Abstract
Background: Shigellosis is the leading cause of diarrheal deaths worldwide and is particularly dangerous in children under 5 years of age in low- and middle-income countries. Additionally, the rise in antibiotic resistance has highlighted the need for an effective Shigella vaccine. Previously, we have used the Multiple Antigen-Presenting System (MAPS) technology to generate monovalent and quadrivalent Salmonella MAPS vaccines that induce functional antibodies against Salmonella components. Methods: In this work, we detail the development of several monovalent vaccines using O-specific polysaccharides (OSPs) from four dominant serotypes, S. flexneri 2a, 3a, and 6, and S. sonnei. We tested several rhizavidin (rhavi) fusion proteins and selected a Shigella-specific protein IpaB. Quadrivalent MAPS were made with Rhavi-IpaB protein and tested in rabbits for immunogenicity. Results: Individual MAPS vaccines generated robust, functional antibody responses against both IpaB and the individual OSP component. Antibodies to IpaB were effective across Shigella serotypes. We also demonstrate that the OSP antibodies generated are specific to each homologous Shigella O type by performing ELISA and bactericidal assays. We combined the components of each MAPS vaccine to formulate a quadrivalent MAPS vaccine which elicited similar antibody and bactericidal responses compared to their monovalent counterparts. Finally, we show that the quadrivalent MAPS immune sera are functional against several clinical isolates of the serotypes used in the vaccine. Conclusions: This quadrivalent MAPS Shigella vaccine is immunogenicity and warrants further study.
1. Introduction
Shigella is the leading bacterial cause of diarrheal deaths worldwide and is associated with a large global burden of disease [1]. Shigellosis is particularly lethal in children under 5 years old in low- and middle-income countries (LMICs) with an estimated 93,000 child deaths out of 148,000 total deaths globally [2]. Shigella spreads by the fecal–oral route and contaminated food and water; only a small amount of bacteria, estimated to be between 10 and 100 colony-forming units, is necessary to cause disease [3]. A large number of Shigella serotypes can cause disease, therefore presenting a challenge for the development of serotype-specific vaccines [4]. Shigella flexneri is the most common diarrhea-causing species of Shigella in LMICs and consists of at least 15 serotypes. The most common disease-causing S. flexneri serotypes in LMICs are 2a, 3a, and 6. Shigella sonnei is dominant in developing countries and only has one serotype [5]. Shigella dysenteriae and Shigella boydii have been less frequently identified as causes of shigellosis in recent years. Antibiotic resistance is on the rise in Shigella isolates, further complicating therapeutic options [6]. For all these reasons, the development of an effective vaccine against Shigella, particularly one suited for deployment in LMICs, is an urgent priority [7].
Studies of natural Shigella infection have shown that protection after infection is serotype-specific, driven by O-specific polysaccharide antibodies [8,9]. A multivalent Shigella vaccine would be necessary to protect against a majority of diarrheal infections globally [1]. Data from the Global Enterics Multicenter Study (GEMS) suggest that quadrivalent vaccines containing O-antigens from S. flexneri 2a, 3a, and 6, and S. sonnei would offer excellent coverage while limiting the number of serotypes included [5,10]. The OSP repeating units for 2a and 3a share the same backbone of a tetrasaccharide (three rhamnose residues and one N-acetylglucosamine) and a branch α-D-Glcp from different rhamnose residues. The repeating units for 6 are two rhamnose residues, Galactopyranose and N-acetyl Galactopyranose. S. sonnei OSP is composed of two amino sugars: 2-acetamido-2-deoxy-l-altruronic acid (l-AltpNAcA) and a 2-acetamido-4-amino-2,4,6-trideoxy-d-galactopyranose [11,12,13,14].
An alternative approach to O-antigen-based Shigella vaccines is the addition of Ipa proteins, such as IpaB, IpaC, and IpaD, which form part of the external portion of the Shigella type III secretion system that is essential for Shigella uptake into epithelial cells [15]. Immunization with IpaB can protect mice from invasive infection with Shigella strains [16]. The investigation of antibodies in human sera following natural exposure to and immunization with the auxotrophic live Shigella flexneri Y vaccine has demonstrated that Ipa proteins are immunogenic [17,18]. Controlled human infection model studies with candidate Shigella vaccines indicate that IgG to IpaB can correlate with protection [19,20].
In this work, we used the Multiple Antigen-Presenting System (MAPS) technology to develop a Shigella vaccine. The MAPS technology allows for the incorporation of pathogen-specific saccharides and proteins in a multivalent vaccine, through a biotin–rhizavidin interaction, as described previously [21]. We first evaluated and compared several fusion proteins for incorporation into the MAPS and selected the fusion protein IpaB. When IpaB fusion protein was affinity-linked to the OSP from the most prominent Shigella serotypes (S. flexneri 2a, S. flexneri 3a, S. flexneri 6, and S. sonnei) to create monovalent MAPS constructs, we showed that each generates robust IgG antibodies against the polysaccharide and protein in the vaccine. Using bactericidal assays, we demonstrated that immunization of rabbits with monovalent Shigella MAPS candidate vaccines generate functional antibodies against the relevant Shigella serotype. We also show that a combination of these four monovalent MAPS constructs into a quadrivalent MAPS vaccine elicits broad immune responses, including functional antibodies, against Shigella.
2. Materials and Methods
Bacterial strains and reagents: Shigella flexneri strains were obtained from Dr. Sharon Tennant from the University of Maryland. Shigella sonnei 53G was obtained from Dr. Eileen Barry from the University of Maryland. Shigella sonnei Mosely was obtained from Dr. Robert Kaminski from the Walter Reed Institute of Research in the US Army. 1-cyano-4-dimethylaminopyridinium tetrafluoroborate (CDAP) was acquired from Thermo Fisher (Waltham, MA, USA). Restriction endonucleases and T7 Shuffle Express competent cells were obtained from New England Biolabs (Ipswich, MA, USA). Aluminum phosphate (alum) was sourced from Brenntag North America (Reading, PA, USA). The plasmid pETDuet and additional reagents were purchased from Sigma (St. Louis, MO, USA).
DNA fragments encoding His-IpaB and IpgC (the chaperone for IpaB), or His-SseB and SseA (the chaperone for SseB), were codon-optimized, synthesized, and inserted into a pETDuet vector containing Rhizavidin (Rhavi) after digestion by restriction enzymes and ligation. Rhavi-IpaB or Rhavi-SseB was cloned between the NcoI and NotI sites, and IpgC or SseA was cloned between the SpeI and XhoI sites. The plasmids were sequencing-verified and introduced into E. coli T7 Shuffle Express cells. The transformed cells were grown to an OD600 of 1 at 30 °C, and the protein expression was then induced with 0.2 mM IPTG at 16 °C overnight. Cells were collected by centrifugation and pellets were resuspended in lysis buffer (20 mM Tris-HCl, 500 mM NaCl, pH 8.0). Cells were lysed by sonication. The supernatant was applied to a Ni-NTA column, and chaperone proteins were washed off with 0.1% sodium deoxycholate (SDOC) in lysis buffer. Rhavi-SseB and Rhavi-IpaB were eluted with lysis buffer containing 300 mM imidazole. The eluted proteins were pooled and further purified using a gel-filtration column. The dimer fractions were combined and used for MAPS assembly.
OSP purification: OSPs were purified as prescribed previously with modifications [22]. Briefly, bacteria were grown in Luria Broth overnight at 37 °C 220 RPM and centrifuged at 5000 RPM for 10 min to collect cells. A 6% acetic acid solution was used to resuspend cell pellets. The resuspension was boiled for 3 h at 100 °C and then neutralized by adding ammonium hydroxide. After centrifuging at 12,000× g for 30 min, the supernatant was dialyzed extensively against water. Sample was concentrated by lyophilization and then treated with Q cassette and ethanol precipitation. Sample was dialyzed against water a final time and loaded on a gel-filtration column to separate the OSPs with larger sizes. The final OSP was lyophilized and frozen in −20 °C. The strain ID used for OSP purification were 200071 S. flexneri 2a, 603518 S. flexneri 3a, 603345 S. flexneri 6, S. sonnei 53G.
