A Population-Based Study of SARS-CoV-2 IgG Antibody Responses to Vaccination in Manitoba
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. Specimens and Serological Assays
2.3. Data Analysis
3. Results
3.1. Specimen Demographics
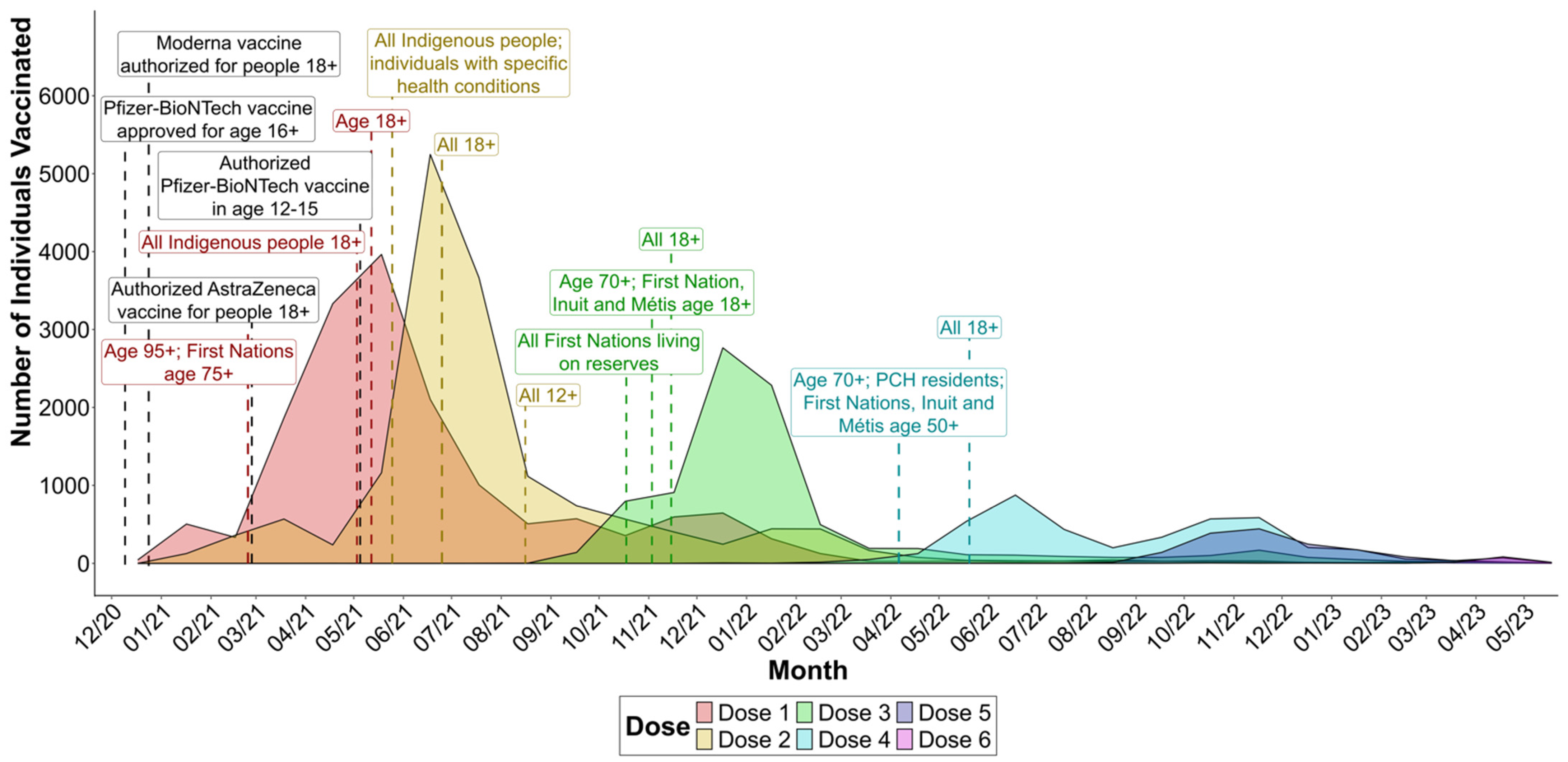
