Enhanced Systemic and Mucosal Immune Responses to Haemophilus parasuis by Intranasal Administration of Lactic-Co-Glycolic Acid Microspheres
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Culture
2.2. Microsphere Preparation
2.3. Observation of Microsphere Morphology and Determination of Particle Size
2.4. Determination of Encapsulation Efficiency and Loading Capacity of PLGA Microspheres
2.5. Animal Vaccination Programs
2.6. Sample Collection
2.7. Evaluation of Serum Humoral Response
2.8. Evaluation of Mucosal Immune Response
2.9. Determination Titers of Cytokines
2.10. Challenge Test in Mice
2.11. Bacteriological Analysis
2.12. Statistical Analysis
3. Results
3.1. Morphology Observation and Encapsulation Efficiency of the Microspheres
3.2. Evaluation of Serum Antibody Levels Induced by the Microspheres
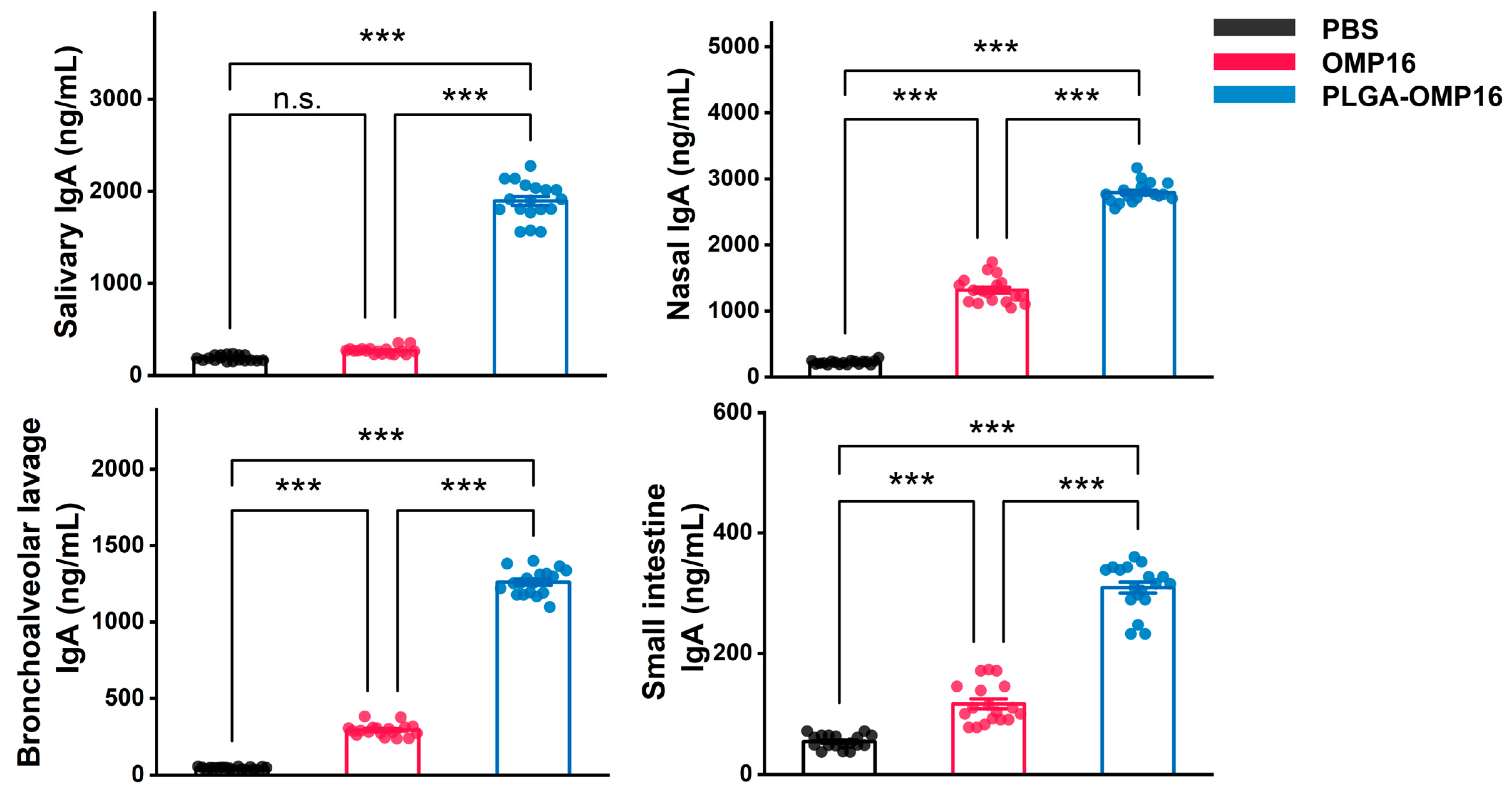
3.3. Evaluation of Mucosal Antibody Concentration
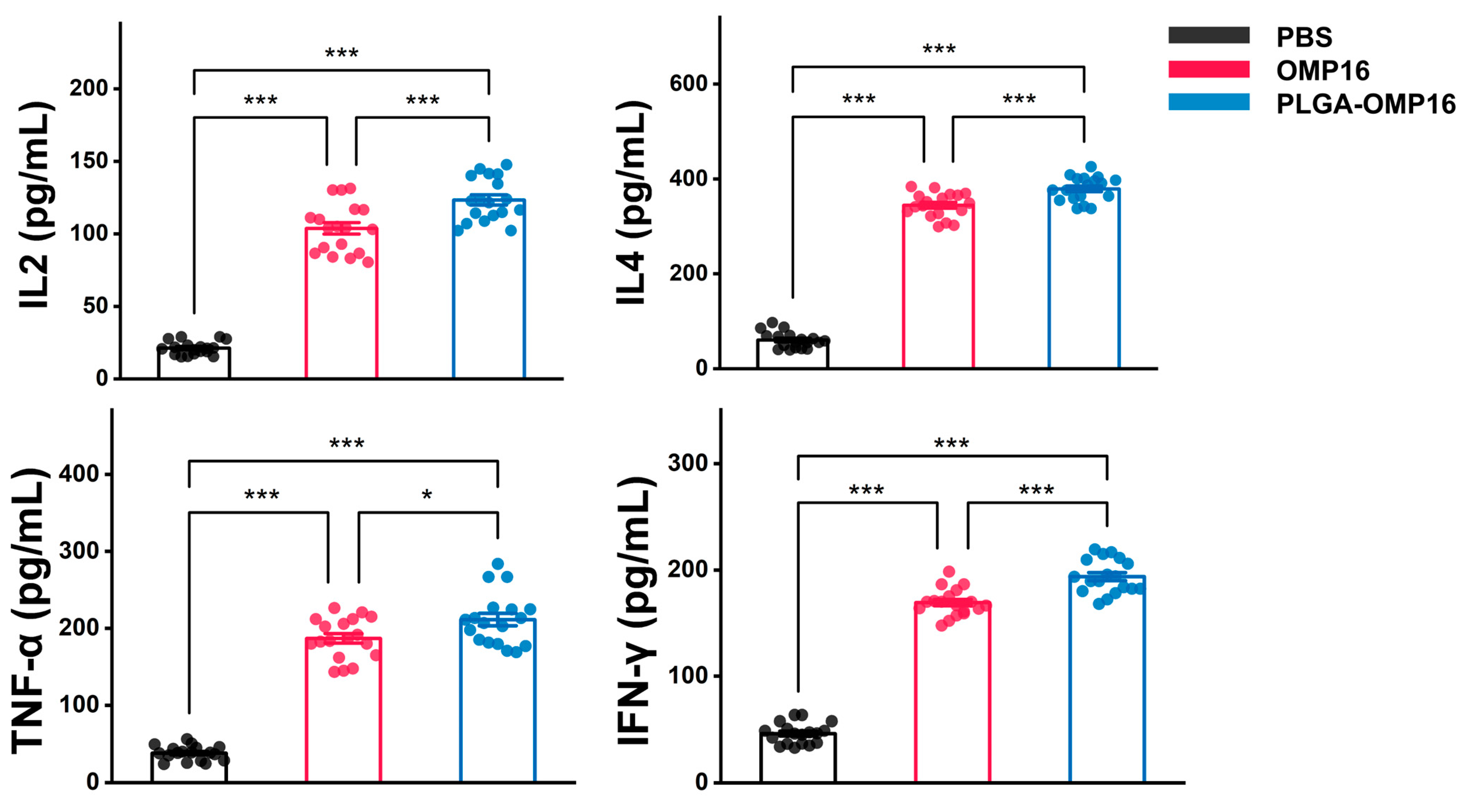
3.4. Estimation of Cytokine Concentration
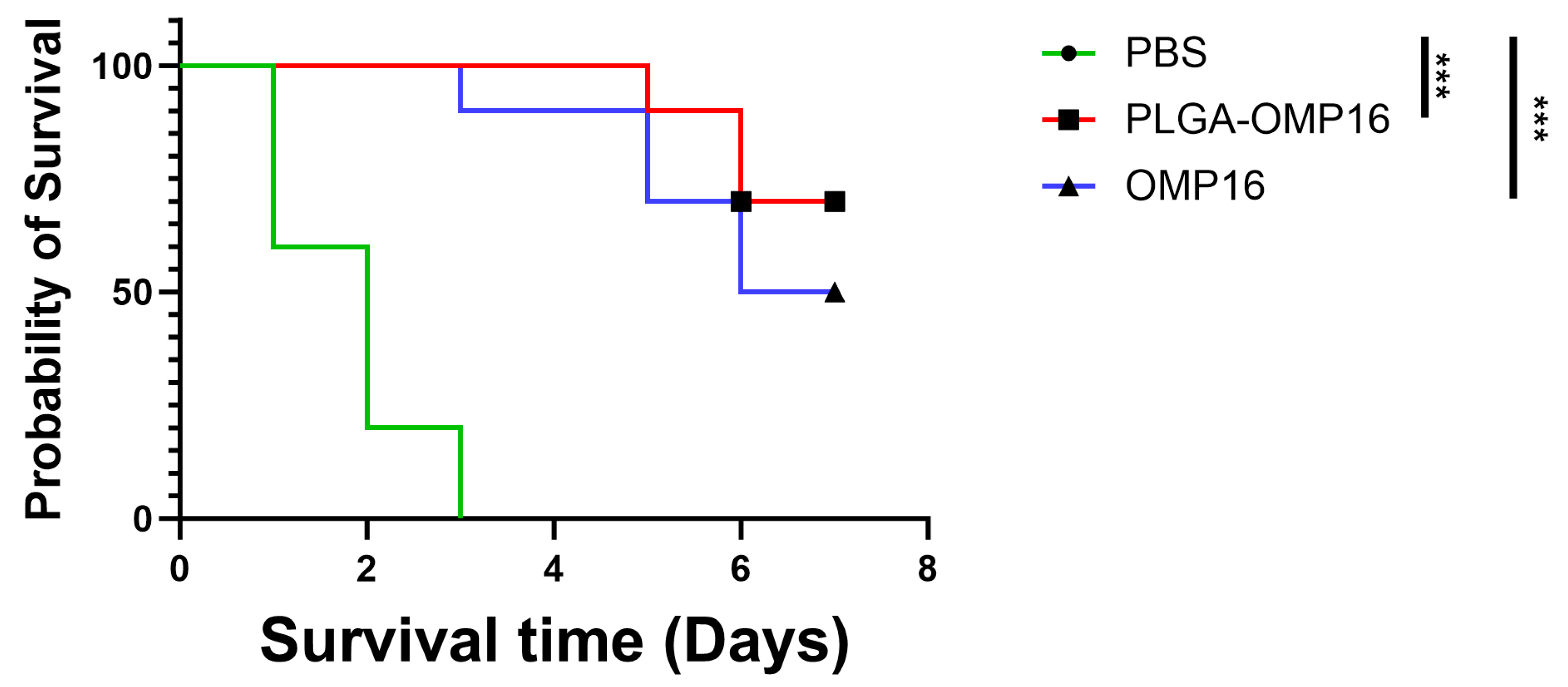
3.5. Challenge Test
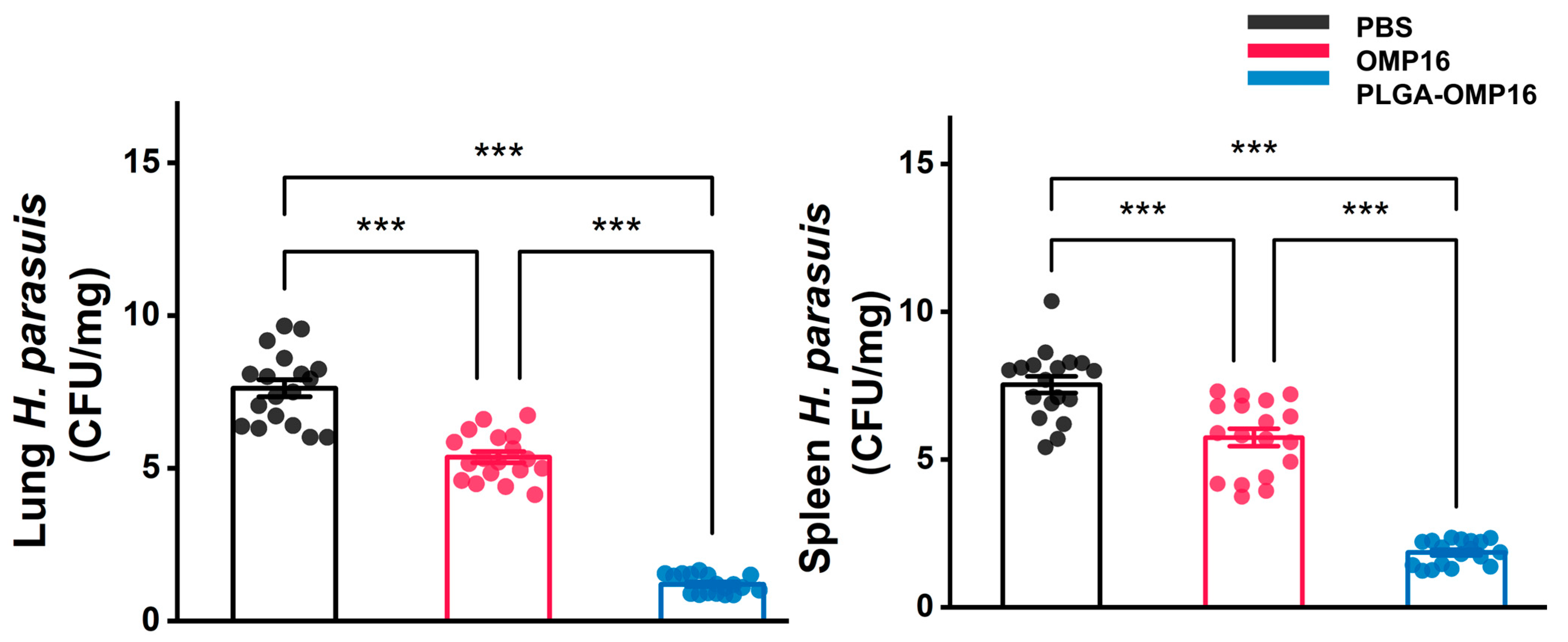
3.6. Bacteriological Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Costa-Hurtado, M.; Barba-Vidal, E.; Maldonado, J.; Aragon, V. Update on Glasser’s disease: How to control the disease under restrictive use of antimicrobials. Vet. Microbiol. 2020, 242, 108595. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, S.; Li, C.; Wang, C.; Liu, Y.; Wang, G.; He, X.; Hu, L.; Liu, Y.; Cui, M.; et al. Secondary Haemophilus parasuis infection enhances highly pathogenic porcine reproductive and respiratory syndrome virus (HP-PRRSV) infection-mediated inflammatory responses. Vet. Microbiol. 2017, 204, 35–42. [Google Scholar] [CrossRef]