OSP quantification: The concentration of OSP was determined by the anthrone assay for S. flexneri 2a, 3a, and 6 [23]. Briefly, OSP samples were mixed with 1.5 mg/mL of anthrone in 98% sulfuric acid and incubated at 100 °C for 20 min. The reaction was cooled to room temperature and an OD reading was taken at 620 nm with an ELISA reader. The concentration of S. sonnei OSP was determined by HPAEC-PAD as described previously [24].
Biotinylation of OSP: S. flexneri 2a, S. flexneri 3a, S. flexneri 6, and S. sonnei OSP were biotinylated with CDAP using the protocol as described previously [21,25]. All procedures occur at room temperature. Amounts of 2 mg/mL OSP and 100 mg/mL CDAP were stirred for 30 s. An amount of 50 mM sodium borate was stirred into the reaction for 2 min. Amine-PEG3-biotin (40 mg/mL) was added at a ratio of 1:1 (w:w, OSP:biotin) and the reaction continued for 2 h. Glycine was added at a final concentration of 20 mM to stop the reaction. Biotinylated OSP was dialyzed against saline intensively. A No-weigh HABA/avidin Premix Biotin Quantification Kit was used to determine biotin concentration.
MAPS formulation: MAPSs were assembled by combining fusion proteins and biotinylated polysaccharides in a 4:1 (w:w) ratio, rotated overnight at 4 °C and run through a size-exclusion chromatography to separate MAPSs from free protein and OSP. MAPS fractions were combined, and protein and OSP concentrations were determined using the methods mentioned above.
Antigen preparation and immunization procedures: MAPS vaccines were mixed with aluminum phosphate (alum, 0.5 mg/mL final concentration) and saline in a 5 mL tube, and then rotated end-over-end overnight at 4 °C. Rabbit immunization experiments were performed at Cocalico Biologicals Inc. (Denver, PA, USA). Rabbits were immunized intramuscularly with a dose equivalent to 5 μg of OSP per 500 μL injection two times with a three-week interval. Blood draw was taken before each immunization (Pre and P1) and three weeks after the last immunization (P2). All animal experiments were approved by the local Animals Care and Use Committee.
Enzyme-linked immunosorbent assay (ELISA): IgG antibody titers against S. flexneri 2a, S. flexneri 3a, S. flexneri 6, and S. sonnei OSP and proteins IpaB, SseB, and CP1 were measured by ELISA as previously described [26,27]. Briefly, NUNC Maxisorp plates were coated overnight at room temperature. Coating antigens were 100 µL of proteins at 1 µg/mL or OSPS at 10 µg/mL in PBS. Plates were blocked with 200 µL of PBS 1% BSA for 1 h after washing with PBS/Tween solution (PBST). Serum samples were added and incubated for 2 h at room temperature. Plates were washed with PBST and then incubated with secondary antibody diluted 1:10,000 in PBST for 1 h. Plates were developed with Sureblue and stopped with 1M HCl. The absorbances were read at 450 nm.
Bactericidal assay (SBAs): The activity of antisera against Shigella strains in this study was analyzed with SBAs [28]. Briefly, dilutions of heat-inactivated rabbit serum were incubated with the relevant bacteria and complement for two hours at 37 °C. Surviving bacteria were enumerated after overnight incubation on blood agar plates. The killing titer is defined as the inverse of the lowest concentration of serum where 50% of the killing occurred.
Opsonophagocytic assay (OPAs): Activity of antisera from IpaB-immunized rabbits was analyzed using OPAs [29,30]. Briefly, the serum was heat-inactivated before use. Serum was then diluted and incubated with target bacteria to allow the antibody to bind. Next, differentiated HL60 cells and baby rabbit complement were added to the reaction, and killing was continued at 37 °C for 2 h. The reaction was then terminated by the addition of Saponin to 1% final concentration. The surviving bacteria were counted after overnight incubation of the reaction on blood agar plates. The killing titer analysis was conducted the same as in the SBAs.
Statistical analysis: Statistical analysis was carried out using the Mann–Whitney U test and PRISM (version 10.1, GraphPad Software, Inc., Boston, MA, USA).
3. Results
3.1. Selection of Fusion Protein in S. flexneri 3a MAPS Complex
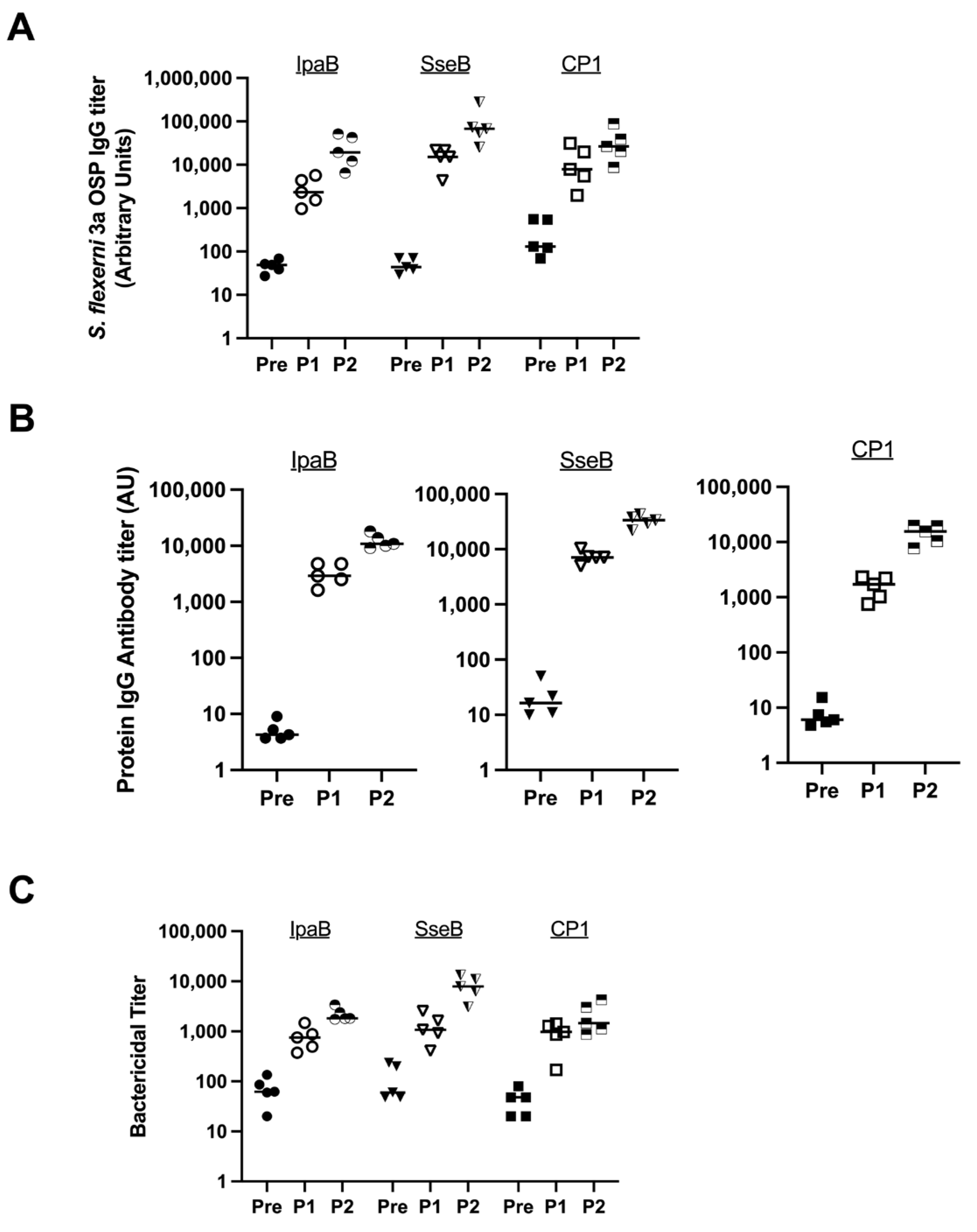
We randomly chose S. flexneri 3a to evaluate three fusion proteins to determine which was the most immunogenic MAPS complex with respect to the OSP. We evaluated Rhavi-IpaB, Rhavi-SseB (an analog of IpaB in Salmonella species), and CP1 (Rhavi-SP1500-SP0785, where SP1500 and SP0785 are two pneumococcal proteins; this fusion protein is included in a 24-valent pneumococcal MAPS vaccine currently in clinical trials) [30,31]. MAPS constructs were formulated at a 4:1 (w:w) ratio of protein to polysaccharide. Each immunization contained 5 µg polysaccharide/dose. In Figure 1, rabbits (n = 5 per group) were immunized with one of three MAPS vaccines twice three weeks apart; sera were collected before the first injection (Pre, solid symbols), three weeks after the first injection (P1, open symbols), and three weeks after the second injection (P2, half-filled symbols). IgG antibody titers against the OSP and relevant proteins in all sera were analyzed using ELISAs.