3.2. Vaccine Rollout and Timeline
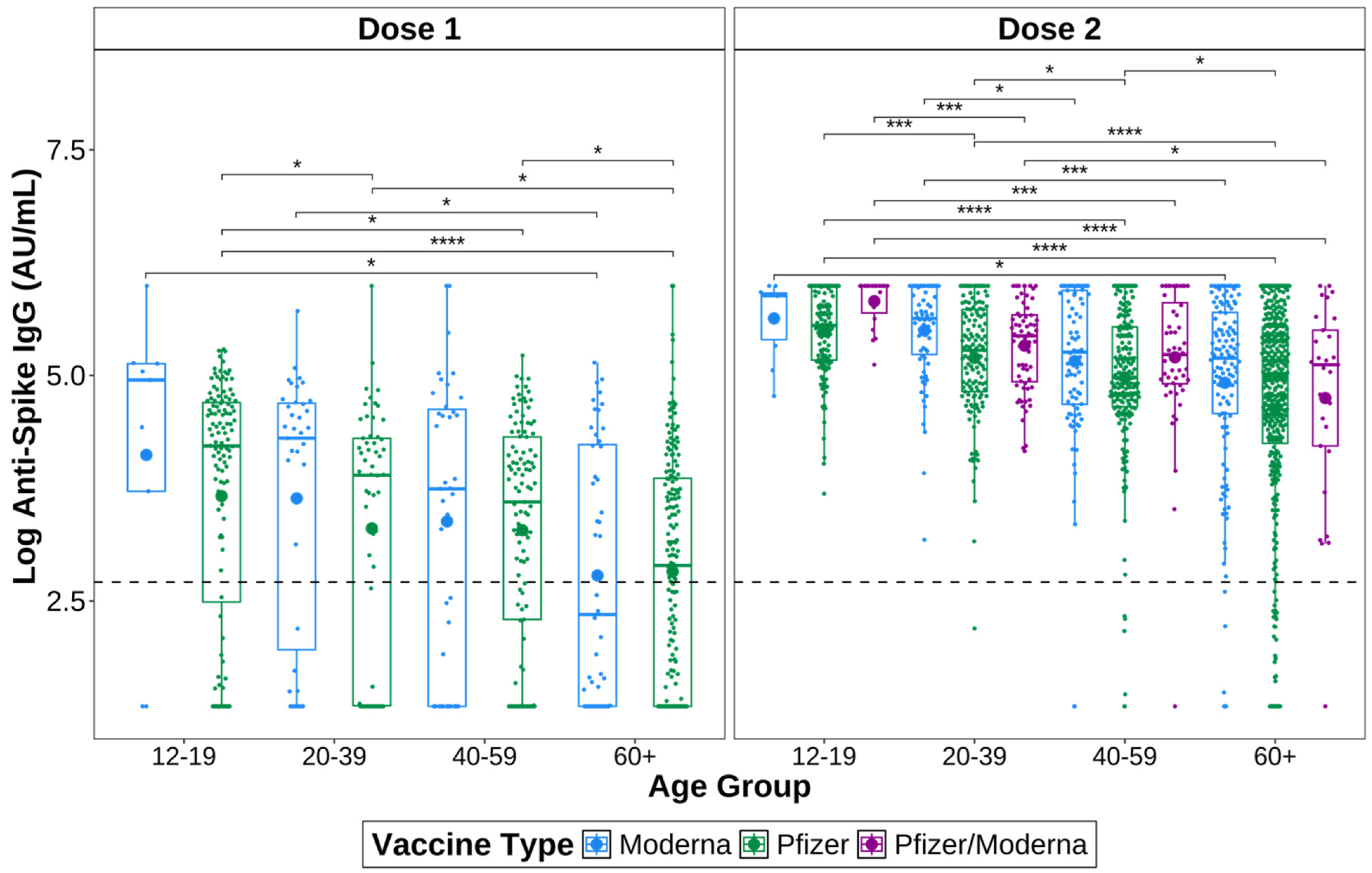
3.3. IgG Titres Vary by Vaccine Type
3.4. IgG Titres Vary by Age
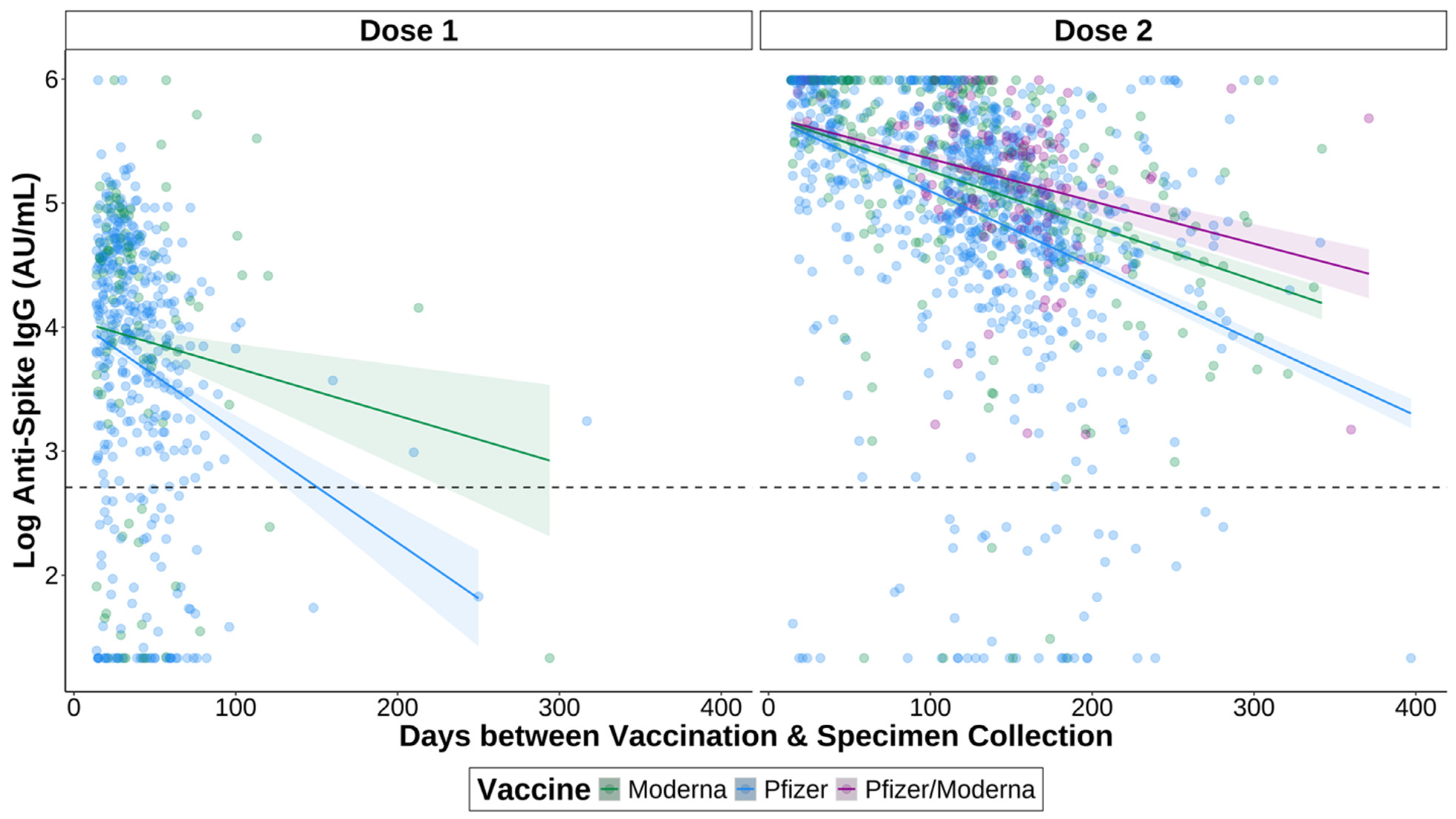
3.5. IgG Titres Vary over Time
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int (accessed on 24 July 2023).