- Liu, S.; Li, W.; Wang, Y.; Gu, C.; Liu, X.; Charreyre, C.; Fan, S.; He, Q. Coinfection with Haemophilus parasuis serovar 4 increases the virulence of porcine circovirus type 2 in piglets. Virol. J. 2017, 14, 227. [Google Scholar] [CrossRef]
- Aragon, V.; Segales, J.; Oliveira, S. Glasser’s disease. In Diseases of Swine, 10th ed.; Zimmerman, J.J., Karriker, L.A., Ramirez, A., Schwartz, K.J., Stevenson, G.W., Zhang, J., Eds.; Wiley-Blackwell: Chichester, UK, 2012. [Google Scholar]
- Costa-Hurtado, M.; Garcia-Rodriguez, L.; Lopez-Serrano, S.; Aragon, V. Haemophilus parasuis VtaA2 is involved in adhesion to extracellular proteins. Vet. Res. 2019, 50, 69. [Google Scholar] [CrossRef]
- Mullins, M.A.; Register, K.B.; Bayles, D.O.; Butler, J.E. Haemophilus parasuis exhibits IgA protease activity but lacks homologs of the IgA protease genes of Haemophilus influenzae. Vet. Microbiol. 2011, 153, 407–412. [Google Scholar] [CrossRef]
- Wang, Z.; Gu, J.; Xiao, K.; Zhu, W.; Lin, Y.; Wen, S.; He, Q.; Xu, X.; Cai, X. Glaesserella parasuis autotransporters EspP1 and EspP2 are novel IgA-specific proteases. Front. Microbiol. 2022, 13, 1041774. [Google Scholar] [CrossRef]
- Olvera, A.; Ballester, M.; Nofrarías, M.; Sibila, M.; Aragon, V. Differences in phagocytosis susceptibility in Haemophilus parasuis strains. Vet Res. 2009, 40, 24. [Google Scholar] [CrossRef] [PubMed]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef]