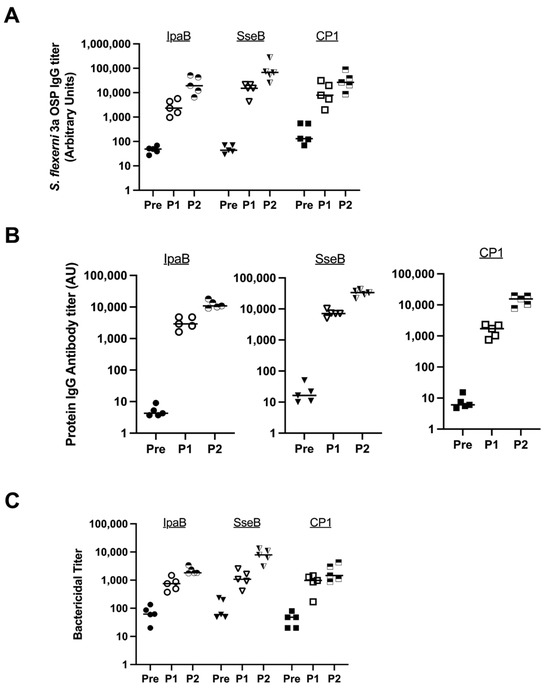
Figure 1.
Comparison of different fusion proteins in MAPS formulations. Pre: rabbit sera prior to MAPS immunization (closed symbols). P1: rabbit sera post one immunization (open symbols). P2: rabbit sera post two immunizations (half-open symbols). (A) Measurement of S. flexneri 3a OSP IgG antibody titer generated from immunization of rabbits with different fusion proteins in the MAPS construct. (B) Protein IgG antibody titer analysis after MAPS constructs immunization. All three proteins are immunogenic. (C) Bactericidal assay to compare killing titers generated from sera of rabbits immunized with different MAPS constructs. Data were collected from two or more experiments; a representative result is shown. Isolate ID for SBA: 603518 S. flexneri 3a.
All three MAPS constructs generated antibodies against the S. flexneri 3a OSP, with responses representing more than a 100-fold increase from baseline after two doses (Figure 1A). There was no statistically significant difference between the antibody titers after two doses between the three MAPS constructs. All three fusion proteins were immunogenic (Figure 1B). The functionality of the antibodies was tested by serum bactericidal assays (SBAs). Each vaccine generated similar and robust killing titers against S. flexneri 3a (Figure 1C).
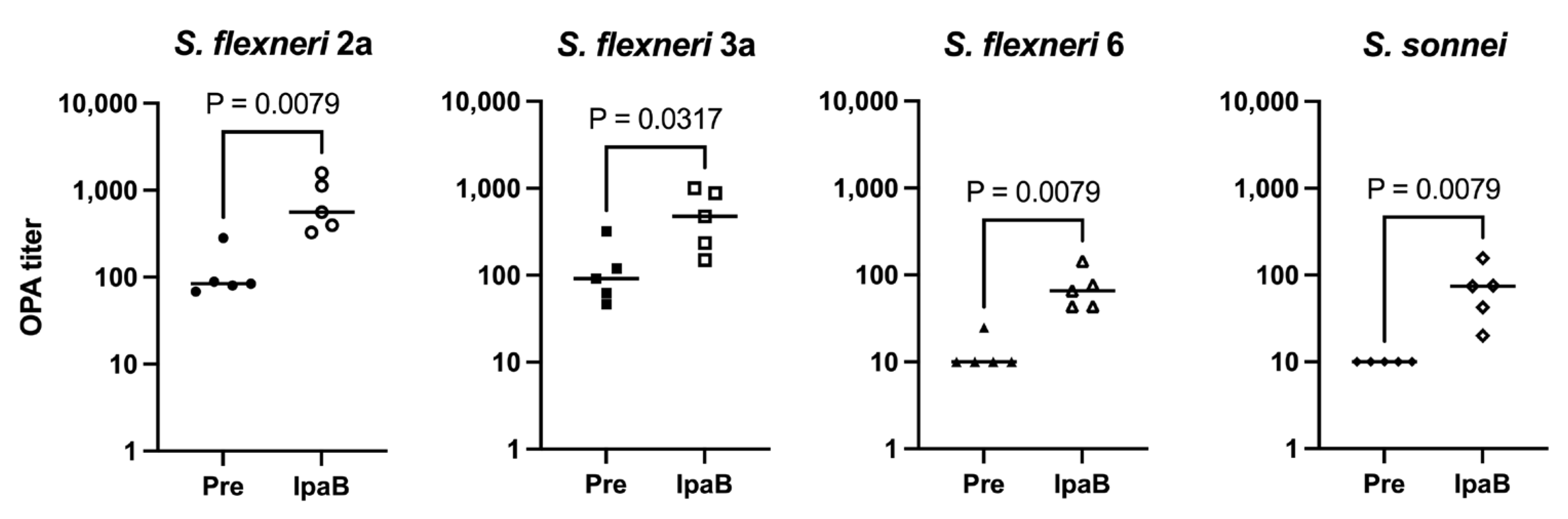
Due to the similar immunogenicity and functional antibody responses between each construct, we evaluated whether the inclusion of IpaB, a protein specific for Shigella, provides additional killing activity. To test this, opsonophagocytic assays (OPAs) were performed against all four Shigella serotypes. Figure 2 shows that immunization with IpaB generates some, albeit modest, killing activity against all four Shigella strains tested compared to sera obtained before immunization. We have also noticed some killing activity from pre-immunization sera against S. flexneri 2a and 3a strains, which leads to different background killing levels. Therefore, we chose IpaB as our fusion protein in the MAPS constructs, hoping that the induction of an anti-IpaB antibody would provide an additional protective effect in addition to the antibody against OSP, as shown from the analysis of human sera from controlled human infection model studies [20,32].
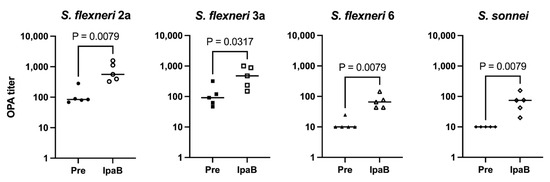
Figure 2.
Ability of IpaB to generate functional antibodies. Pre: rabbit sera prior to IpaB immunization (closed symbols). IpaB: rabbit sera after two IpaB immunizations (open symbols). Opsonophagocytic activity of sera from rabbits immunized with IpaB against S. flexneri 2a, S. flexneri 3a, S. flexneri 6, and S. sonnei. Data were collected from two or more experiments; a representative result is shown. Isolate ID for OPA: 200071 S. flexneri 2a, 603518 S. flexneri 3a, 603345 S. flexneri 6, and S. sonnei 53G.
