Sequential Immune Acquisition of Monoclonal Antibodies Enhances Phagocytosis of Acinetobacter baumannii by Recognizing ATP Synthase
Abstract
:1. Introduction
2. Materials and Methods
2.1. MLST Serotype and Virulence Gene Assignment of A. baumannii
2.2. Production of Monoclonal Antibodies
2.3. Whole-Cell ELISA
2.4. Western Blot
2.5. Co-Immunoprecipitation
2.6. LC-MS/MS Analysis
2.7. In Vitro Complement Susceptibility and Phagocytosis Assay
2.8. Mouse Infection Model
2.9. Cytokine Assay
2.10. Statistical Analysis
3. Results
3.1. MLST Serotype and Virulence Genes of Clinically Isolated A. baumannii
3.2. Monoclonal Antibodies Obtained by Sequential Immunization Provide an Effectively Cross-Recognition Effect
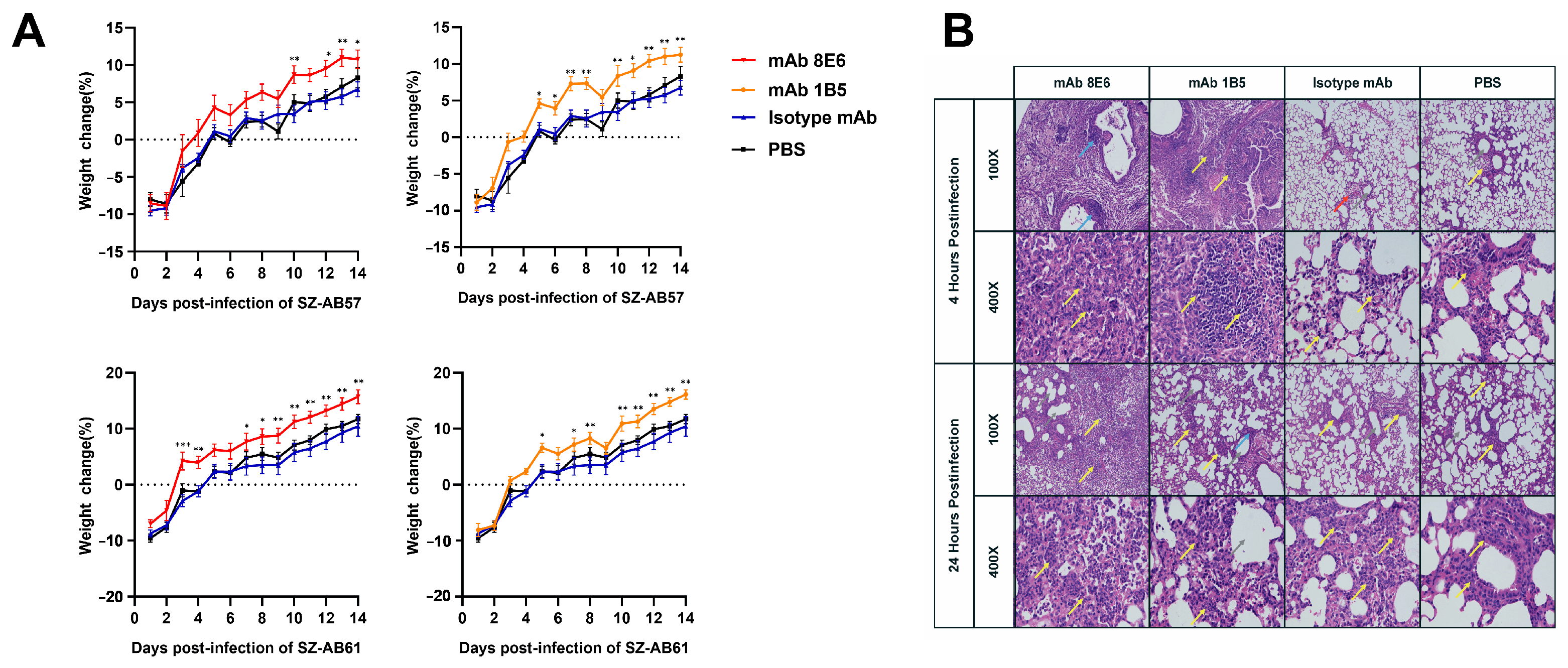
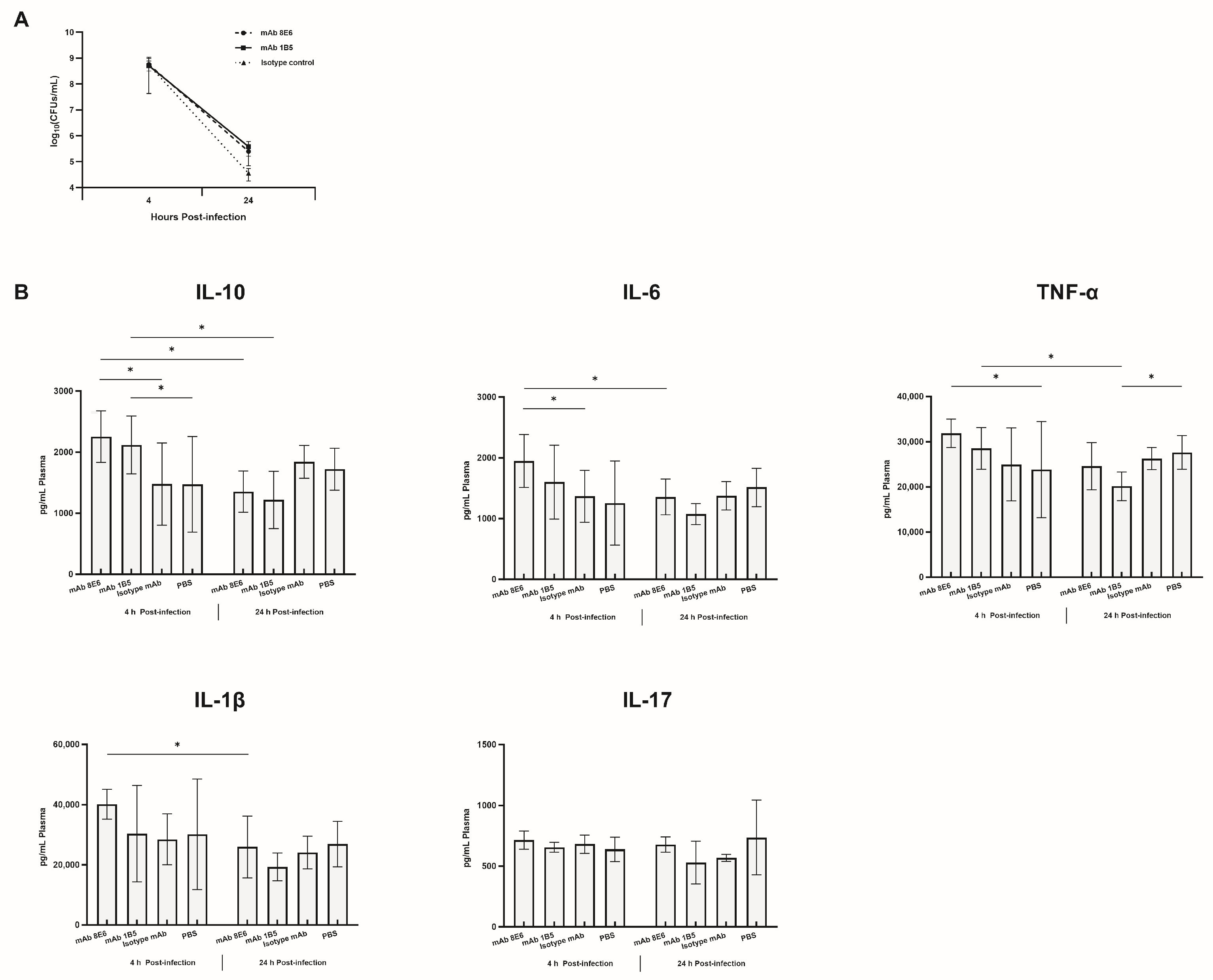
3.3. Monoclonal Antibodies Obtained by Sequential Immunization Provide an Effective Cross-Opsonophagocytosis Effect
3.4. The mAbs against A. baumannii Infection through the Potentiation of Inflammatory Mediator Release
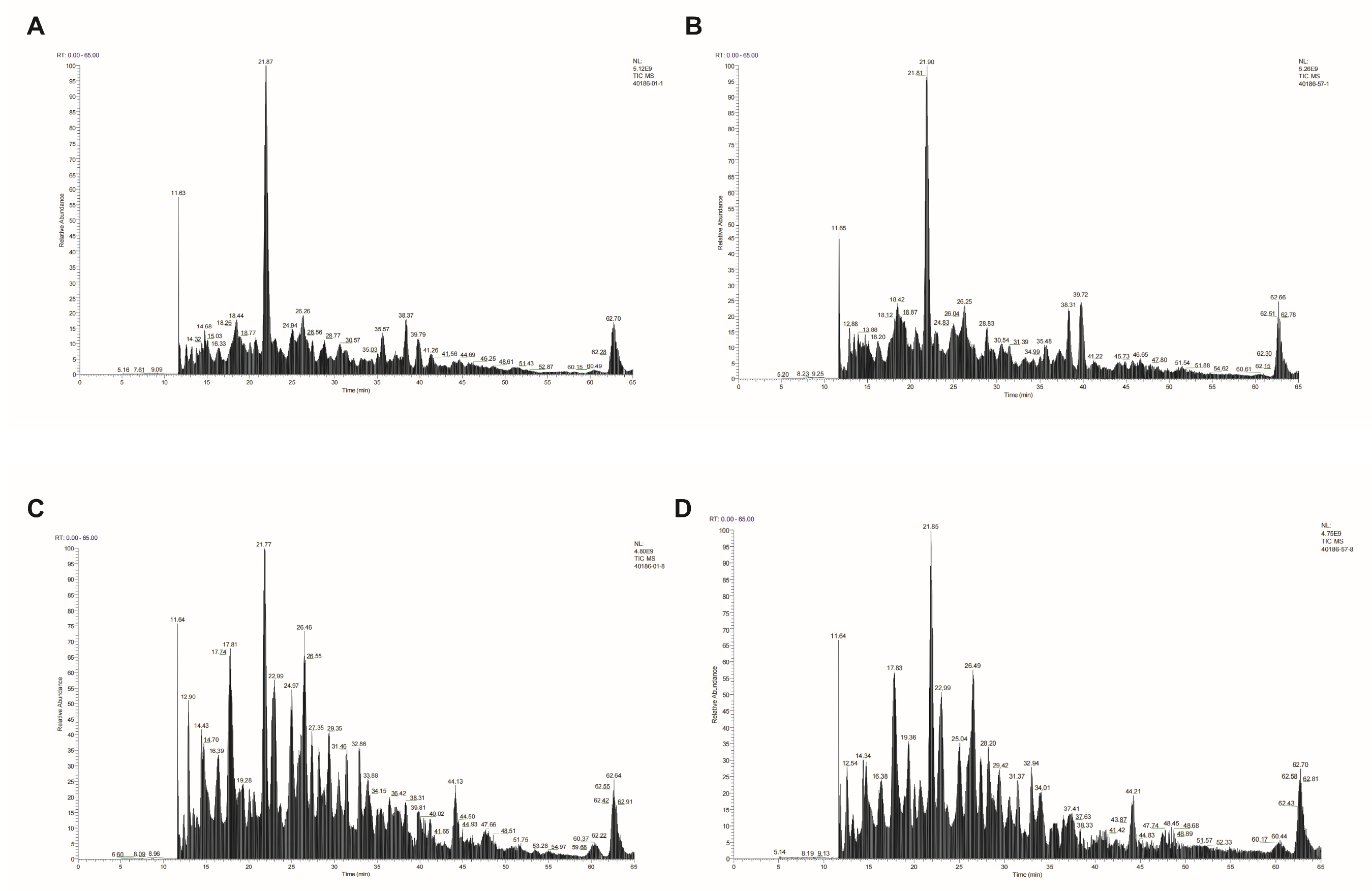
3.5. Cross-Recognition Bacterial Epitope Identification
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- OECD. Embracing a One Health Framework to Fight Antimicrobial Resistance. In OECD Health Policy Studies; OECD Publishing: Paris, France, 2023. [Google Scholar] [CrossRef]
- Evans, B.A.; Hamouda, A.; Amyes, S.G. The rise of carbapenem-resistant Acinetobacter baumannii. Curr. Pharm. Des. 2013, 19, 22338. [Google Scholar] [CrossRef]
- National Center for Emerging and Zoonotic Infectious Diseases (U.S.); Division of Healthcare Quality Promotion; Division of Healthcare Quality Promotion. COVID-19: US Impact on Antimicrobial Resistance, Special Report 2022; CDC: Atlanta, GA, USA, 2022. [CrossRef]
- Wang-Lin, S.X.; Olson, R.; Beanan, J.M.; MacDonald, U.; Balthasar, J.P.; Russo, T.A. The Capsular Polysaccharide of Acinetobacter baumannii Is an Obstacle for Therapeutic Passive Immunization Strategies. Infect. Immun. 2017, 85, e00591-17. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Zhao, Q.; Wu, Y.; Zhou, H.; Ding, S.; Zhu, K. Mucoid Acinetobacter baumannii enhances anti-phagocytosis through reducing C3b deposition. Front. Med. 2022, 9, 879361. [Google Scholar] [CrossRef] [PubMed]