- Jin, H.; Zhou, R.; Kang, M.; Luo, R.; Cai, X.; Chen, H. Biofilm formation by field isolates and reference strains of Haemophilus parasuis. Vet. Microbiol. 2006, 118, 117–123. [Google Scholar] [CrossRef]
- Cerda-Cuellar, M.; Aragon, V. Serum-resistance in Haemophilus parasuis is associated with systemic disease in swine. Vet. J. 2008, 175, 384–389. [Google Scholar] [CrossRef]
- Bello-Orti, B.; Costa-Hurtado, M.; Martinez-Moliner, V.; Segales, J.; Aragon, V. Time course Haemophilus parasuis infection reveals pathological differences between virulent and non-virulent strains in the respiratory tract. Vet. Microbiol. 2014, 170, 430–437. [Google Scholar] [CrossRef]
- Takahashi, K.; Naga, S.; Yagihashi, T.; Ikehata, T.; Nakano, Y.; Senna, K.; Maruyama, T.; Murofushi, J. A cross-protection experiment in pigs vaccinated with Haemophilus parasuis serovars 2 and 5 bacterins, and evaluation of a bivalent vaccine under laboratory and field conditions. J. Vet. Med. Sci. 2001, 63, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Hau, S.J.; Eberle, K.C.; Brockmeier, S.L. Importance of strain selection in the generation of heterologous immunity to Glaesserella (Haemophilus) parasuis. Vet. Immunol. Immunopathol. 2021, 234, 110205. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xue, Q.; Zeng, Q.; Zhao, Z. Haemophilus parasuis vaccines. Vet. Immunol. Immunopathol. 2016, 180, 53–58. [Google Scholar] [CrossRef]
- Shakya, A.K.; Chowdhury, M.; Tao, W.; Gill, H.S. Mucosal vaccine delivery: Current state and a pediatric perspective. J. Control. Release 2016, 240, 394–413. [Google Scholar] [CrossRef]