3.2. Immunogenicity and Functional Analysis of Antibodies after Immunization with Monovalent MAPS
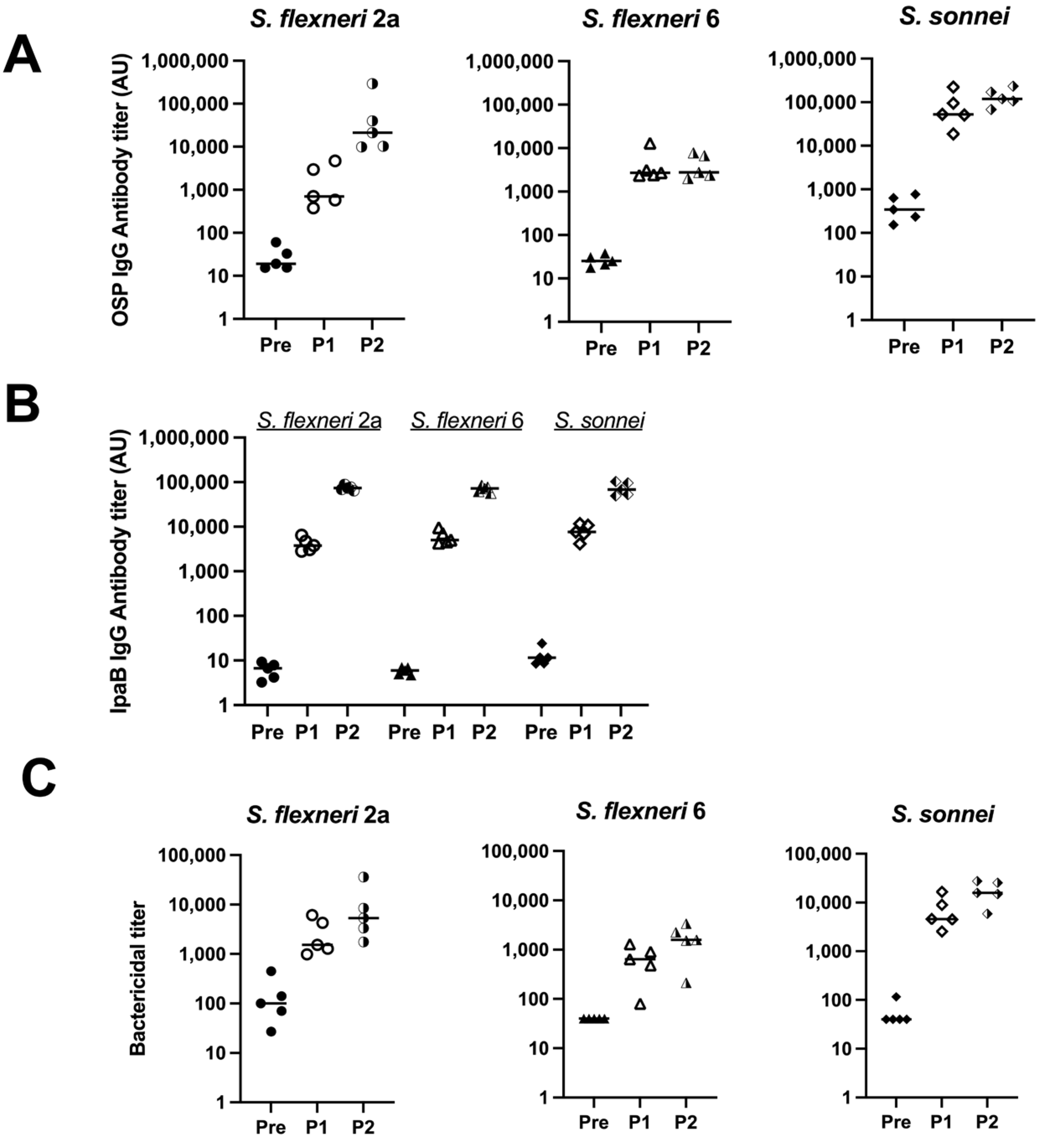
To test the immunogenicity of the MAPS with respect to S. flexneri 2a, S. flexneri 6, and S. sonnei, OSPs were purified from each organism. MAPS constructs were formed using IpaB as the rhizavidin fusion protein. The serum antibody levels against each OSP were analyzed after each rabbit immunization. Robust IgG antibody titers were generated after two doses of each vaccine (Figure 3A, half-filled symbols). The antibodies are specific to each organism within the MAPS, with little to no cross-reactivity between species and serotypes (Figure S1). Each monovalent MAPS construct elicited robust antibodies to the IpaB protein. (Figure 3B). The functionality of generated antibodies was analyzed with SBAs with each organism (Figure 3C). Each MAPS construct individually generated functional antibodies against their respective organism. These data, taken together with Figure 1, demonstrate that MAPS constructs including OSP from S. flexneri 2a, S. flexneri 3a, S. flexneri 6, or S. sonnei and IpaB elicit significant levels of functional antibodies to each component.
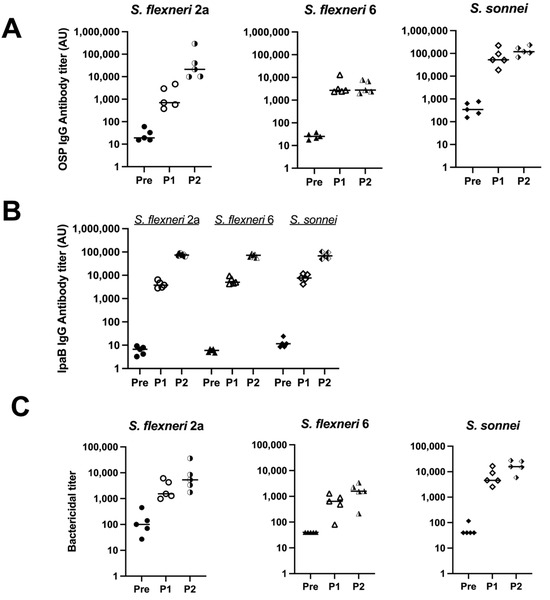
Figure 3.
Quantity and function of IgG antibodies generated from rabbit immunization with monovalent MAPS. Pre: rabbit sera prior to MAPS immunization (closed symbols). P1: rabbit sera post one immunization (open symbols). P2: rabbit sera post two immunizations (half-open symbols). (A) S. flexneri 2a, S. flexneri 6, and S. sonnei OSP antibody titers after each monovalent MAPS constructs rabbit immunization. (B) IpaB IgG antibody titer analysis after each monovalent MAPS constructs immunization. (C) Bactericidal assays to determine activity of antibodies in rabbit sera after one and two doses of MAPS constructs. Data were collected from two or more experiments; a representative result is shown. Isolate ID for SBA: 200071 S. flexneri 2a, 603345 S. flexneri 6, S. sonnei 53G.