- Kamoshida, G.; Kikuchi-Ueda, T.; Tansho-Nagakawa, S.; Nakano, R.; Nakano, A.; Kikuchi, H.; Ubagai, T.; Ono, Y. Acinetobacter baumannii escape from neutrophil extracellular traps (NETs). J. Infect. Chemother. 2015, 21, 43–49. [Google Scholar] [CrossRef]
- Tan, Y.C.; Lahiri, C. Promising Acinetobacter baumannii Vaccine Candidates and Drug Targets in Recent Years. Front. Immunol. 2022, 13, 900509. [Google Scholar] [CrossRef]
- Russo, T.A.; Beanan, J.M.; Olson, R.; MacDonald, U.; Cox, A.D.; St Michael, F.; Vinogradov, E.V.; Spellberg, B.; Luke-Marshall, N.R.; Campagnari, A.A. The K1 capsular polysaccharide from Acinetobacter baumannii is a potential therapeutic target via passive immunization. Infect. Immun. 2013, 81, 915–922. [Google Scholar] [CrossRef]
- Nielsen, T.B.; Pantapalangkoor, P.; Luna, B.M.; Bruhn, K.W.; Yan, J.; Dekitani, K.; Hsieh, S.; Yeshoua, B.; Pascual, B.; Vinogradov, E.; et al. Monoclonal Antibody Protects Against Acinetobacter baumannii Infection by Enhancing Bacterial Clearance and Evading Sepsis. J. Infect. Dis. 2017, 216, 489–501. [Google Scholar] [CrossRef]
- Nielsen, T.B.; Yan, J.; Slarve, M.; Lu, P.; Li, R.; Ruiz, J.; Lee, B.; Burk, E.; Talyansky, Y.; Oelschlaeger, P.; et al. Monoclonal Antibody Therapy against Acinetobacter baumannii. Infect. Immun. 2021, 89, e0016221. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, T.B.; Yan, J.; Slarve, M.; Li, R.; Junge, J.A.; Luna, B.M.; Wilkinson, I.; Yerramalla, U.; Spellberg, B. Development of a Bispecific Antibody Targeting Clinical Isolates of Acinetobacter baumannii. J. Infect. Dis. 2023, 227, 1042–1049. [Google Scholar] [CrossRef]
- Yang, F.L.; Lou, T.C.; Kuo, S.C.; Wu, W.L.; Chern, J.; Lee, Y.T.; Chen, S.T.; Zou, W.; Lin, N.T.; Wu, S.H. A medically relevant capsular polysaccharide in Acinetobacter baumannii is a potential vaccine candidate. Vaccine 2017, 35, 1440–1447. [Google Scholar] [CrossRef]