- Russell, M.W.; Mestecky, J. Mucosal Vaccines: An Overview. In Mucosal Immunology, 4th ed.; Academic Press: Boston, MA, USA, 2015; pp. 1039–1046. [Google Scholar]
- Criscuolo, E.; Caputo, V.; Diotti, R.A.; Sautto, G.A.; Kirchenbaum, G.A.; Clementi, N. Alternative Methods of Vaccine Delivery: An Overview of Edible and Intradermal Vaccines. J. Immunol. Res. 2019, 2019, 8303648. [Google Scholar] [CrossRef]
- Correa, V.A.; Portilho, A.I.; De Gaspari, E. Vaccines, adjuvants and key factors for mucosal immune response. Immunology 2022, 167, 124–138. [Google Scholar] [CrossRef]
- Bastola, R.; Noh, G.; Keum, T.; Bashyal, S.; Seo, J.E.; Choi, J.; Oh, Y.; Cho, Y.; Lee, S. Vaccine adjuvants: Smart components to boost the immune system. Arch. Pharm. Res. 2017, 40, 1238–1248. [Google Scholar] [CrossRef]
- Lamichhane, A.; Azegami, T.; Kiyono, H. The mucosal immune system for vaccine development. Vaccine 2014, 32, 6711–6723. [Google Scholar] [CrossRef]
- Kammona, O.; Kiparissides, C. Recent advances in nanocarrier-based mucosal delivery of biomolecules. J. Control. Release 2012, 161, 781–794. [Google Scholar] [CrossRef]
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; Le Breton, A.; Preat, V. PLGA-based nanoparticles: An overview of biomedical applications. J. Control. Release 2012, 161, 505–522. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-H.; Zhao, G.-Z.; Qiu, L.-X.; Dai, A.-L.; Wu, W.-W.; Yang, X.-Y. Protective Efficacy of an Inactive Vaccine Based on the LY02 Isolate against Acute Haemophilus parasuis Infection in Piglets. Biomed Res. Int. 2015, 2015, 649878. