Evaluation and Immunogenicity of Combined Liposome-Based Vaccine Candidates against Hepatitis E and B Viruses in Rhesus Monkeys
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Primates
2.3. Candidate Vaccines
2.3.1. Construction of Recombinant DNA Plasmids
2.3.2. Recombinant Proteins
2.3.3. Liposome Preparations
2.4. Monkey Immunization
2.5. Challenge of Immunized Monkeys
2.6. Monitoring of Monkeys
2.7. Serological Assays
2.8. Molecular Assays
2.9. Statistical Analyses
3. Results
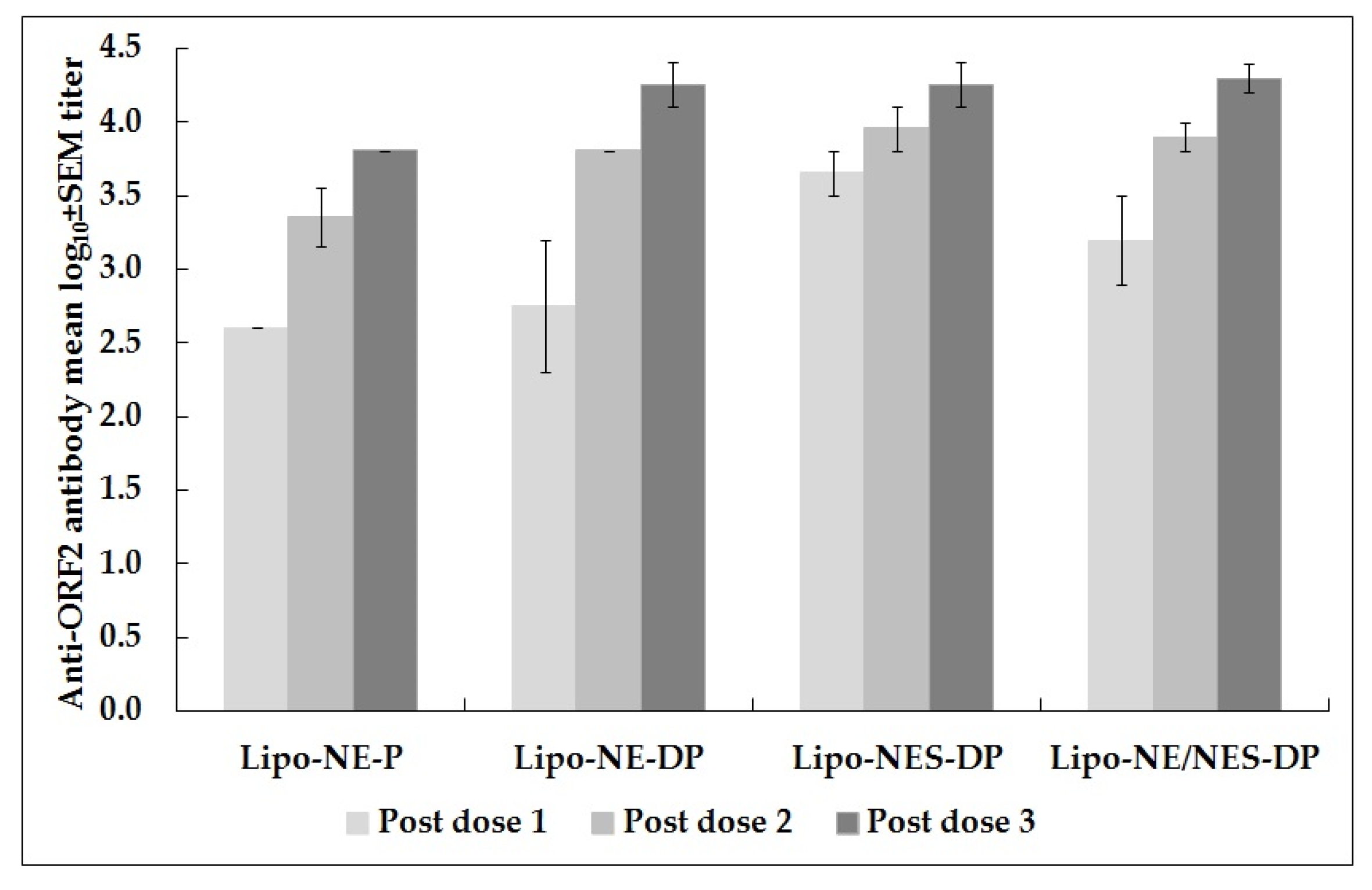
3.1. Immune Response to Anti-HEV Vaccine Candidates in Monovalent and Combined Approaches
3.2. Immune Response to Anti-HBV Vaccine Candidate in Combined Approach
3.3. Dynamics of HEV Infection in Control Monkeys
3.4. Assessment of HEV Infection in Challenged Monkeys
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wong, D.C.; Purcell, R.H.; Sreenivasan, M.A.; Prasad, S.R.; Pavri, K.M. Epidemic and endemic hepatitis in India: Evidence for a non-A, non-B hepatitis aetiology. Lancet 1980, 316, 876–879. [Google Scholar] [CrossRef]
- Aggarwal, R.; Goel, A. Hepatitis E: Current Status in India. Clin. Liver Dis. 2021, 18, 168–172. [Google Scholar] [CrossRef]
- Ma, Z.; de Man, R.A.; Kamar, N.; Pan, Q. Chronic hepatitis E: Advancing research and patient care. J. Hepatol. 2022, 77, 1109–1123. [Google Scholar] [CrossRef]
- Viral Hepatitis—The Silent Disease Facts and Treatment Guidelines. Available online: https://ncdc.mohfw.gov.in/linkimages/guideline_hep20158117187417.pdf (accessed on 20 November 2023).
- Nasir, M.; Wu, G.Y. HEV and HBV Dual Infection: A Review. J. Clin. Transl. Hepatol. 2020, 8, 313–321. [Google Scholar] [CrossRef]
- Premkumar, M.; Chawla, Y.K. Chronic Hepatitis B: Challenges and Successes in India. Clin. Liver Dis. 2021, 18, 111–116. [Google Scholar] [CrossRef]
- Innis, B.L.; Lynch, J.A. Immunization against Hepatitis E. Cold Spring Harb. Perspect. Med. 2018, 8, a032573. [Google Scholar] [CrossRef]