- Yang, X.; Wei, R.; Liu, H.; Wei, T.; Zeng, P.; Cheung, Y.C.; Heng, H.; Chan, E.W.; Li, X.; Chen, S. Discovery of a Monoclonal Antibody That Targets Cell-Surface Pseudaminic Acid of Acinetobacter baumannii with Direct Bactericidal Effect. ACS Cent. Sci. 2024, 10, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Krammer, F. Strategies to induce broadly protective antibody responses to viral glycoproteins. Expert Rev. Vaccines 2017, 16, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Huang, Y.; Yuan, S.; Li, Y.; Wu, S.; Xu, J.; Huang, R. Sequential immunization with consensus influenza hemagglutinins raises cross-reactive neutralizing antibodies against various heterologous HA strains. Vaccine 2017, 35, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Moe, G.R.; Zuno-Mitchell, P.; Hammond, S.N.; Granoff, D.M. Sequential immunization with vesicles prepared from heterologous Neisseria meningitidis strains elicits broadly protective serum antibodies to group B strains. Infect. Immun. 2002, 70, 6021–6031. [Google Scholar] [CrossRef] [PubMed]
- Kamuyu, G.; Suen Cheng, Y.; Willcocks, S.; Kewcharoenwong, C.; Kiratisin, P.; Taylor, P.W.; Wren, B.W.; Lertmemongkolchai, G.; Stabler, R.A.; Brown, J. Sequential Vaccination with Heterologous Acinetobacter baumannii Strains Induces Broadly Reactive Antibody Responses. Front. Immunol. 2021, 12, 705533. [Google Scholar] [CrossRef]
- Ying, J.; Lu, J.; Zong, L.; Li, A.; Pan, R.; Cheng, C.; Li, K.; Chen, L.; Ying, J.; Tou, H.; et al. Molecular Epidemiology and Characterization of Genotypes of Acinetobacter baumannii Isolates from Regions of South China. Jpn. J. Infect. Dis. 2016, 69, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Fu, Y.; Hua, X.; Xu, Q.; Lan, P.; Jiang, Y.; Yu, Y.; Zhou, Z. Acinetobacter baumannii strains isolated from cerebrospinal fluid (CSF) and bloodstream analysed by cgMLST: The dominance of clonal complex CC92 in CSF infections. Int. J. Antimicrob. Agents 2021, 58, 106404. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Wang, H.; Wang, X.; Jing, H.; Duan, R.; Qin, S.; Lv, D.; Fan, Y.; Huang, Z.; Stirling, K.; et al. Molecular characterization and antibiotic resistance of Acinetobacter baumannii in cerebrospinal fluid and blood. PLoS ONE 2021, 16, e0247418. [Google Scholar] [CrossRef] [PubMed]