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Yang, X.; Li, X.; Qiu, G.H.; Dai, A.; Huang, Q.; Huang, C.; Guo, X. OMP16-based vaccine encapsulated by alginate-chitosan microspheres provides significant protection against Haemophilus parasuis in mice. Vaccine 2017, 35, 1417–1423. [Google Scholar] [CrossRef] [PubMed]
- Genta, I.; Colonna, C.; Conti, B.; Caliceti, P.; Salmaso, S.; Speziale, P.; Pietrocola, G.; Chiesa, E.; Modena, T.; Dorati, R. CNA loaded PLGA nanoparticles improve humoral response against, S. aureus-mediated infections in a mouse model: Subcutaneous vs nasal administration strategy. J. Microencapsul. 2016, 33, 750–762. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.W.; Ehsan, M.; Wang, Q.; Haseeb, M.; Lakho, S.A.; Haider, A.; Lu, M.; Xu, L.; Song, X.; Yan, R.; et al. PLGA-Encapsulated Haemonchus contortus Antigen ES-15 Augments Immune Responses in a Murine Model. Vaccines 2023, 11, 1794. [Google Scholar] [CrossRef]
- Jaganathan, K.S.; Vyas, S.P. Strong systemic and mucosal immune responses to surface-modified PLGA microspheres containing recombinant hepatitis B antigen administered intranasally. Vaccine 2006, 24, 4201–4211. [Google Scholar] [CrossRef]
- Hu, M.; Zhang, Y.; Xie, F.; Li, G.; Li, J.; Si, W.; Liu, S.; Hu, S.; Zhang, Z.; Shen, N.; et al. Protection of piglets by a Haemophilus parasuis ghost vaccine against homologous challenge. Clin. Vaccine Immunol. 2013, 20, 795–802. [Google Scholar] [CrossRef]