- Lynch, J.A.; Lim, J.K.; Peter, P.E.; Wartel, T.A.; Marti, M.; Yakubu, B.; Rees, H.; Talaat, K.; Kmush, B.L.; Aggarwal, R.; et al. Hepatitis E Vaccine Illuminating the Barriers to Use. PLoS Negl. Trop. Dis. 2023, 17, e0010969. [Google Scholar] [CrossRef]
- Clinical Trials Registry—India (CTRI). 2007. Available online: https://ctri.nic.in/Clinicaltrials/advsearch2.php (accessed on 14 August 2023).
- Arankalle, V.A.; Lole, K.S.; Deshmukh, T.M.; Srivastava, S.; Shaligram, U.S. Challenge Studies in Rhesus Monkeys Immunized with Candidate Hepatitis E Vaccines: DNA, DNA-Prime-Protein-Boost and DNA-Protein Encapsulated in Liposomes. Vaccine 2009, 27, 1032–1039. [Google Scholar] [CrossRef]
- Shrivastava, S.; Lole, K.S.; Tripathy, A.S.; Shaligram, U.S.; Arankalle, V.A. Development of Candidate Combination Vaccine for Hepatitis E and Hepatitis B: A Liposome Encapsulation Approach. Vaccine 2009, 27, 6582–6588. [Google Scholar] [CrossRef]
- Deshmukh, T.M.; Lole, K.S.; Tripathy, A.S.; Arankalle, V.A. Immunogenicity of Candidate Hepatitis E Virus DNA Vaccine Expressing Complete and Truncated ORF2 in Mice. Vaccine 2007, 25, 4350–4360. [Google Scholar] [CrossRef]
- Shouval, D. Immunization against Hepatitis A. Cold Spring Harb. Perspect. Med. 2018, 9, a031682. [Google Scholar] [CrossRef]
- Pattyn, J.; Hendrickx, G.; Vorsters, A.; Van Damme, P. Hepatitis B Vaccines. J. Infect. Dis. 2021, 224 (Suppl. S4), S343–S351. [Google Scholar] [CrossRef]
- Van Damme, P.; Van Herck, K. A Review of the Efficacy, Immunogenicity and Tolerability of a Combined Hepatitis A and B Vaccine. Expert Rev. Vaccines 2004, 3, 249–267. [Google Scholar] [CrossRef]
- Technical Guidelines for Diagnosis & Management of Hepatitis B. 2019. Available online: https://main.mohfw.gov.in/sites/default/files/Technical%20and%20Operational%20_LOW%20RISE_0.pdf (accessed on 24 November 2023).