- Watson, O.J.; Barnsley, G.; Toor, J.; Hogan, A.B.; Winskill, P.; Ghani, A.C. Global Impact of the First Year of COVID-19 Vaccination: A Mathematical Modelling Study. Lancet Infect. Dis. 2022, 22, 1293–1302. [Google Scholar] [CrossRef] [PubMed]
- Buchan, S.A.; Chung, H.; Brown, K.A.; Austin, P.C.; Fell, D.B.; Gubbay, J.B.; Nasreen, S.; Schwartz, K.L.; Sundaram, M.E.; Tadrous, M.; et al. Estimated Effectiveness of COVID-19 Vaccines Against Omicron or Delta Symptomatic Infection and Severe Outcomes. JAMA Netw. Open 2022, 5, e2232760. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention Science Brief: COVID-19 Vaccines and Vaccination. Available online: https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/fully-vaccinated-people.html (accessed on 17 July 2023).
- Levine-Tiefenbrun, M.; Yelin, I.; Alapi, H.; Katz, R.; Herzel, E.; Kuint, J.; Chodick, G.; Gazit, S.; Patalon, T.; Kishony, R. Viral Loads of Delta-Variant SARS-CoV-2 Breakthrough Infections after Vaccination and Booster with BNT162b2. Nat. Med. 2021, 27, 2108–2110. [Google Scholar] [CrossRef] [PubMed]
- Mlcochova, P.; Kemp, S.A.; Dhar, M.S.; Papa, G.; Meng, B.; Ferreira, I.A.T.M.; Datir, R.; Collier, D.A.; Albecka, A.; Singh, S.; et al. SARS-CoV-2 B.1.617.2 Delta Variant Replication and Immune Evasion. Nature 2021, 599, 114–119. [Google Scholar] [CrossRef]
- Accorsi, E.K.; Britton, A.; Fleming-Dutra, K.E.; Smith, Z.R.; Shang, N.; Derado, G.; Miller, J.; Schrag, S.J.; Verani, J.R. Association Between 3 Doses of mRNA COVID-19 Vaccine and Symptomatic Infection Caused by the SARS-CoV-2 Omicron and Delta Variants. JAMA 2022, 327, 639–651. [Google Scholar] [CrossRef]
- Meister, T.; Kolde, A.; Fischer, K.; Pisarev, H.; Kolde, R.; Kalda, R.; Suija, K.; Tisler, A.; Uusküla, A. A Retrospective Cohort Study of Incidence and Risk Factors for Severe SARS-CoV-2 Breakthrough Infection among Fully Vaccinated People. Sci. Rep. 2023, 13, 8531. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention COVID-19 Vaccine Effectiveness Update. Available online: https://covid.cdc.gov/covid-data-tracker/#vaccine-effectiveness (accessed on 17 July 2023).
- Health Canada Health Canada Authorizes First COVID-19 Vaccine. Available online: https://www.canada.ca/en/health-canada/news/2020/12/health-canada-authorizes-first-covid-19-vaccine0.html (accessed on 23 November 2023).
- Health Canada Health Canada Authorizes Moderna COVID-19 Vaccine. Available online: https://www.canada.ca/en/health-canada/news/2020/12/health-canada-authorizes-moderna-covid-19-vaccine.html (accessed on 24 November 2023).
- Province of Manitoba|News Releases|COVID-19 Vaccine Bulletin #1. Available online: https://news.gov.mb.ca/news/?archive=&item=50057 (accessed on 13 October 2023).
- Province of Manitoba|News Releases|COVID-19 Vaccine Bulletin #44. Available online: https://news.gov.mb.ca/news/index.html?item=50883&posted=2021-02-26 (accessed on 27 November 2023).
- Coronavirus Vaccination Now Open to Manitobans 95 and over, First Nations 75 and over—Winnipeg|Globalnews.Ca. Available online: https://globalnews.ca/news/7659967/coronavirus-manitoba-vaccination-plan-general-population/ (accessed on 27 November 2023).