- Angen, O.; Oliveira, S.; Ahrens, P.; Svensmark, B.; Leser, T.D. Development of an improved species specific PCR test for detection of Haemophilus parasuis. Vet. Microbiol. 2007, 119, 266–276. [Google Scholar] [CrossRef]
- Pang, M.; Tu, T.; Wang, Y.; Zhang, P.; Ren, M.; Yao, X.; Luo, Y.; Yang, Z. Design of a multi-epitope vaccine against Haemophilus parasuis based on pan-genome and immunoinformatics approaches. Front. Vet. Sci. 2022, 9, 1053198. [Google Scholar] [CrossRef]
- Lycke, N. Recent progress in mucosal vaccine development: Potential and limitations. Nat. Rev. Immunol. 2012, 12, 592–605. [Google Scholar] [CrossRef]
- Miquel-Clopes, A.; Bentley, E.G.; Stewart, J.P.; Carding, S.R. Mucosal vaccines and technology. Clin. Exp. Immunol. 2019, 196, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Hariri, B.M.; Cohen, N.A. New insights into upper airway innate immunity. Am. J. Rhinol. Allergy 2016, 30, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Zhang, M.; Zhu, H.; Du, X.; Wang, J. Mucosal vaccine delivery: A focus on the breakthrough of specific barriers. Acta Pharm. Sin. B 2022, 12, 3456–3474. [Google Scholar] [CrossRef] [PubMed]
- Eshaghi, B.; Schudel, A.; Sadeghi, I.; Chen, Z.; Lee, A.H.; Kanelli, M.; Tierney, F.; Han, J.; Ingalls, B.; Francis, D.M.; et al. The role of engineered materials in mucosal vaccination strategies. Nature Reviews. Materials 2024, 9, 29–45. [Google Scholar] [CrossRef]
- Bhat, A.A.; Seth, R.K.; Babu, J.; Biswas, S.; Rao, D.N. Induction of mucosal and systemic humoral immune responses in murine system by intranasal immunization with peptide antigens of, P. vivax and CpG oligodeoxynucleotide (ODN) in microparticle delivery. Int. Immunopharmacol. 2009, 9, 1197–1208. [Google Scholar] [CrossRef] [PubMed]