- Verma, R.; Khanna, P.; Dhankar, M. Vaccination during pregnancy: Today’s need in India. Hum Vaccine Immunother. 2016, 12, 668–670. [Google Scholar] [CrossRef]
- Ferguson, M.; Walker, D.; Mast, E.; Fields, H. Report of a Collaborative Study to Assess the Suitability of a Reference Reagent for Antibodies to Hepatitis E Virus. Biologicals 2002, 30, 43–48. [Google Scholar] [CrossRef]
- Purcell, R.H.; Nguyen, H.; Shapiro, M.; Engle, R.E.; Govindarajan, S.; Blackwelder, W.C.; Wong, D.C.; Jean-Paul Prieels, J.-P.; Emerson, S.U. Pre-clinical immunogenicity and efficacy trial of a recombinant hepatitis E vaccine. Vaccine 2003, 21, 2607–2615. [Google Scholar] [CrossRef]
- Li, S.W.; Zhang, J.; Li, Y.M.; Ou, S.H.; Huang, G.Y.; He, Z.Q.; Ge, S.X.; Xian, Y.L.; Pang, S.Q.; Ng, M.H.; et al. A bacterially expressed particulate hepatitis E vaccine: Antigenicity, immunogenicity and protectivity on primates. Vaccine 2005, 23, 2893–2901. [Google Scholar] [CrossRef]
- Shrestha, M.P.; Scott, R.M.; Joshi, D.M.; Mammen, M.P.; Thapa, G.B.; Thapa, N.; Myint, K.S.A.; Fourneau, M.; Kuschner, R.A.; Shrestha, S.K.; et al. Safety and Efficacy of a Recombinant Hepatitis E Vaccine. N. Engl. J. Med. 2007, 356, 895–903. [Google Scholar] [CrossRef]
- Zhu, F.C.; Zhang, J.; Zhang, X.F.; Zhou, C.; Wang, Z.Z.; Huang, S.J.; Wang, H.; Yang, C.L.; Jiang, H.M.; Cai, J.P.; et al. Efficacy and Safety of a Recombinant Hepatitis E Vaccine in Healthy Adults: A Large-Scale, Randomised, Double-Blind Placebo-Controlled, Phase 3 Trial. Lancet 2010, 376, 895–902. [Google Scholar] [CrossRef]