- Public Health Agency of Canada COVID-19 Vaccination: Vaccination Coverage. Available online: https://health-infobase.canada.ca/covid-19/vaccination-coverage/#a3 (accessed on 24 July 2023).
- Canada, P.H.A. of Demographics: COVID-19 Vaccination Coverage in Canada—Canada.Ca. Available online: https://health-infobase.canada.ca/covid-19/vaccination-coverage/archive/2023-09-11/#a5 (accessed on 14 December 2023).
- Public Health Agency of Canada COVID-19 Vaccination Technical Notes—Canada.Ca. Available online: https://health-infobase.canada.ca/covid-19/vaccination-coverage/technical-notes.html#a5 (accessed on 28 March 2024).
- Duong, S.; Burtniak, J.; Gretchen, A.; Mai, A.; Klassen, P.; Wei, Y.; Loeppky, C.; Shaw, S.Y.; Bullard, J.; Van Caeseele, P.; et al. Riding High: Seroprevalence of SARS-CoV-2 after 4 Pandemic Waves in Manitoba, Canada, April 2020–February 2022. BMC Public Health 2023, 23, 2420. [Google Scholar] [CrossRef]
- DiaSorin LIASON SARS-CoV-2 S1/S2 IgG ([REF] 311460) 2021. Available online: https://www.fda.gov/media/137359/download (accessed on 22 March 2024).
- Abbott SARS-CoV-2 IgG—For Use with Architect 2022. Available online: https://www.fda.gov/media/137383/download (accessed on 22 March 2024).
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- van den Brand, T. Ggh4x: Hacks for “Ggplot2”. 2023. Available online: https://teunbrand.github.io/ggh4x/index.html (accessed on 30 November 2023).
- Clarke, E.; Sherrill-Mix, S.; Dawson, C. Ggbeeswarm: Categorical Scatter (Violin Point) Plots 2023. Available online: https://cran.r-project.org/web/packages/ggbeeswarm/index.html (accessed on 30 November 2023).
- Sjoberg, D.D.; Whiting, K.; Curry, M.; Lavery, J.A.; Larmarange, J. Reproducible Summary Tables with the Gtsummary Package. R J. 2021, 13, 570–580. [Google Scholar] [CrossRef]
- Lenth, R.V.; Bolker, B.; Buerkner, P.; Giné-Vázquez, I.; Herve, M.; Jung, M.; Love, J.; Miguez, F.; Riebl, H.; Singmann, H. Emmeans: Estimated Marginal Means, Aka Least-Squares Means. 2024. Available online: https://cran.r-project.org/web/packages/emmeans/index.html (accessed on 30 November 2023).
- Kassambara, A. Rstatix: Pipe-Friendly Framework for Basic Statistical Tests. 2023. Available online: https://rpkgs.datanovia.com/rstatix/ (accessed on 30 November 2023).
- R Core Team R: A Language and Environment for Statistical Computing 2023. Available online: https://www.R-project.org (accessed on 30 November 2023).
- Province of Manitoba|News Releases|Manitoba Opens First COVID-19 Immunization Clinic. Available online: https://news.gov.mb.ca/news/index.html?item=50068&posted=2020-12-16 (accessed on 17 January 2024).
- Province of Manitoba|News Releases|COVID-19 Vaccine Bulletin #77. Available online: https://news.gov.mb.ca/news/index.html?item=51254&posted=2021-05-12 (accessed on 27 November 2023).
- Province of Manitoba|News Releases|Province Begins Booking COVID-19 Immunization Appointments to Protect First Priority Group. Available online: https://news.gov.mb.ca/news/?archive=&item=50022 (accessed on 13 October 2023).
- Province of Manitoba|News Releases|COVID-19 Vaccine Bulletin #47. Available online: https://news.gov.mb.ca/news/index.html?item=50922&posted=2021-03-05 (accessed on 27 November 2023).
- Province of Manitoba|News Releases|COVID-19 Vaccine Bulletin #96. Available online: https://news.gov.mb.ca/news/index.html?item=51496&posted=2021-06-25 (accessed on 27 November 2023).
- Province of Manitoba|News Releases|COVID-19 Vaccine Bulletin #139. Available online: https://news.gov.mb.ca/news/index.html?item=52729&posted=2021-11-15 (accessed on 27 November 2023).
- Manitoba Government [@MBGov] Manitobans 18+ Can Now Get Their #COVID19Vaccine Booster Dose Sooner: 4 Months after Being Fully Vaccinated or after Receiving Their 1st Booster Dose. Twitter 2022. Available online: https://x.com/MBGov/status/1527763596075094016 (accessed on 17 January 2024).