- You, Q.; Du, X.; Hu, N.; Zhang, Y.; Zhang, N.; Wang, F.; Li, J.; Sun, Y.; Wang, F.; Shi, H. Local characteristics of molecular epidemiolgy of Acinetobacter baumannii in Jilin province (northeast China). BMC Microbiol. 2023, 23, 19. [Google Scholar] [CrossRef]
- Cui, F.; Liu, L.; Miao, X.; Qian, H.; Lu, S.; Tian, J.; Qiao, G.; Shao, B.; Li, Q.; Zhang, R.; et al. Epidemiological and comparative genomic analyses of Multidrug-Resistant Acinetobacter baumannii collected between 2020 and 2022 in Liaocheng City, Shandong Province, China. J. Biosaf. Biosecurity 2023, 5, 60–66. [Google Scholar] [CrossRef]
- Jia, H.; Chen, Y.; Wang, J.; Xie, X.; Ruan, Z. Emerging challenges of whole-genome-sequencing–powered epidemiological surveillance of globally distributed clonal groups of bacterial infections, giving Acinetobacter baumannii ST195 as an example. Int. J. Med. Microbiol. 2019, 309, 151339. [Google Scholar] [CrossRef]
- Gao, Y.; Li, H.; Chen, H.; Zhang, J.; Wang, R.; Wang, Z.; Wang, H. Origin, Phylogeny, and Transmission of the Epidemic Clone ST208 of Carbapenem-Resistant Acinetobacter baumannii on a Global Scale. Microbiol. Spectr. 2022, 10, e0260421. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.V.; Kumanovics, A.; Wiencek, J.; Melanson, S.E.F.; Love, T.; Wu, A.H.B.; Zhao, Z.; Meng, Q.H.; Koch, D.D.; Apple, F.S.; et al. Performance of Three Anti-SARS-CoV-2 Anti-S and One Anti-N Immunoassays for the Monitoring of Immune Status and Vaccine Response. Viruses 2024, 16, 292. [Google Scholar] [CrossRef] [PubMed]
- Massik, A.; Hibaoui, L.; Benboubker, M.; Yahyaoui, G.; Oumokhtar, B.; Mahmoud, M. Acinetobacter baumannii Carbapenemase Producers in Morocco: Genetic Diversity. Cureus 2023, 15, e43629. [Google Scholar] [CrossRef] [PubMed]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; DeZonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 2017, 18, 529. [Google Scholar] [CrossRef]