3.3. Quadrivalent Shigella MAPS Vaccine
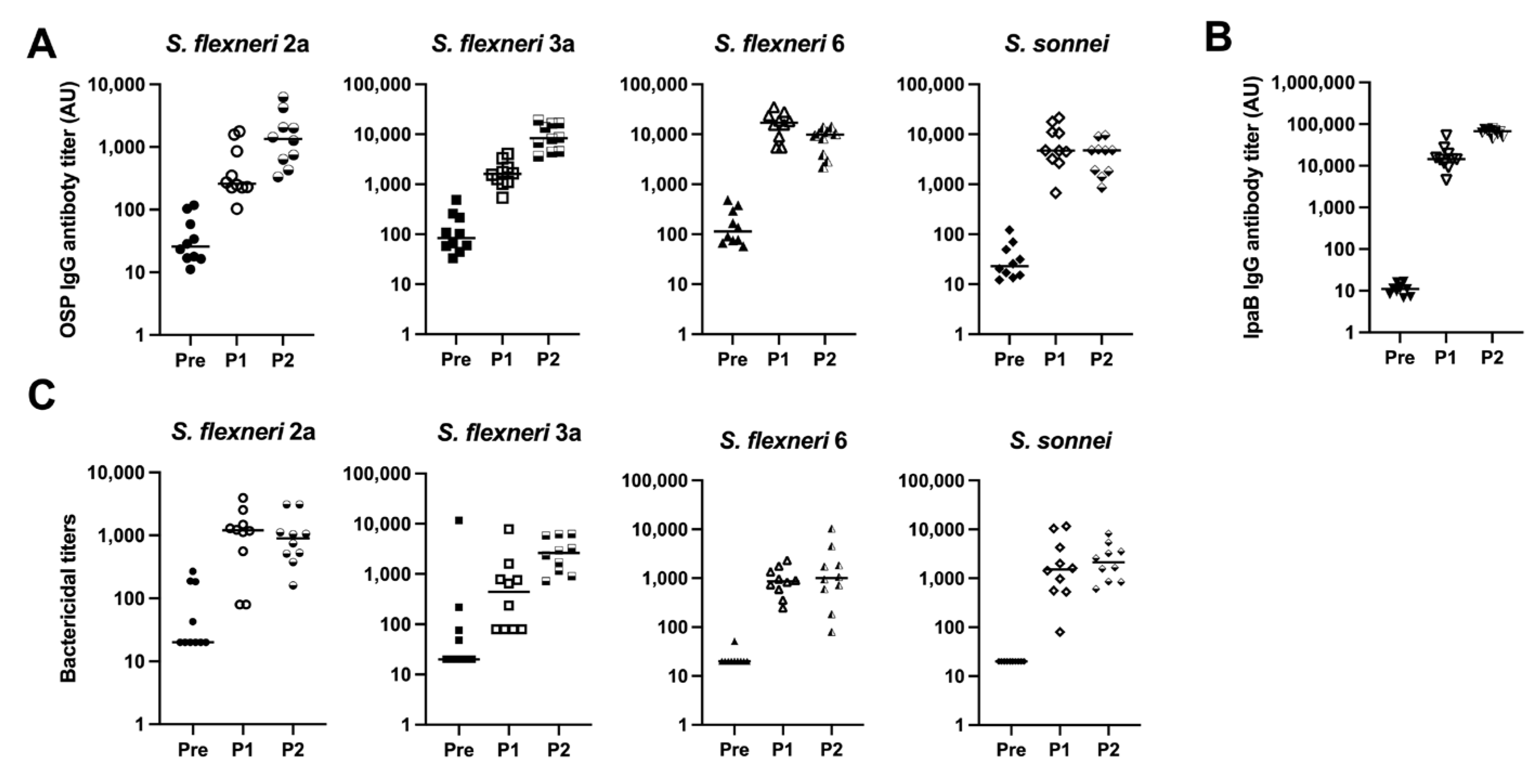
Given the immunogenicity of the monovalent MAPS vaccines, we combined the four monovalent MAPS constructs into one vaccine and immunized rabbits with 5 µg per OSP per dose. The OSP antibody titers were similar to their monovalent counterparts after two doses (Figure 4A). IpaB was immunogenic as expected (Figure 4B). The function of OSP IgG antibodies generated with the quadrivalent MAPS vaccine was studied using bactericidal assays and demonstrated similar killing activity to the monovalent MAPS (Figure 4C). The quadrivalent MAPS sera also provided robust killing against additional clinical strains of each serotype (Figure 5). These data suggest that a quadrivalent Shigella MAPS vaccine is immunogenic and capable of generating functional antibodies to target several clinical strains of the homologous serotype.
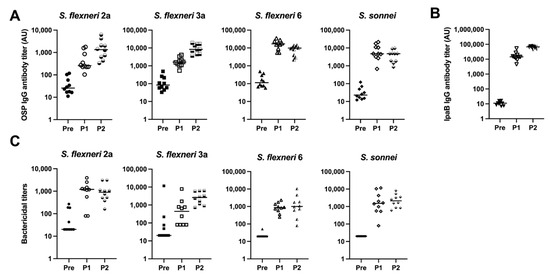
Figure 4.
Analysis of rabbit sera after immunization with Quadrivalent Shigella MAPS. Pre: rabbit sera prior to MAPS immunization (closed symbols). P1: rabbit sera post one immunization (open symbols). P2: rabbit sera post two immunizations (half-open symbols). (A) S. flexneri 2a, S. flexneri 3a, S. flexneri 6, and S. sonnei OSP antibody titers after two rabbit immunizations of quadrivalent MAPS vaccine. (B) IpaB IgG antibody titer analysis after two quadrivalent MAPS vaccine immunizations. (C) Bactericidal assays to determine activity of antibodies in rabbit sera after two doses of quadrivalent MAPS construct. Data were collected from two or more experiments; a representative result is shown. Isolate ID for SBA: 200071 S. flexneri 2a, 603518 S. flexneri 3a, 603345 S. flexneri 6, S. sonnei 53G.
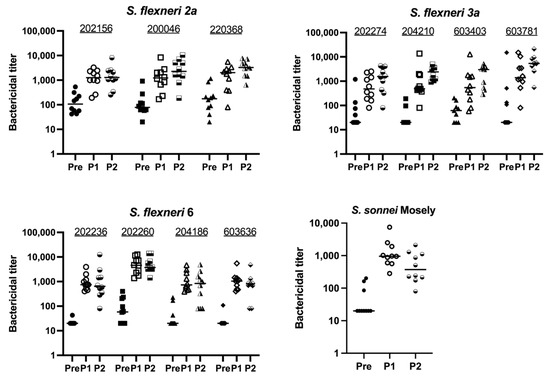
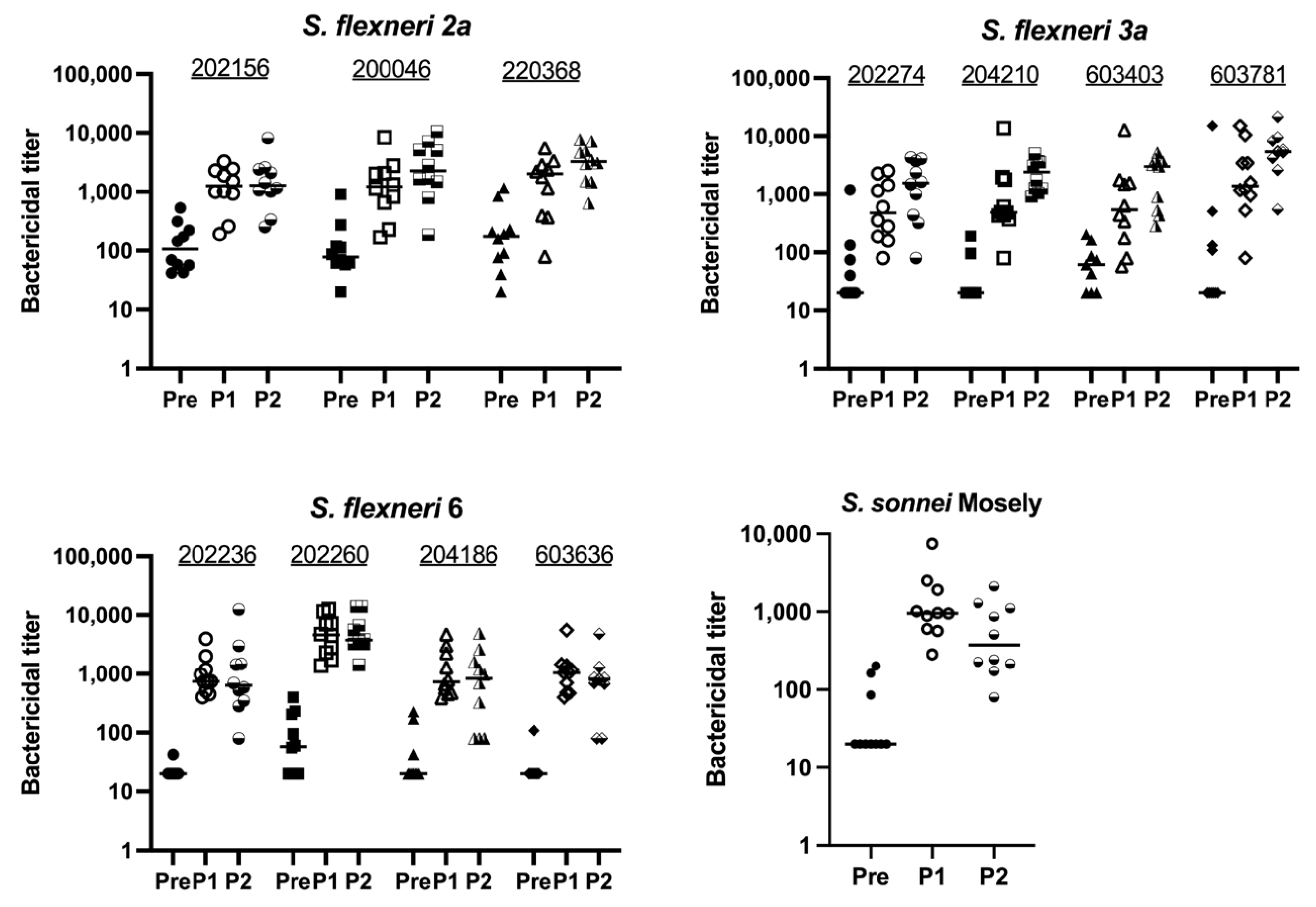
Figure 5.