- Montoya, J.G.; Adams, A.E.; Bonetti, V.; Deng, S.; Link, N.A.; Pertsch, S.; Olson, K.; Li, M.; Dillon, E.C.; Frosch, D.L. Differences in IgG Antibody Responses Following BNT162b2 and mRNA-1273 SARS-CoV-2 Vaccines. Microbiol. Spectr. 2021, 9, e01162-21. [Google Scholar] [CrossRef]
- Steensels, D.; Pierlet, N.; Penders, J.; Mesotten, D.; Heylen, L. Comparison of SARS-CoV-2 Antibody Response Following Vaccination With BNT162b2 and mRNA-1273. JAMA 2021, 326, 1533–1535. [Google Scholar] [CrossRef]
- Stuart, A.S.V.; Shaw, R.H.; Liu, X.; Greenland, M.; Aley, P.K.; Andrews, N.J.; Cameron, J.C.; Charlton, S.; Clutterbuck, E.A.; Collins, A.M.; et al. Immunogenicity, Safety, and Reactogenicity of Heterologous COVID-19 Primary Vaccination Incorporating mRNA, Viral-Vector, and Protein-Adjuvant Vaccines in the UK (Com-COV2): A Single-Blind, Randomised, Phase 2, Non-Inferiority Trial. Lancet Lond. Engl. 2022, 399, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, K.L.; Fink, A.L.; Plebanski, M.; Klein, S.L. Sex and Gender Differences in the Outcomes of Vaccination over the Life Course. Annu. Rev. Cell Dev. Biol. 2017, 33, 577–599. [Google Scholar] [CrossRef] [PubMed]
- Pellini, R.; Venuti, A.; Pimpinelli, F.; Abril, E.; Blandino, G.; Campo, F.; Conti, L.; De Virgilio, A.; De Marco, F.; Di Domenico, E.G.; et al. Initial Observations on Age, Gender, BMI and Hypertension in Antibody Responses to SARS-CoV-2 BNT162b2 Vaccine. EClinicalMedicine 2021, 36. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M.D.C.R.; Vasconcelos, G.S.; de Melo, A.C.L.; Matsui, T.C.; Caetano, L.F.; de Carvalho Araújo, F.M.; Fonseca, M.H.G. Influence of Age, Gender, Previous SARS-CoV-2 Infection, and Pre-Existing Diseases in Antibody Response after COVID-19 Vaccination: A Review. Mol. Immunol. 2023, 156, 148–155. [Google Scholar] [CrossRef]
- Trevisan, C.; Raparelli, V.; Malara, A.; Abbatecola, A.M.; Noale, M.; Palmieri, A.; Fedele, G.; Di Lonardo, A.; Leone, P.; Schiavoni, I.; et al. Sex Differences in the Efficacy and Safety of SARS-CoV-2 Vaccination in Residents of Long-Term Care Facilities: Insights from the GeroCovid Vax Study. Intern. Emerg. Med. 2023, 18, 1337–1347. [Google Scholar] [CrossRef]
- Northern Health Region. Northern Health Region Community Health Assessment 2019; Northern Health Region: Flin Flon, MB, Canada, 2019.
- Southern Health-Santé Sud. Southern Health-Santé Sud Community Health Assessment 2019; Southern Health-Santé Sud: Southport, MB, Canada, 2019.
- Southern Health-Santé Sud. Southern Health-Santé Sud (SH-SS) 2019 Community Health Assessment Winkler District; Southern Health-Santé Sud: Southport, MB, Canada, 2019.
- Southern Health-Santé Sud. Southern Health-Santé Sud (SH-SS) 2019 Community Health Assessment Stanley District; Southern Health-Santé Sud: Southport, MB, Canada, 2019.
- Froese, I. This Manitoba Community Has a Vaccination Rate of 24% against COVID-19. Here’s Why. CBC News, 29 September 2021. [Google Scholar]
- CTV News Winnipeg INTERACTIVE MAP: COVID-19 Vaccine Uptake in Manitoba. Available online: https://winnipeg.ctvnews.ca/interactive-map-covid-19-vaccine-uptake-in-manitoba-1.5465089 (accessed on 29 May 2024).
- Neustaeter, B. Regions in Alberta, Manitoba Reporting Lowest Vaccination Rates across Canada. Available online: https://www.ctvnews.ca/health/coronavirus/regions-in-alberta-manitoba-reporting-lowest-vaccination-rates-across-canada-1.5541605 (accessed on 29 May 2024).
- Sugiyama, A.; Kurisu, A.; Nagashima, S.; Hando, K.; Saipova, K.; Akhmedova, S.; Abe, K.; Imada, H.; Hussain, M.R.A.; Ouoba, S.; et al. Seroepidemiological Study of Factors Affecting Anti-Spike IgG Antibody Titers after a Two-Dose mRNA COVID-19 Vaccination in 3744 Healthy Japanese Volunteers. Sci. Rep. 2022, 12, 16294. [Google Scholar] [CrossRef]
- Levin, E.G.; Lustig, Y.; Cohen, C.; Fluss, R.; Indenbaum, V.; Amit, S.; Doolman, R.; Asraf, K.; Mendelson, E.; Ziv, A.; et al. Waning Immune Humoral Response to BNT162b2 Covid-19 Vaccine over 6 Months. N. Engl. J. Med. 2021, 385, e84. [Google Scholar] [CrossRef]
- Pérez-Alós, L.; Armenteros, J.J.A.; Madsen, J.R.; Hansen, C.B.; Jarlhelt, I.; Hamm, S.R.; Heftdal, L.D.; Pries-Heje, M.M.; Møller, D.L.; Fogh, K.; et al. Modeling of Waning Immunity after SARS-CoV-2 Vaccination and Influencing Factors. Nat. Commun. 2022, 13, 1614. [Google Scholar] [CrossRef]
- Richards, N.E.; Keshavarz, B.; Workman, L.J.; Nelson, M.R.; Platts-Mills, T.A.E.; Wilson, J.M. Comparison of SARS-CoV-2 Antibody Response by Age Among Recipients of the BNT162b2 vs the mRNA-1273 Vaccine. JAMA Netw. Open 2021, 4, e2124331. [Google Scholar] [CrossRef]