- UniProt Consortium. UniProt: The Universal Protein Knowledgebase in 2023. Nucleic Acids Res. 2023, 51, D523–D531. [Google Scholar] [CrossRef] [PubMed]
- Klausen, M.S.; Anderson, M.V.; Jespersen, M.C.; Nielsen, M.; Marcatili, P. LYRA, a webserver for lymphocyte receptor structural modeling. Nucleic Acids Res. 2015, 43, W349–W355. [Google Scholar] [CrossRef] [PubMed]
- Bienert, S.; Waterhouse, A.; de Beer, T.A.; Tauriello, G.; Studer, G.; Bordoli, L.; Schwede, T. The SWISS-MODEL Repository-new features and functionality. Nucleic Acids Res. 2017, 45, D313–D319. [Google Scholar] [CrossRef] [PubMed]
- Kozakov, D.; Hall, D.; Xia, B.; Porter, K.A.; Padhorny, D.; Yueh, C.; Beglov, D.; Vajda, S. The ClusPro web server for protein–protein docking. Nat. Protocol 2017, 12, 255–278. [Google Scholar] [CrossRef]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Zurawski, D.V.; McLendon, M.K. Monoclonal Antibodies as an Antibacterial Approach Against Bacterial Pathogens. Antibiotics 2020, 9, 155. [Google Scholar] [CrossRef] [PubMed]
- Munro, C.; Zilberberg, M.D.; Shorr, A.F. Bloodstream Infection in the Intensive Care Unit: Evolving Epidemiology and Microbiology. Antibiotics 2024, 13, 123. [Google Scholar] [CrossRef] [PubMed]
- Magda, M.; Bettoni, S.; Laabei, M.; Fairley, D.; Russo, T.A.; Riesbeck, K.; Blom, A.M. Clinical Isolates of Acinetobacter spp. Are Highly Serum Resistant Despite Efficient Recognition by the Complement System. Front. Immunol. 2022, 13, 814193. [Google Scholar] [CrossRef]
- Noris, M.; Remuzzi, G. Overview of Complement Activation and Regulation. Semin. Nephrol. 2013, 33, 479–492. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, T.B.; Yan, J.; Luna, B.M.; Talyansky, Y.; Slarve, M.; Bonomo, R.A.; Spellberg, B. Monoclonal Antibody Requires Immunomodulation for Efficacy Against Acinetobacter baumannii Infection. J. Infect. Dis. 2021, 224, 2133–2147. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Xiang, C.; Wang, N.; Zhang, X.; Xie, Y.; Yang, H.; Guo, G.; Liu, K.; Li, Y.; Shi, Y. Acinetobacter baumannii reinforces the pathogenesis by promoting IL-17 production in a mouse pneumonia model. Med. Microbiol. Immunol. 2023, 212, 65–73. [Google Scholar] [CrossRef]