Bactericidal activity of Quadrivalent Shigella MAPS immunized rabbit sera against different clinical strains. Pre: rabbit sera prior to MAPS immunization (closed symbols). P1: rabbit sera post one immunization (open symbols). P2: rabbit sera post two immunizations (half-open symbols). Sera from rabbits immunized with quadrivalent Shigella MAPS show similar bactericidal activity against other clinical strains of the same serotypes. Data were collected from two or more experiments; a representative result is shown. Isolate IDs are listed on graphs.
4. Discussion
A vaccine against prominent Shigella serotypes could aid in controlling the burden of shigellosis in LMICs. A S. sonnei O-antigen glycoconjugate vaccine was developed by the National Institutes for Health (NIH) and protected Israeli military recruits and children older than three years [33,34]. This work laid the foundations for the development of second-generation O-antigen-based parenteral Shigella vaccines [7]. There are several vaccines being evaluated clinically and preclinically, targeting different Shigella serotypes.
Conjugate and subunit vaccines are being actively tested, due to their low reactivity and in theory a robust immunogenic response [7]. The bioconjugate S. flexneri 2a candidate vaccine, Flexyn2a, has been shown to be safe, immunogenic, and protective against moderate to severe shigellosis in a controlled human intentional challenge model (CHIM) study (NCT02646371, NCT02388009) [35,36]. This vaccine technology is now being used in a tetravalent bioconjugate candidate vaccine Shigella4V by LimmaTech Biologics AG to study its safety, immunogenicity, and dose in Kenyan infants (NCT04056117). InvaplexAR and InvaplexAR-DETOX are subunit vaccines containing IpaB, IpaC, and either wild-type LPS or under-acylated LPS, respectively [37]. Both were safe and immunogenic in phase I studies (NCT02445963, NCT03869333). InvaplexAR generated bactericidal antibodies against S. flexneri 2a and other Shigella species and antibodies were present in the serum of test subjects almost 18 months later [37,38]. Further clinical studies are being designed to evaluate immunogenicity in target populations. Finally, preclinical studies and a Phase 1 study of synthetic carbohydrate-based conjugate vaccine called Sf2a-TT15, a chemically synthesized oligosaccharide linked to tetanus toxoid, yielded promising results [39]. Currently, Sf2a-TT15 is being evaluated in young children and infants in Kenya (NCT04602975), and a 10ug O-antigen equivalent dose vaccine is being evaluated in a CHIM study in North American adults (NCT04078022).
The GSK Vaccines Institute for Global Health (GVGH) has been working on two Generalized Modules for Membrane Antigens (GMMA) vaccines to target Shigella. A Shigella sonnei GMMA vaccine, 1790GAHB, was well tolerated in two Phase 1 studies in healthy adults (NCT02017899, NCT02034500) [40], but gave disappointing results in a CHIM study (NCT03527173) [41]. A Phase 1/2 clinical study of the 4-valent GVGH altSonflex 1-2-3 GMMA vaccine is underway to determine the appropriate dose for further vaccine development (NCT05073003) [4].
Based on the experience with the pneumococcal MAPS, the MAPS technology elicits a strong immune response to each of included antigens, is well tolerated, and is economic to produce [31,42]. Using the MAPS technology, we combined two immunogenic Shigella antigens, IpaB and O-specific polysaccharides, from four dominant serotypes.
Carrier proteins are important for the induction of anti-polysaccharide antibodies for conjugate vaccines [43]. Thus, we have tested different fusion proteins for the shigella MAPS, as we have done previously for the bivalent Vi/Paratyphi A vaccine and the quadrivalent Salmonella Vaccine [29,30]. Consistently with our previous results, the MAPS made with all three fusion proteins induced good anti-OSP antibody which are bactericidal (Figure 1). The inclusion of IpaB appears to generate some cross-serotype opsonophagocytic killing activity (Figure 2), but clinical studies would be required to confirm this effect in humans. We then tested Rhavi-IpaB in combination with the other three OSPs from S. flexneri 2a, 6, and S. sonnei, and found that MAPS immunization generated a high level of antibodies against the three OSPs and the antibodies were highly functional (Figure 3). We also showed that the antibodies generated against these OSPs are very specific, and not much cross-activity was observed (Figure S1).
Combining the four monovalent MAPSs into one quadrivalent vaccine yielded functional antibodies similar to those generated by monovalent constructs (Figure 4). Thus, this suggests that there is little or no interference between these MAPSs. Our quadrivalent Shigella MAPS generates antibodies that exhibit successful bactericidal activity against several clinical strains of the same serotypes (Figure 5), which further proves the specificity of MAPS sera against the included serotypes. In addition, the killing activity of MAPS sera against clinical strains suggests that MAPS vaccine may be functional in clinical settings. While these antibodies demonstrate bactericidal activity in vitro, it is unknown to what extent these will correlate with protection from disease in humans and particularly in young children.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/vaccines12101091/s1, Figure S1: Analysis of OSP IgG antibody specificity to parent strain’s OSP included in MAPS formulation.
Author Contributions
Conceptualization, C.A.M., F.Z., R.M. and Y.-J.L.; Data curation, E.M.B., J.G., R.M. and Y.-J.L.; Formal analysis, E.M.B., F.Z., R.M. and Y.-J.L.; Funding acquisition, Y.-J.L.; Investigation, E.M.B., J.G., B.R., Z.H., L.B., B.S. and S.F.M.; Methodology, E.M.B., F.Z., R.M. and Y.-J.L.; Project administration, C.A.M.; Supervision, Y.-J.L.; Writing—original draft, E.M.B.; Writing—review and editing, C.A.M., R.M. and Y.-J.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Bill and Melinda Gates Foundation.
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Review Board of Cocalico Biologicals Inc.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available upon request.
Acknowledgments
We thank Sharon Tennant for the clinical Shigella strains; Eileen Barry for the S. sonnei 53G strain; and Robert Kaminski for the S. sonnei Mosley strain. We thank Velupillai Puvanesarajah and Robert Corder for providing help with OSP purification and identification. We thank Wendy Picking for the discussion of IpaB purification.
Conflicts of Interest
F.Z., R.M. and Y.-J.L. are inventors of the MAPS technology, which is now owned by GSK. F.Z., R.M. and Y.-J.L. are consultants to GSK.