- Cox, L.S.; Bellantuono, I.; Lord, J.M.; Sapey, E.; Mannick, J.B.; Partridge, L.; Gordon, A.L.; Steves, C.J.; Witham, M.D. Tackling Immunosenescence to Improve COVID-19 Outcomes and Vaccine Response in Older Adults. Lancet Healthy Longev. 2020, 1, e55–e57. [Google Scholar] [CrossRef]
- Velásquez García, H.A.; Wilton, J.; Smolina, K.; Chong, M.; Rasali, D.; Otterstatter, M.; Rose, C.; Prystajecky, N.; David, S.; Galanis, E.; et al. Mental Health and Substance Use Associated with Hospitalization among People with COVID-19: A Population-Based Cohort Study. Viruses 2021, 13, 2196. [Google Scholar] [CrossRef] [PubMed]
- Skorupa, M.; Szczepanek, J.; Goroncy, A.; Jarkiewicz-Tretyn, J.; Ptaszyńska, B.; Rajewski, P.; Koper, W.; Pałgan, K.; Tretyn, A. The Dynamics of Changes in the Concentration of IgG against the S1 Subunit in Polish Healthcare Workers in the Period from 1 to 12 Months after Injection, Including Four COVID-19 Vaccines. Vaccines 2022, 10, 506. [Google Scholar] [CrossRef] [PubMed]
- Jeulin, H.; Labat, C.; Duarte, K.; Toupance, S.; Nadin, G.; Craus, D.; Georgiopoulos, I.; Gantois, I.; Goehringer, F.; Benetos, A. Anti-Spike IgG Antibody Kinetics Following the Second and Third Doses of BNT162b2 Vaccine in Nursing Home Residents. J. Am. Geriatr. Soc. 2022, 70, 2552–2560. [Google Scholar] [CrossRef] [PubMed]
- Mubarak, A.; Almutairi, S.; Al-Dhabbah, A.D.; Aldabas, S.Y.; Bhat, R.; Alqoufail, M.M.; Abdel-Maksoud, M.A.; Almanaa, T.N.; Farrag, M.A.; Alturaiki, W. Durability of SARS-CoV-2 Specific IgG Antibody Responses Following Two Doses of Match and Mixed COVID-19 Vaccines Regimens in Saudi Population. Infect. Drug Resist. 2022, 15, 3791–3800. [Google Scholar] [CrossRef]
- Menni, C.; May, A.; Polidori, L.; Louca, P.; Wolf, J.; Capdevila, J.; Hu, C.; Ourselin, S.; Steves, C.J.; Valdes, A.M.; et al. COVID-19 Vaccine Waning and Effectiveness and Side-Effects of Boosters: A Prospective Community Study from the ZOE COVID Study. Lancet Infect. Dis. 2022, 22, 1002–1010. [Google Scholar] [CrossRef]
- Lapuente, D.; Winkler, T.H.; Tenbusch, M. B-Cell and Antibody Responses to SARS-CoV-2: Infection, Vaccination, and Hybrid Immunity. Cell. Mol. Immunol. 2024, 21, 144–158. [Google Scholar] [CrossRef]
- Iyer, A.S.; Jones, F.K.; Nodoushani, A.; Kelly, M.; Becker, M.; Slater, D.; Mills, R.; Teng, E.; Kamruzzaman, M.; Garcia-Beltran, W.F.; et al. Persistence and Decay of Human Antibody Responses to the Receptor Binding Domain of SARS-CoV-2 Spike Protein in COVID-19 Patients. Sci. Immunol. 2020, 5, eabe0367. [Google Scholar] [CrossRef]
- Hartley, G.E.; Edwards, E.S.J.; Aui, P.M.; Varese, N.; Stojanovic, S.; McMahon, J.; Peleg, A.Y.; Boo, I.; Drummer, H.E.; Hogarth, P.M.; et al. Rapid Generation of Durable B Cell Memory to SARS-CoV-2 Spike and Nucleocapsid Proteins in COVID-19 and Convalescence. Sci. Immunol. 2020, 5, eabf8891. [Google Scholar] [CrossRef]
- Turner, J.S.; Kim, W.; Kalaidina, E.; Goss, C.W.; Rauseo, A.M.; Schmitz, A.J.; Hansen, L.; Haile, A.; Klebert, M.K.; Pusic, I.; et al. SARS-CoV-2 Infection Induces Long-Lived Bone Marrow Plasma Cells in Humans. Nature 2021, 595, 421–425. [Google Scholar] [CrossRef]
- Feikin, D.R.; Higdon, M.M.; Abu-Raddad, L.J.; Andrews, N.; Araos, R.; Goldberg, Y.; Groome, M.J.; Huppert, A.; O’Brien, K.L.; Smith, P.G.; et al. Duration of Effectiveness of Vaccines against SARS-CoV-2 Infection and COVID-19 Disease: Results of a Systematic Review and Meta-Regression. Lancet Lond. Engl. 2022, 399, 924–944. [Google Scholar] [CrossRef]
- Keshavarz, B.; Richards, N.E.; Workman, L.J.; Patel, J.; Muehling, L.M.; Canderan, G.; Murphy, D.D.; Brovero, S.G.; Ailsworth, S.M.; Eschenbacher, W.H.; et al. Trajectory of IgG to SARS-CoV-2 After Vaccination With BNT162b2 or mRNA-1273 in an Employee Cohort and Comparison With Natural Infection. Front. Immunol. 2022, 13, 850987. [Google Scholar] [CrossRef] [PubMed]
- Self, W.H.; Tenforde, M.W.; Rhoads, J.P.; Gaglani, M.; Ginde, A.A.; Douin, D.J.; Olson, S.M.; Talbot, H.K.; Casey, J.D.; Mohr, N.M.; et al. Comparative Effectiveness of Moderna, Pfizer-BioNTech, and Janssen (Johnson & Johnson) Vaccines in Preventing COVID-19 Hospitalizations Among Adults Without Immunocompromising Conditions—United States, March–August 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1337–1343. [Google Scholar] [CrossRef] [PubMed]
- Public Health Agency of Canada COVID-19 Vaccines: Canadian Immunization Guide. Available online: https://www.canada.ca/en/public-health/services/publications/healthy-living/canadian-immunization-guide-part-4-active-vaccines/page-26-covid-19-vaccine.html (accessed on 16 January 2024).