- Cao, Y.F.; Tao, H.; Hu, Y.M.; Shi, C.B.; Wu, X.; Liang, Q.; Chi, C.P.; Li, L.; Liang, Z.L.; Meng, J.H.; et al. A phase 1 randomized open-label clinical study to evaluate the safety and tolerability of a novel recombinant hepatitis E vaccine. Vaccine 2017, 35, 5073–5080. [Google Scholar] [CrossRef]
- Arankalle, V.A.; Chadha, M.S.; Dama, B.M.; Tsarev, S.A.; Purcell, R.H.; Banerjee, K. Role of immune serum globulins in pregnant women during an epidemic of hepatitis E. J. Viral Hepat. 1998, 5, 199–204. [Google Scholar] [CrossRef]
- Abbasi, J. India’s New COVID-19 DNA Vaccine for Adolescents and Adults Is a First. JAMA 2021, 326, 1365. [Google Scholar] [CrossRef]
- Jack, A.D.; Hall, A.J.; Maine, N.; Mendy, M.; Whittle, H.C. What Level of Hepatitis B Antibody Is Protective? J. Infect. Dis. 1999, 179, 489–492. [Google Scholar] [CrossRef]
- Arankalle, V.A.; Chadha, M.S.; Banerjee, K.; Srinivasan, M.A.; Chobe, L.P. Hepatitis E virus infection in pregnant rhesus monkeys. Indian J. Med. Res. 1993, 97, 4–8. [Google Scholar]
- Zhang, J.; Zhang, X.-F.; Zhou, C.; Wang, Z.-Z.; Huang, S.-J.; Yao, X.; Liang, Z.-L.; Wu, T.; Li, J.-X.; Yan, Q.; et al. Protection against Hepatitis E Virus Infection by Naturally Acquired and Vaccine-Induced Immunity. Clin. Microbiol. Infect. Dis. 2014, 20, PO397–PO405. [Google Scholar] [CrossRef]
- Kulkarni, S.P.; Sharma, M.; Tripathy, A.S. Antibody and Memory B Cell Responses in Hepatitis E Recovered Individuals, 1–30 Years Post Hepatitis E Virus Infection. Sci. Rep. 2019, 9, 4090. [Google Scholar] [CrossRef]
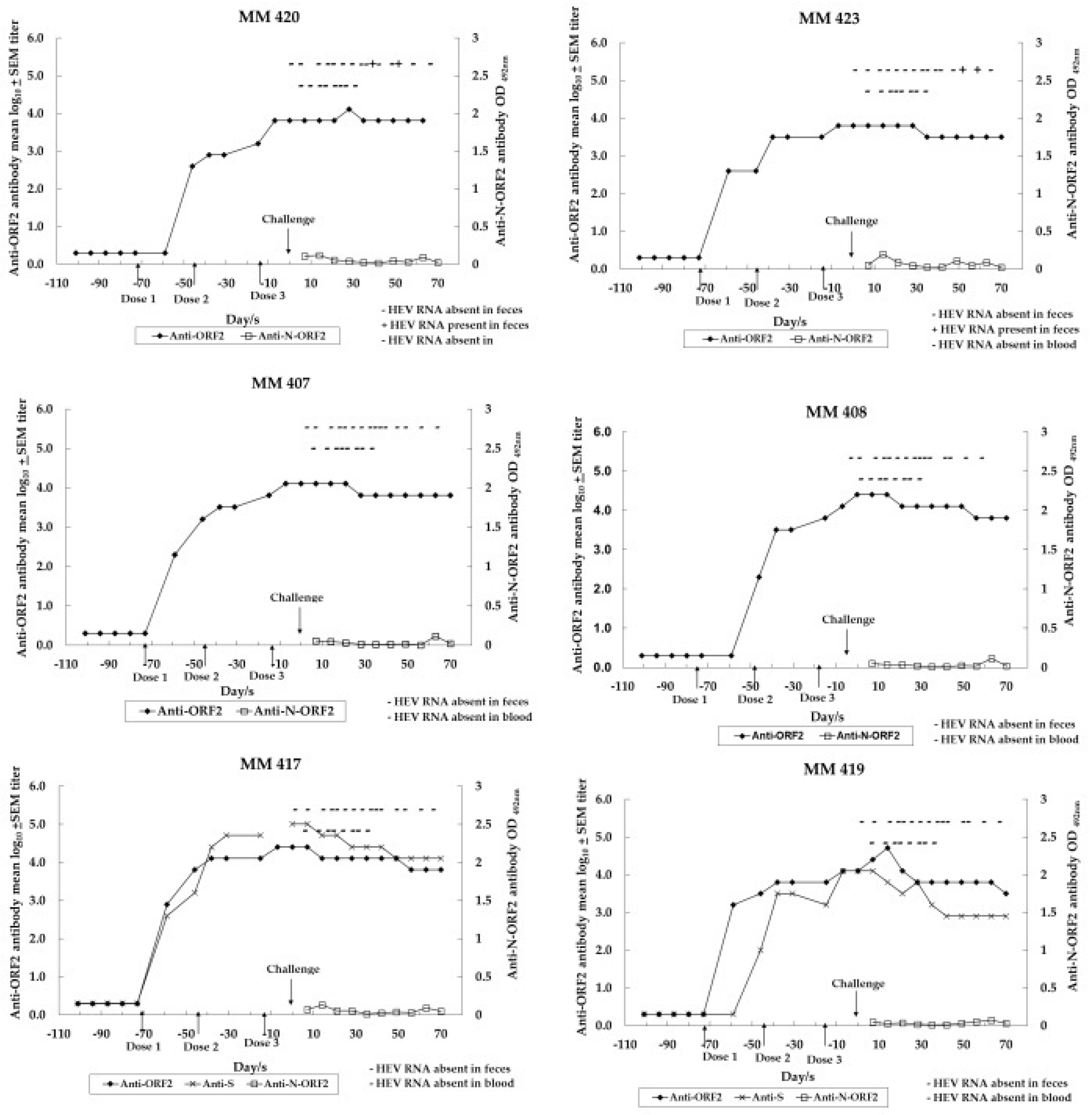
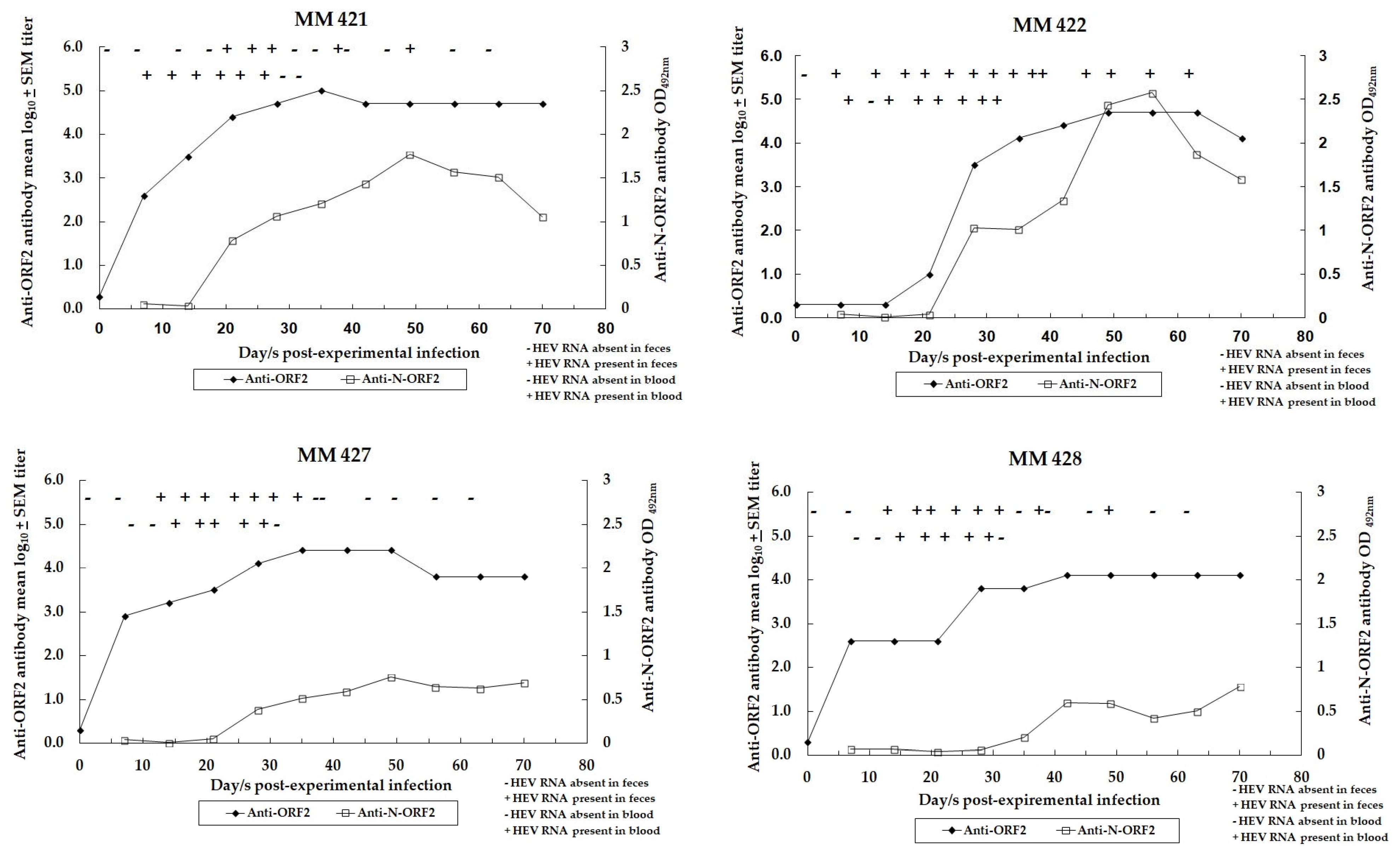