References
- Fleming, J.A.; Gurley, N.; Knudson, S.; Kabore, L.; Bawa, J.T.; Dapaah, P.; Kumar, S.; Uranw, S.; Tran, T.; Mai, L.T.P.; et al. Exploring Shigella vaccine priorities and preferences: Results from a mixed-methods study in low- and middle-income settings. Vaccine X 2023, 15, 100368. [Google Scholar] [CrossRef] [PubMed]
- Vos, T.; Lim, S.S.; Abbafati, C.; Abbas, K.M.; Abbasi, M.; Abbasifard, M.; Abbasi-Kangevari, M.; Abbastabar, H.; Abd-Allah, F.; Abdelalim, A.; et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef] [PubMed]
- DuPont, H.L.; Levine, M.M.; Hornick, R.B.; Formal, S.B. Inoculum size in shigellosis and implications for expected mode of transmission. J. Infect. Dis. 1989, 159, 1126–1128. [Google Scholar] [CrossRef] [PubMed]
- Micoli, F.; Nakakana, U.N.; Berlanda Scorza, F. Towards a Four-Component GMMA-Based Vaccine against Shigella. Vaccines 2022, 10, 328. [Google Scholar] [CrossRef]
- Kotloff, K.L.; Nataro, J.P.; Blackwelder, W.C.; Nasrin, D.; Farag, T.H.; Panchalingam, S.; Wu, Y.; Sow, S.O.; Sur, D.; Breiman, R.F.; et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): A prospective, case-control study. Lancet 2013, 382, 209–222. [Google Scholar] [CrossRef]
- Vaja, M.D.; Chokshi, H.A.; Jansari, J.J.; Dixit, O.S.; Savaliya, S.S.; Patel, D.P.; Patel, F.S. Study of Antimicrobial Resistance (AMR) in Shigella spp. in India. Recent Adv. Anti-Infect. Drug Discov. 2024, 19, 182–196. [Google Scholar] [CrossRef]
- MacLennan, C.A.; Grow, S.; Ma, L.F.; Steele, A.D. The Shigella Vaccines Pipeline. Vaccines 2022, 10, 1376. [Google Scholar] [CrossRef]
- Formal, S.B.; Oaks, E.V.; Olsen, R.E.; Wingfield-Eggleston, M.; Snoy, P.J.; Cogan, J.P. Effect of prior infection with virulent Shigella flexneri 2a on the resistance of monkeys to subsequent infection with Shigella sonnei. J. Infect. Dis. 1991, 164, 533–537. [Google Scholar] [CrossRef]
- Cohen, D.; Bassal, R.; Goren, S.; Rouach, T.; Taran, D.; Schemberg, B.; Peled, N.; Keness, Y.; Ken-Dror, S.; Vasilev, V.; et al. Recent trends in the epidemiology of shigellosis in Israel. Epidemiol. Infect. 2014, 142, 2583–2594. [Google Scholar] [CrossRef]
- Liu, J.; Platts-Mills, J.A.; Juma, J.; Kabir, F.; Nkeze, J.; Okoi, C.; Operario, D.J.; Uddin, J.; Ahmed, S.; Alonso, P.L.; et al. Use of quantitative molecular diagnostic methods to identify causes of diarrhoea in children: A reanalysis of the GEMS case-control study. Lancet 2016, 388, 1291–1301. [Google Scholar] [CrossRef]
- Kubler-Kielb, J.; Vinogradov, E.; Chu, C.; Schneerson, R. O-Acetylation in the O-specific polysaccharide isolated from Shigella flexneri serotype 2a. Carbohydr. Res. 2007, 342, 643–647. [Google Scholar] [CrossRef] [PubMed]
- Perepelov, A.V.; Shekht, M.E.; Liu, B.; Shevelev, S.D.; Ledov, V.A.; Senchenkova, S.N.; L’Vov, V.L.; Shashkov, A.S.; Feng, L.; Aparin, P.G.; et al. Shigella flexneri O-antigens revisited: Final elucidation of the O-acetylation profiles and a survey of the O-antigen structure diversity. FEMS Immunol. Med. Microbiol. 2012, 66, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Knirel, Y.A.; Feng, L.; Perepelov, A.V.; Senchenkova, S.N.; Wang, Q.; Reeves, P.R.; Wang, L. Structure and genetics of Shigella O antigens. FEMS Microbiol. Rev. 2008, 32, 627–653. [Google Scholar] [CrossRef] [PubMed]
- Kontrohr, T.; Kocsis, B. Structure of the hexose region of Shigella sonnei phase II lipopolysaccharide with 3-deoxy-D-manno-octulosonic acid as possible immunodeterminant and its relation to Escherichia coli R1 core. Eur. J. Biochem. 1978, 88, 267–273. [Google Scholar] [CrossRef]
- Ménard, R.; Prévost, M.C.; Gounon, P.; Sansonetti, P.; Dehio, C. The secreted Ipa complex of Shigella flexneri promotes entry into mammalian cells. Proc. Natl. Acad. Sci. USA 1996, 93, 1254–1258. [Google Scholar] [CrossRef]
- Desalegn, G.; Kapoor, N.; Pill-Pepe, L.; Bautista, L.; Yin, L.; Ndungo, E.; Oaks, E.V.; Fairman, J.; Pasetti, M.F. A Novel Shigella O-Polysaccharide-IpaB Conjugate Vaccine Elicits Robust Antibody Responses and Confers Protection against Multiple Shigella Serotypes. mSphere 2023, 8, e0001923. [Google Scholar] [CrossRef]
- Li, A.; Rong, Z.C.; Ekwall, E.; Forsum, U.; Lindberg, A.A. Serum antibody responses against Shigella lipopolysaccharides and invasion plasmid-coded antigens in Shigella infected Swedish patients. Scand. J. Infect. Dis. 1993, 25, 569–577. [Google Scholar] [CrossRef]
- Van de Verg, L.L.; Herrington, D.A.; Boslego, J.; Lindberg, A.A.; Levine, M.M. Age-specific prevalence of serum antibodies to the invasion plasmid and lipopolysaccharide antigens of Shigella species in Chilean and North American populations. J. Infect. Dis. 1992, 166, 158–161. [Google Scholar] [CrossRef]
- Ndungo, E.; Randall, A.; Hazen, T.H.; Kania, D.A.; Trappl-Kimmons, K.; Liang, X.; Barry, E.M.; Kotloff, K.L.; Chakraborty, S.; Mani, S.; et al. A Novel Shigella Proteome Microarray Discriminates Targets of Human Antibody Reactivity following Oral Vaccination and Experimental Challenge. mSphere 2018, 3, 10-1128. [Google Scholar] [CrossRef]
- Bernshtein, B.; Ndungo, E.; Cizmeci, D.; Xu, P.; Kovac, P.; Kelly, M.; Islam, D.; Ryan, E.T.; Kotloff, K.L.; Pasetti, M.F.; et al. Systems approach to define humoral correlates of immunity to Shigella. Cell Rep. 2022, 40, 111216. [Google Scholar] [CrossRef]
- Zhang, F.; Lu, Y.J.; Malley, R. Multiple antigen-presenting system (MAPS) to induce comprehensive B- and T-cell immunity. Proc. Natl. Acad. Sci. USA 2013, 110, 13564–13569. [Google Scholar] [CrossRef] [PubMed]
- Micoli, F.; Rondini, S.; Gavini, M.; Pisoni, I.; Lanzilao, L.; Colucci, A.M.; Giannelli, C.; Pippi, F.; Sollai, L.; Pinto, V.; et al. A scalable method for O-antigen purification applied to various Salmonella serovars. Anal. Biochem. 2013, 434, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Roe, J.H. The determination of sugar in blood and spinal fluid with anthrone reagent. J. Biol. Chem. 1955, 212, 335–343. [Google Scholar] [CrossRef]
- Giannelli, C.; Raso, M.M.; Palmieri, E.; De Felice, A.; Pippi, F.; Micoli, F. Development of a Specific and Sensitive HPAEC-PAD Method for Quantification of Vi Polysaccharide Applicable to other Polysaccharides Containing Amino Uronic Acids. Anal. Chem. 2020, 92, 6304–6311. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.J.; Forte, S.; Thompson, C.M.; Anderson, P.W.; Malley, R. Protection against Pneumococcal colonization and fatal pneumonia by a trivalent conjugate of a fusion protein with the cell wall polysaccharide. Infect. Immun. 2009, 77, 2076–2083. [Google Scholar] [CrossRef] [PubMed]