- Vinh, D.C.; Gouin, J.-P.; Cruz-Santiago, D.; Canac-Marquis, M.; Bernier, S.; Bobeuf, F.; Sengupta, A.; Brassard, J.-P.; Guerra, A.; Dziarmaga, R.; et al. Real-World Serological Responses to Extended-Interval and Heterologous COVID-19 mRNA Vaccination in Frail, Older People (UNCoVER): An Interim Report from a Prospective Observational Cohort Study. Lancet Healthy Longev. 2022, 3, e166–e175. [Google Scholar] [CrossRef] [PubMed]
- Chauvin, C.; Retnakumar, S.V.; Bayry, J. Obesity Negatively Impacts Maintenance of Antibody Response to COVID-19 Vaccines. Cell Rep. Med. 2023, 4, 101117. [Google Scholar] [CrossRef]
- Tong, M.Z.; Sng, J.D.; Carney, M.; Cooper, L.; Brown, S.; Lineburg, K.E.; Chew, K.Y.; Collins, N.; Ignacio, K.; Airey, M.; et al. Elevated BMI Reduces the Humoral Response to SARS-CoV-2 Infection. Clin. Transl. Immunol. 2023, 12, e1476. [Google Scholar] [CrossRef]

| Characteristic | N | Female, N = 10,308 1 | Male, N = 10,057 1 | Overall 1 |
|---|---|---|---|---|
| Age Group | 20,365 | |||
| 1 to 11 | 1443 (14%) | 1483 (15%) | 2926 (14%) | |
| 12 to 19 | 2040 (20%) | 1843 (18%) | 3883 (19%) | |
| 20 to 39 | 2409 (23%) | 2180 (22%) | 4589 (23%) | |
| 40 to 59 | 2248 (22%) | 2298 (23%) | 4546 (22%) | |
| 60+ | 2168 (21%) | 2253 (22%) | 4421 (22%) | |
| Age | 20,365 | |||
| Median (IQR) | 33 (17, 56) | 36 (17, 58) | 34 (17, 57) | |
| Range | 1, 102 | 1, 101 | 1, 102 | |
| Vaccinated before Specimen Collection | 20,365 | 5194 (50%) | 5228 (52%) | 10,422 (51%) |
| First Dose | 16,558 | |||
| AstraZeneca | 420 (5.0%) | 598 (7.3%) | 1018 (6.1%) | |
| Moderna | 1715 (20%) | 1911 (23%) | 3626 (22%) | |
| Pfizer | 5599 (67%) | 4973 (61%) | 10,572 (64%) | |
| Other | 657 (7.8%) | 685 (8.4%) | 1342 (8.1%) | |
| Second Dose | 15,840 | |||
| Moderna | 2392 (30%) | 2737 (35%) | 5129 (32%) | |
| Pfizer | 5110 (63%) | 4480 (58%) | 9590 (61%) | |
| Other | 551 (6.8%) | 570 (7.3%) | 1121 (7.1%) | |
| Third Dose | 8701 | |||
| Moderna | 761 (18%) | 884 (20%) | 1645 (19%) | |
| Pfizer | 2728 (63%) | 2625 (60%) | 5353 (62%) | |
| Moderna Bivalent | 65 (1.5%) | 72 (1.6%) | 137 (1.6%) | |
| Pfizer Bivalent | 115 (2.7%) | 139 (3.2%) | 254 (2.9%) | |
| Other | 631 (15%) | 681 (15%) | 1312 (15%) | |
| Fourth Dose | 4236 | |||
| Moderna | 103 (5.0%) | 149 (6.9%) | 252 (5.9%) | |
| Pfizer | 842 (41%) | 909 (42%) | 1751 (41%) | |
| Moderna Bivalent | 445 (21%) | 502 (23%) | 947 (22%) | |
| Pfizer Bivalent | 529 (26%) | 418 (19%) | 947 (22%) | |
| Other | 154 (7.4%) | 185 (8.6%) | 339 (8.0%) | |
| Fifth Dose | 1618 | |||
| Moderna | 3 (0.4%) | 5 (0.6%) | 8 (0.5%) | |
| Pfizer | 8 (1.0%) | 14 (1.6%) | 22 (1.4%) | |
| Moderna Bivalent | 257 (34%) | 310 (36%) | 567 (35%) | |
| Pfizer Bivalent | 489 (64%) | 501 (59%) | 990 (61%) | |
| Other | 8 (1.0%) | 23 (2.7%) | 31 (1.9%) | |
| Sixth Dose | 129 | |||
| Moderna | 0 (0%) | 1 (1.4%) | 1 (0.8%) | |
| Pfizer | 0 (0%) | 0 (0%) | 0 (0%) | |
| Moderna Bivalent | 2 (3.3%) | 3 (4.3%) | 5 (3.9%) | |
| Pfizer Bivalent | 52 (87%) | 55 (80%) | 107 (83%) | |
| Other | 6 (10%) | 10 (14%) | 16 (12%) | |
| Total Doses Received | 20,365 | |||
| 0 | 1917 (19%) | 1890 (19%) | 3807 (19%) | |