| Monkey ID No. | Vaccine Candidate Formulations in Liposomes or Empty Liposomes | Challenged/Experimentally Infected (HEV RNA Copies/mL) |
|---|---|---|
| MM 420, 423 | Lipo-NE-P (Protein of HEV) | Yes (106) |
| MM 407, 408 | Lipo-NE-DP (DNA + Protein of HEV) | Yes (106) |
| MM 417, 419 | Lipo-NES-DP (DNA + Protein of each of HEV and HBV) | Yes (106) |
| Unimmunized Controls MM 421, 422, 427, 428 | PBS with empty liposomes | Yes (106) |
| Groups | Monkey ID No. | Anti-ORF2 (NE) Antibody Reciprocal and (GMTs/Mean log10 ± SEM) Titers | |||||
|---|---|---|---|---|---|---|---|
| Post-Dose 1 | Post-Dose 2 | Post-Dose 3 | 1 Month PC/EI ** | ~11 Months PC/EI | ~2 Years 3 Months PC/EI | ||
| Control | MM 421 | NA * | NA | NA | 51,200 (12,800) | 12,800 (6400) | 3200 |
| MM 422 | NA | NA | NA | 3200 (400) | 6400 (400) | 3200 | |
| MM 427 | NA | NA | NA | 12,800 (12,800) | 800 (800) | 800 | |
| MM 428 | NA | NA | NA | 6400 (6400) | 3200 (800) | 400 | |
| NA | NA | NA | 10,763.5/4.0 ± 0.3 (4525.5/3.7 ± 0.4) | 3805.5/3.6 ± 0.3 (1131.4/3.1 ± 0.3) | 1345.4/3.1 ± 0.2 | ||
| Lipo-NE-P | MM 420 | 400 (800) | 1600 (1600) | 6400 (6400) | 12,800 (6400) | 200 (400) | 200 |
| MM 423 | 400 (400) | 3200 (3200) | 6400 (6400) | 6400 (6400) | 1600 (800) | 800 | |
| 400/2.6 ± 0.0 (565.7/2.8 ± 0.2) | 2262.7/3.4 ± 0.2 (2262.7/3.4 ± 0.2) | 6400/3.8 ± 0.0 (6400/3.8 ± 0.0) | 9051/4.0 ± 0.2 (6400/3.8 ± 0.0) | 565.7/2.8 ± 0.5 (565.7/2.8 ± 0.2) | 400/2.6 ± 0.3 | ||
| Lipo-NE-DP | MM 407 | 1600 (800) | 6400 (6400) | 12,800 (6400) | 6400 (6400) | 1600 (3200) | 800 |
| MM 408 | 200 (800) | 6400 (6400) | 25,600 (12,800) | 12,800 (6400) | 1600 (400) | 400 | |