- Douedi, S.; Chaudhri, M.; Miskoff, J. Anti-interleukin-6 monoclonal antibody for cytokine storm in COVID-19. Ann. Thorac. Med. 2020, 15, 171–173. [Google Scholar] [CrossRef]
- Vestergaard, M.; Bald, D.; Ingmer, H. Targeting the ATP synthase in bacterial and fungal pathogens: Beyond Mycobacterium tuberculosis. J. Glob. Antimicrob. Resist. 2022, 29, 29–41. [Google Scholar] [CrossRef]
- Demmer, J.K.; Phillips, B.P.; Uhrig, O.L.; Filloux, A.; Allsopp, L.P.; Bublitz, M.; Meier, T. Structure of ATP synthase from ESKAPE pathogen Acinetobacter baumannii. Sci. Adv. 2022, 8, eabl5966. [Google Scholar] [CrossRef] [PubMed]
- Jones, D. Tuberculosis success. Nat. Rev. Drug Discov. 2013, 12, 175–176. [Google Scholar] [CrossRef]
- Zhang, Y.; Lai, Y.; Zhou, S.; Ran, T.; Zhang, Y.; Zhao, Z.; Feng, Z.; Yu, L.; Xu, J.; Shi, K.; et al. Inhibition of M. tuberculosis and human ATP synthase by BDQ and TBAJ-587. Nature 2024, 631, 409–414. [Google Scholar] [CrossRef]
- Yun, S.H.; Choi, C.W.; Kwon, S.O.; Park, G.W.; Cho, K.; Kwon, K.H.; Kim, J.Y.; Yoo, J.S.; Lee, J.C.; Choi, J.S.; et al. Quantitative proteomic analysis of cell wall and plasma membrane fractions from multidrug-resistant Acinetobacter baumannii. J. Proteome Res. 2011, 10, 459–469. [Google Scholar] [CrossRef] [PubMed]





| Isolate Strains | Isolate Source | Allelic Distribution * | STs | Susceptibility | Global Clonal | Year/Country | Virulence Genes | ||
|---|---|---|---|---|---|---|---|---|---|
| OXA-23 | OXA-24 | OXA-51 | |||||||
| SZ-AB57 | Sputum | 1, 3, 3, 2, 2, 97, 3 | 208 | PDR | GC2 | 2021/Shenzhen, China | + | − | + |
| SZ-AB61 | Sputum | 1, 15, 2, 28, 1, 107, 32 | 229 | PDR | GC1 | 2021/Shenzhen, China | + | − | + |
| SZ-AB22 | Sputum | 1, 3, 3, 2, 2, 96, 3 | 195 | PDR | GC2 | 2021/Shenzhen, China | + | − | + |
| ZH-AB74 | Sputum | 1, 3, 3, 2, 2, 97, 3 | 208 | MDR | GC2 | 2022/Zhuhai, China | + | − | + |
| ZH-AB64 | Sputum | 1, 3, 3, 2, 2, 97, 3 | 208 | MDR | GC2 | 2022/Zhuhai, China | + | − | + |