- Konadu, E.; Shiloach, J.; Bryla, D.A.; Robbins, J.B.; Szu, S.C. Synthesis, characterization, and immunological properties in mice of conjugates composed of detoxified lipopolysaccharide of Salmonella paratyphi A bound to tetanus toxoid with emphasis on the role of O acetyls. Infect. Immun. 1996, 64, 2709–2715. [Google Scholar] [CrossRef]
- Lu, Y.J.; Zhang, F.; Sayeed, S.; Thompson, C.M.; Szu, S.; Anderson, P.W.; Malley, R. A bivalent vaccine to protect against Streptococcus pneumoniae and Salmonella typhi. Vaccine 2012, 30, 3405–3412. [Google Scholar] [CrossRef]
- Nahm, M.H.; Yu, J.; Weerts, H.P.; Wenzel, H.; Tamilselvi, C.S.; Chandrasekaran, L.; Pasetti, M.F.; Mani, S.; Kaminski, R.W. Development, Interlaboratory Evaluations, and Application of a Simple, High-Throughput Shigella Serum Bactericidal Assay. mSphere 2018, 3, e00146-18. [Google Scholar] [CrossRef]
- Boerth, E.M.; Gong, J.; Roffler, B.; Thompson, C.M.; Song, B.; Malley, S.F.; Hirsch, A.; MacLennan, C.A.; Zhang, F.; Malley, R.; et al. Induction of Broad Immunity against Invasive Salmonella Disease by a Quadrivalent Combination Salmonella MAPS Vaccine Targeting Salmonella Enterica Serovars Typhimurium, Enteritidis, Typhi, and Paratyphi A. Vaccines 2023, 11, 1671. [Google Scholar] [CrossRef]
- Zhang, F.; Boerth, E.M.; Gong, J.; Ma, N.; Lucas, K.; Ledue, O.; Malley, R.; Lu, Y.J. A Bivalent MAPS Vaccine Induces Protective Antibody Responses against Salmonella Typhi and Paratyphi A. Vaccines 2022, 11, 91. [Google Scholar] [CrossRef]
- Chichili, G.R.; Smulders, R.; Santos, V.; Cywin, B.; Kovanda, L.; Van Sant, C.; Malinoski, F.; Sebastian, S.; Siber, G.; Malley, R. Phase 1/2 study of a novel 24-valent pneumococcal vaccine in healthy adults aged 18 to 64 years and in older adults aged 65 to 85 years. Vaccine 2022, 40, 4190–4198. [Google Scholar] [CrossRef] [PubMed]
- Bernshtein, B.; Kelly, M.; Cizmeci, D.; Zhiteneva, J.A.; Macvicar, R.; Kamruzzaman, M.; Bhuiyan, T.R.; Chowdhury, F.; Khan, A.I.; Qadri, F.; et al. Determinants of immune responses predictive of protection against shigellosis in an endemic zone: A systems analysis of antibody profiles and function. Lancet Microbe 2024, 100889. [Google Scholar] [CrossRef] [PubMed]
- Passwell, J.H.; Ashkenazi, S.; Banet-Levi, Y.; Ramon-Saraf, R.; Farzam, N.; Lerner-Geva, L.; Even-Nir, H.; Yerushalmi, B.; Chu, C.; Shiloach, J.; et al. Age-related efficacy of Shigella O-specific polysaccharide conjugates in 1–4-year-old Israeli children. Vaccine 2010, 28, 2231–2235. [Google Scholar] [CrossRef] [PubMed]
- Cohen, D.; Ashkenazi, S.; Green, M.S.; Gdalevich, M.; Robin, G.; Slepon, R.; Yavzori, M.; Orr, N.; Block, C.; Ashkenazi, I.; et al. Double-blind vaccine-controlled randomised efficacy trial of an investigational Shigella sonnei conjugate vaccine in young adults. Lancet 1997, 349, 155–159. [Google Scholar] [CrossRef]
- Riddle, M.S.; Kaminski, R.W.; Di Paolo, C.; Porter, C.K.; Gutierrez, R.L.; Clarkson, K.A.; Weerts, H.E.; Duplessis, C.; Castellano, A.; Alaimo, C.; et al. Safety and Immunogenicity of a Candidate Bioconjugate Vaccine against Shigella flexneri 2a Administered to Healthy Adults: A Single-Blind, Randomized Phase I Study. Clin. Vaccine Immunol. 2016, 23, 908–917. [Google Scholar] [CrossRef]
- Talaat, K.R.; Alaimo, C.; Martin, P.; Bourgeois, A.L.; Dreyer, A.M.; Kaminski, R.W.; Porter, C.K.; Chakraborty, S.; Clarkson, K.A.; Brubaker, J.; et al. Human challenge study with a Shigella bioconjugate vaccine: Analyses of clinical efficacy and correlate of protection. EBioMedicine 2021, 66, 103310. [Google Scholar] [CrossRef]
- Turbyfill, K.R.; Clarkson, K.A.; Oaks, E.V.; Kaminski, R.W. From Concept to Clinical Product: A Brief History of the Novel Shigella Invaplex Vaccine’s Refinement and Evolution. Vaccines 2022, 10, 548. [Google Scholar] [CrossRef]
- Duplessis, C.; Clarkson, K.A.; Ross Turbyfill, K.; Alcala, A.N.; Gutierrez, R.; Riddle, M.S.; Lee, T.; Paolino, K.; Weerts, H.P.; Lynen, A.; et al. GMP manufacture of Shigella flexneri 2a Artificial Invaplex (Invaplex(AR)) and evaluation in a Phase 1 Open-label, dose escalating study administered intranasally to healthy, adult volunteers. Vaccine 2023, 41, 6261–6271. [Google Scholar] [CrossRef]
- Meron-Sudai, S.; Asato, V.; Adler, A.; Bialik, A.; Goren, S.; Ariel-Cohen, O.; Reizis, A.; Mulard, L.A.; Phalipon, A.; Cohen, D. A Shigella flexneri 2a synthetic glycan-based vaccine induces a long-lasting immune response in adults. NPJ Vaccines 2023, 8, 35. [Google Scholar] [CrossRef]
- Launay, O.; Lewis, D.J.M.; Anemona, A.; Loulergue, P.; Leahy, J.; Sciré, A.S.; Maugard, A.; Marchetti, E.; Zancan, S.; Huo, Z.; et al. Safety Profile and Immunologic Responses of a Novel Vaccine against Shigella sonnei Administered Intramuscularly, Intradermally and Intranasally: Results from Two Parallel Randomized Phase 1 Clinical Studies in Healthy Adult Volunteers in Europe. EBioMedicine 2017, 22, 164–172. [Google Scholar] [CrossRef]
- Frenck, R.W., Jr.; Conti, V.; Ferruzzi, P.; Ndiaye, A.G.W.; Parker, S.; McNeal, M.M.; Dickey, M.; Granada, J.P.; Cilio, G.L.; De Ryck, I.; et al. Efficacy, safety, and immunogenicity of the Shigella sonnei 1790GAHB GMMA candidate vaccine: Results from a phase 2b randomized, placebo-controlled challenge study in adults. EClinicalMedicine 2021, 39, 101076. [Google Scholar] [CrossRef] [PubMed]
- Borys, D.; Rupp, R.; Smulders, R.; Chichili, G.R.; Kovanda, L.L.; Santos, V.; Malinoski, F.; Siber, G.; Malley, R.; Sebastian, S. Safety, tolerability and immunogenicity of a novel 24-valent pneumococcal vaccine in toddlers: A phase 1 randomized controlled trial. Vaccine 2024, 42, 2560–2571. [Google Scholar] [CrossRef] [PubMed]
- Pecetta, S.; Lo Surdo, P.; Tontini, M.; Proietti, D.; Zambonelli, C.; Bottomley, M.J.; Biagini, M.; Berti, F.; Costantino, P.; Romano, M.R.; et al. Carrier priming with CRM 197 or diphtheria toxoid has a different impact on the immunogenicity of the respective glycoconjugates: Biophysical and immunochemical interpretation. Vaccine 2015, 33, 314–320. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).