| 565.7/2.8 ± 0.5 (800/2.9 ± 0.0) | 6400/3.8 ± 0.0 (6400/3.8 ± 0.0) | 18,101.9/4.3 ± 0.2 (6400/3.8 ± 0.0) | 9051/4.0 ± 0.2 (6400/3.8 ± 0.0) | 1600/3.2 ± 0.0 (1131.4/3.1 ± 0.5) | 565.7/2.8 ± 0.2 | ||
| Lipo-NES-DP | MM 417 | 6400 (1600) | 12,800 (6400) | 25,600 (12,800) | 12,800 (12,800) | 6400 | 1600 |
| MM 419 | 3200 (1600) | 6400 (6400) | 12,800 (6400) | 6400 (3200) | 1600 | 400 | |
| 4525.5/3.7 ± 0.2 (1600/3.2 ± 0.0) | 9051/4.0 ± 0.2 (6400/3.8 ± 0.0) | 18,101.9/4.3 ± 0.2 (9051/4.0 ± 0.2) | 9051/4.0 ± 0.2 (6400/3.8 ± 0.3) | 3200/3.5 ± 0.3 | 800/2.9 ± 0.3 | ||
| Lipo-NE/NES-DP | MM 407 | 1600 (800) | 6400 (6400) | 12,800 (6400) | 6400 (6400) | 1600 (3200) | 800 |
| MM 408 | 200 (800) | 6400 (6400) | 25,600 (12,800) | 12,800 (6400) | 1600 (400) | 400 | |
| MM 417 | 6400 (1600) | 12,800 (6400) | 25,600 (12,800) | 12,800 (12,800) | 6400 | 1600 | |
| MM 419 | 3200 (1600) | 6400 (6400) | 12,800 (6400) | 6400 (3200) | 1600 | 400 | |
| 1600.0/3.2 ± 0.3 (1131.4/3.1 ± 0.1) | 7610.9/3.9 ± 0.1 (6400/3.8 ± 0.0) | 18,101.9/4.3 ± 0.1 (7610.9/3.9 ± 0.1) | 9051/4.0 ± 0.1 (6400/3.8 ± 0.1) | 2262.7/3.4 ± 0.2 | 672.7/2.8 ± 0.1 | ||
| Group | Monkey ID No. | Anti-HBV Antibody Levels in mIU/mL | |||||
|---|---|---|---|---|---|---|---|
| 4 Weeks Post-Dose 1 | 4 Weeks Post-Dose 2 | 2 Weeks Post-Dose 3 before HEV Challenge | 4 Weeks Post-Dose 3, 2 Weeks Post HEV Challenge | ~11 Months, 2 Weeks Post-Dose 3 | ~2 Years 4 Months Post-Dose 3 | ||
| Lipo-NES-DP | MM417 | 130 | 7559 | 59,786 | 89,703 | 26,767 | - |
| MM419 | 6 | 406 | 6213 | 8967 | 8120 | 1760 | |
