola-virus-disease (accessed on 10 November 2023).
- UNICEF Ebola Vaccine Price Data. Available online: https://www.unicef.org/supply/documents/ebola-vaccine-pricing-data (accessed on 10 November 2023).



(Doses) | Product Shelf-Life (Months) | % Change in Doses Procured | % Change in Doses Wasted | Critical Price per Dose (USD) |
|---|---|---|---|---|
| 500,000 | 36 | 0 | 0 | 75 |
| 42 | −18 | −18 | 91 | |
| 48 | −25 | −33 | 100 | |
| 250,000 | 36 | −50 | −50 | 75 |
| 42 | −55 | −52 | 83 | |
| 48 | −63 | −67 | 100 |
| Parameter | Units | Associated Stakeholder | Baseline Value | Test Value | Average Time to Campaign (Days) | % Change Average from Baseline |
|---|---|---|---|---|---|---|
| Time for WHO PQ review | months | WHO | 4 | T(1, 4, 9) | 1167 | −1.5% |
| Time for NRA to review the dossier | months | DRC | 3 | T(2, 3, 12) | 1185 | 0% |
| Time to publish a position paper | years | WHO | 1 | T(1, 2, 3) | 1422 | 20.0% |
| Time to approve funding | months | GAVI | 2 | T(1, 2, 3) | 1185 | 0% |
| Time to initiate a campaign | months | DRC | 3 | T(2, 3, 6) | 1206 | 1.8% |
| The combined effect of variations across all parameters | 1459 | 23.2% | ||||
| Scenario Number | (Days) | (Days) | (Days) | (Years) | (Years) | % Change in Cases | % Change in Deaths |
|---|---|---|---|---|---|---|---|
| 1 | 2000 | 1095 | 10 | 1 | n/a | 0 | 0 |
| 2 | 2000 | 1095 | 10 | 1 | 3 | 0 | 0 |
| 3 | 2000 | 1095 | 10 | 1 | 2 | −65.6 | −66.0 |
| 4 | 2000 | 1095 | 10 | 1 | 1 | −65.6 | −66.0 |
| 5 | 2000 | 1095 | 10 | 2 | n/a | 0 | 0 |
| 6 | 2000 | 1095 | 10 | 2 | 3 | 0 | 0 |
| 7 | 2000 | 1095 | 10 | 2 | 2 | −83.3 | −83.6 |
| 8 | 2000 | 1095 | 10 | 2 | 1 | −83.3 | −83.6 |
| 9 | 2000 | 1095 | 10 | 3 | n/a | −65.6 | −66.0 |
| 10 | 2000 | 1095 | 10 | 3 | 3 | −65.6 | −66.0 |
| 11 | 2000 | 1095 | 10 | 3 | 2 | −65.6 | −66.0 |
| 12 | 2000 | 1095 | 10 | 3 | 1 | −65.6 | −66.0 |
| Scenario Number | Description | Target Coverage for HCWs (%) | Target Coverage for Non-HCWs (%) | % Change in Cases | % Change in Deaths | Doses Administered per Case Averted |
|---|---|---|---|---|---|---|
| 13 | Low coverage HCWs | 25 | 0 | −37.8 | −37.8 | 2.49 |
| 14 | Medium coverage HCWs | 50 | 0 | −60.8 | −60.9 | 3.09 |
| 15 | High coverage HCWs | 75 | 0 | −74.8 | −75.0 | 3.77 |
| 16 | Full coverage HCWs | 100 | 0 | −83.3 | −83.7 | 4.52 |
| 17 | Low coverage Non-HCWs | 50 | 5 | −82.2 | −83.0 | 365.6 |
| 18 | Medium coverage Non-HCWs | 50 | 10 | −84.0 | −85.2 | 584.2 |
| 19 | High coverage Non-HCWs | 50 | 13 | −84.3 | −85.7 | 702.7 |
| 20 | High coverage for both groups | 100 | 13 | −89.9 | −91.4 | 661.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guttieres, D.; Diepvens, C.; Decouttere, C.; Vandaele, N. Modeling Supply and Demand Dynamics of Vaccines against Epidemic-Prone Pathogens: Case Study of Ebola Virus Disease. Vaccines 2024, 12, 24. https://doi.org/10.3390/vaccines12010024
Guttieres D, Diepvens C, Decouttere C, Vandaele N. Modeling Supply and Demand Dynamics of Vaccines against Epidemic-Prone Pathogens: Case Study of Ebola Virus Disease. Vaccines. 2024; 12(1):24. https://doi.org/10.3390/vaccines12010024
Chicago/Turabian StyleGuttieres, Donovan, Charlot Diepvens, Catherine Decouttere, and Nico Vandaele. 2024. "Modeling Supply and Demand Dynamics of Vaccines against Epidemic-Prone Pathogens: Case Study of Ebola Virus Disease" Vaccines 12, no. 1: 24. https://doi.org/10.3390/vaccines12010024
APA StyleGuttieres, D., Diepvens, C., Decouttere, C., & Vandaele, N. (2024). Modeling Supply and Demand Dynamics of Vaccines against Epidemic-Prone Pathogens: Case Study of Ebola Virus Disease. Vaccines, 12(1), 24. https://doi.org/10.3390/vaccines12010024