- Nedbalcova, K.; Kucerova, Z.; Krejci, J.; Tesarik, R.; Gopfert, E.; Kummer, V.; Leva, L.; Kudlackova, H.; Ondriasova, R.; Faldyna, M. Passive immunisation of post-weaned piglets using hyperimmune serum against experimental Haemophilus parasuis infection. Res. Vet. Sci. 2011, 91, 225–229. [Google Scholar] [CrossRef]
- Gupta, G.; Khan, A.A.; Rao, D.N. Cell-mediated immune response and Th/Th cytokine profile of B-T constructs of F1 and V antigen of Yersinia pestis. Scand. J. Immunol. 2010, 71, 186–198. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, T.; Dai, T.; Zhuang, L.; Liu, Y.; Li, X.; Huang, C.; Zheng, X. Enhanced Systemic and Mucosal Immune Responses to Haemophilus parasuis by Intranasal Administration of Lactic-Co-Glycolic Acid Microspheres. Vaccines 2024, 12, 1103. https://doi.org/10.3390/vaccines12101103
Lei T, Dai T, Zhuang L, Liu Y, Li X, Huang C, Zheng X. Enhanced Systemic and Mucosal Immune Responses to Haemophilus parasuis by Intranasal Administration of Lactic-Co-Glycolic Acid Microspheres. Vaccines. 2024; 12(10):1103. https://doi.org/10.3390/vaccines12101103
Chicago/Turabian StyleLei, Tianyu, Tingting Dai, Liyun Zhuang, Yiting Liu, Xiaohua Li, Cuiqin Huang, and Xintian Zheng. 2024. "Enhanced Systemic and Mucosal Immune Responses to Haemophilus parasuis by Intranasal Administration of Lactic-Co-Glycolic Acid Microspheres" Vaccines 12, no. 10: 1103. https://doi.org/10.3390/vaccines12101103