| Groups | Monkey ID No. | Anti-ORF2 Antibody Titer after Dose 3 before Challenge/5 Weeks Post EI | Peak/Pre-Challenge Ratio of ALT Values (Day after Challenge/EI) | HEV RNA Copies in Feces, Peak Titer/mL in 10% (w/v) Fecal Suspension (Day PC/EI **) |
|---|---|---|---|---|
| Lipo-NE-P | MM 420 | 6400 | 1.0 (12th) | 9.7 × 105 (37th) |
| MM 423 | 6400 | 1.1 (12th) | 1.0 × 105 (49th) | |
| Lipo-NE-DP | MM 407 | 12,800 | 0.9 (12th) | ND a |
| MM 408 | 25,600 | 0.9 (12th) | “ | |
| Lipo-NES-DP | MM 417 | 25,600 | 1.1 (12th) | “ |
| MM 419 | 12,800 | 1.0 (12th) | “ | |
| Control | MM 421 | 102,400 | 1.2 (12th) | 1.4 × 107 (17th) |
| MM 422 | 12,800 | 1.9 (19th) | 8.7 × 108 (17th) | |
| MM 427 | 25,600 | 0.8 (22nd) | 3.0 × 106 (17th) | |
| MM 428 | 6400 | 1.7 (19th) | 2.5 × 106 (20th) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deshmukh, T.; Shah, R.; Devhare, P.; Lole, K.; Arankalle, V. Evaluation and Immunogenicity of Combined Liposome-Based Vaccine Candidates against Hepatitis E and B Viruses in Rhesus Monkeys. Vaccines 2024, 12, 53. https://doi.org/10.3390/vaccines12010053
Deshmukh T, Shah R, Devhare P, Lole K, Arankalle V. Evaluation and Immunogenicity of Combined Liposome-Based Vaccine Candidates against Hepatitis E and B Viruses in Rhesus Monkeys. Vaccines. 2024; 12(1):53. https://doi.org/10.3390/vaccines12010053
Chicago/Turabian StyleDeshmukh, Tejaswini, Rachita Shah, Pradip Devhare, Kavita Lole, and Vidya Arankalle. 2024. "Evaluation and Immunogenicity of Combined Liposome-Based Vaccine Candidates against Hepatitis E and B Viruses in Rhesus Monkeys" Vaccines 12, no. 1: 53. https://doi.org/10.3390/vaccines12010053