| 1 | 338 (3.3%) | 380 (3.8%) | 718 (3.5%) | |
| 2 | 3753 (36%) | 3386 (34%) | 7139 (35%) | |
| 3 | 2227 (22%) | 2238 (22%) | 4465 (22%) | |
| 4 | 1308 (13%) | 1310 (13%) | 2618 (13%) | |
| 5 | 705 (6.8%) | 784 (7.8%) | 1489 (7.3%) | |
| 6 | 60 (0.6%) | 69 (0.7%) | 129 (0.6%) | |
| RHA | 20,365 | |||
| IEHR | 976 (9.5%) | 956 (9.5%) | 1932 (9.5%) | |
| NHR | 847 (8.2%) | 802 (8.0%) | 1649 (8.1%) | |
| PMHR | 1230 (12%) | 1177 (12%) | 2407 (12%) | |
| SHSS | 1447 (14%) | 1376 (14%) | 2823 (14%) | |
| WRHA | 5808 (56%) | 5746 (57%) | 11,554 (57%) | |
| Anti-Spike IgG | 14,089 | |||
| Negative | 2382 (34%) | 2385 (34%) | 4767 (34%) | |
| Positive | 4667 (66%) | 4655 (66%) | 9322 (66%) | |
| Anti-Nucleocapsid IgG | 20,365 | |||
| Negative | 6567 (64%) | 6246 (62%) | 12,813 (63%) | |
| Positive | 3741 (36%) | 3811 (38%) | 7552 (37%) | |
| Positive for Anti-Spike and Anti-Nucleocapsid IgG | 20,365 | 2176 (21%) | 2167 (22%) | 4343 (21%) |
| Positive for Anti-Spike IgG Only | 20,365 | 2491 (24%) | 2488 (25%) | 4979 (24%) |
| Weeks between First Dose and Specimen Collection | 10,561 | |||
| Median (IQR) | 38 (20, 56) | 37 (20, 54) | 38 (20, 55) | |
| Range | 0, 117 | 0, 116 | 0, 117 | |
| Weeks between First and Second Dose | 15,840 | |||
| Median (IQR) | 7.0 (5.0, 10.0) | 7.0 (5.0, 10.0) | 7.0 (5.0, 10.0) | |
| Range | 3.0, 65.0 | 3.0, 78.0 | 3.0, 78.0 | |
| Weeks between Second Dose and Specimen Collection | 9147 | |||
| Median (IQR) | 35 (19, 51) | 34 (19, 49) | 34 (19, 50) | |
| Range | 0, 114 | 0, 113 | 0, 114 | |
| Weeks between Second and Third Dose | 8701 | |||
| Median (IQR) | 28 (27, 33) | 28 (26, 32) | 28 (26, 33) | |
| Range | 4, 109 | 4, 98 | 4, 109 | |
| Weeks between Third Dose and Specimen Collection | 3432 | |||
| Median (IQR) | 19 (9, 36) | 18 (9, 36) | 19 (9, 36) | |
| Range | 0, 78 | 0, 78 | 0, 78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martens, B.; Van Caeseele, P.; Bullard, J.; Loeppky, C.; Wei, Y.; Reimer, J.; McKinnon, L.R.; Shaw, S.Y.; Kindrachuk, J.; Stein, D.R. A Population-Based Study of SARS-CoV-2 IgG Antibody Responses to Vaccination in Manitoba. Vaccines 2024, 12, 1095. https://doi.org/10.3390/vaccines12101095
Martens B, Van Caeseele P, Bullard J, Loeppky C, Wei Y, Reimer J, McKinnon LR, Shaw SY, Kindrachuk J, Stein DR. A Population-Based Study of SARS-CoV-2 IgG Antibody Responses to Vaccination in Manitoba. Vaccines. 2024; 12(10):1095. https://doi.org/10.3390/vaccines12101095
Chicago/Turabian StyleMartens, Brielle, Paul Van Caeseele, Jared Bullard, Carla Loeppky, Yichun Wei, Joss Reimer, Lyle R. McKinnon, Souradet Y. Shaw, Jason Kindrachuk, and Derek R. Stein. 2024. "A Population-Based Study of SARS-CoV-2 IgG Antibody Responses to Vaccination in Manitoba" Vaccines 12, no. 10: 1095. https://doi.org/10.3390/vaccines12101095
APA StyleMartens, B., Van Caeseele, P., Bullard, J., Loeppky, C., Wei, Y., Reimer, J., McKinnon, L. R., Shaw, S. Y., Kindrachuk, J., & Stein, D. R. (2024). A Population-Based Study of SARS-CoV-2 IgG Antibody Responses to Vaccination in Manitoba. Vaccines, 12(10), 1095. https://doi.org/10.3390/vaccines12101095