| ZH-AB59 | Sputum | 1, 3, 3, 2, 2, 96, 3 | 195 | MDR | GC2 | 2022/Zhuhai, China | + | − | + |
| ZH-AB91 | Sputum | 1, 3, 3, 2, 2, 97, 3 | 208 | MDR | GC2 | 2022/Zhuhai, China | + | − | + |
| ZH-AB23 | Sputum | 1, 3, 3, 2, 2, 97, 3 | 208 | MDR | GC2 | 2022/Zhuhai, China | + | − | + |
| ZH-AB323 | Sputum | 1, 3, 3, 2, 2, 96, 3 | 195 | MDR | GC2 | 2022/Zhuhai, China | + | − | + |
| ZH-AB01 | Sputum | 1, 3, 3, 2, 2, 96, 3 | 195 | MDR | GC2 | 2022/Zhuhai, China | + | − | + |
| ZH-AB10 | Drainage fluid | 1, 3, 3, 2, 2, 97, 3 | 208 | MDR | GC2 | 2022/Zhuhai, China | + | − | + |
| ZH-AB36 | Sputum | 1, 3, 3, 2, 2, 97, 3 | 208 | MDR | GC2 | 2022/Zhuhai, China | + | − | + |
| ZH-AB66 | Sputum | 1, 3, 3, 2, 2, 97, 3 | 208 | MDR | GC2 | 2022/Zhuhai, China | + | − | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, D.; Zeng, Z.; Li, Z.; Li, M.; Zhai, L.; Lin, Y.; Xu, R.; Qu, J.; Zhang, B.; Zhao, W.; et al. Sequential Immune Acquisition of Monoclonal Antibodies Enhances Phagocytosis of Acinetobacter baumannii by Recognizing ATP Synthase. Vaccines 2024, 12, 1120. https://doi.org/10.3390/vaccines12101120
Huang D, Zeng Z, Li Z, Li M, Zhai L, Lin Y, Xu R, Qu J, Zhang B, Zhao W, et al. Sequential Immune Acquisition of Monoclonal Antibodies Enhances Phagocytosis of Acinetobacter baumannii by Recognizing ATP Synthase. Vaccines. 2024; 12(10):1120. https://doi.org/10.3390/vaccines12101120
Chicago/Turabian StyleHuang, Dong, Zhujun Zeng, Zhuolin Li, Mengjun Li, Linlin Zhai, Yuhao Lin, Rui Xu, Jiuxin Qu, Bao Zhang, Wei Zhao, and et al. 2024. "Sequential Immune Acquisition of Monoclonal Antibodies Enhances Phagocytosis of Acinetobacter baumannii by Recognizing ATP Synthase" Vaccines 12, no. 10: 1120. https://doi.org/10.3390/vaccines12101120
APA StyleHuang, D., Zeng, Z., Li, Z., Li, M., Zhai, L., Lin, Y., Xu, R., Qu, J., Zhang, B., Zhao, W., & Shen, C. (2024). Sequential Immune Acquisition of Monoclonal Antibodies Enhances Phagocytosis of Acinetobacter baumannii by Recognizing ATP Synthase. Vaccines, 12(10), 1120. https://doi.org/10.3390/vaccines12101120





