The Influence of Initial Immunosuppression on the Kinetics of Humoral Response after SARS-CoV-2 Vaccination in Patients Undergoing Kidney Transplantation
Abstract
:1. Introduction
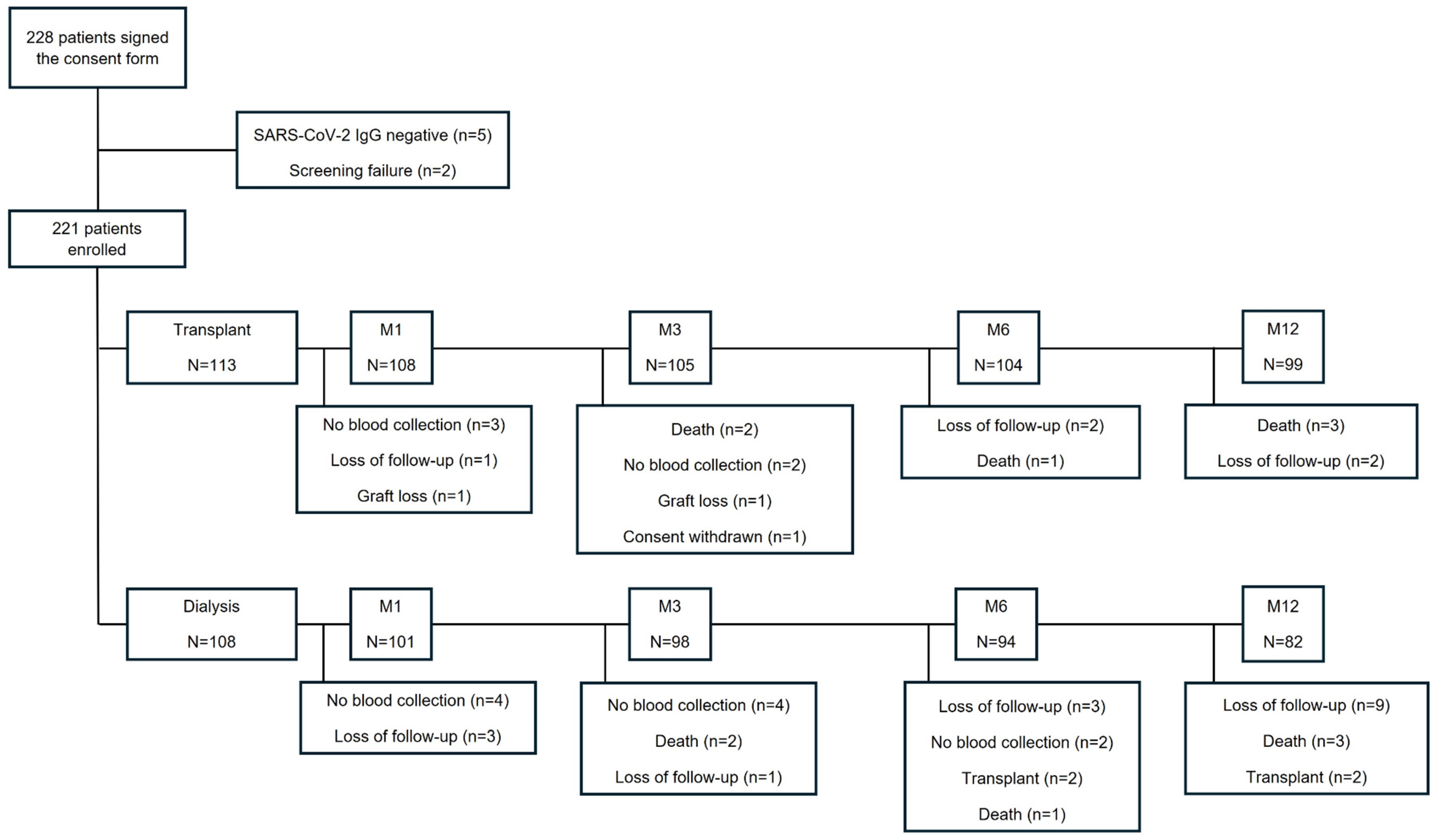
2. Material and Methods
2.1. Study Design and Population
2.2. Primary Endpoint
2.3. Secondary Endpoints
2.4. Immunogenicity Assessment
2.5. Vaccines and Vaccination Strategy
2.6. Immunosuppressive Therapy
2.7. Statistical Analysis
3. Results
3.1. Demographic Characteristics
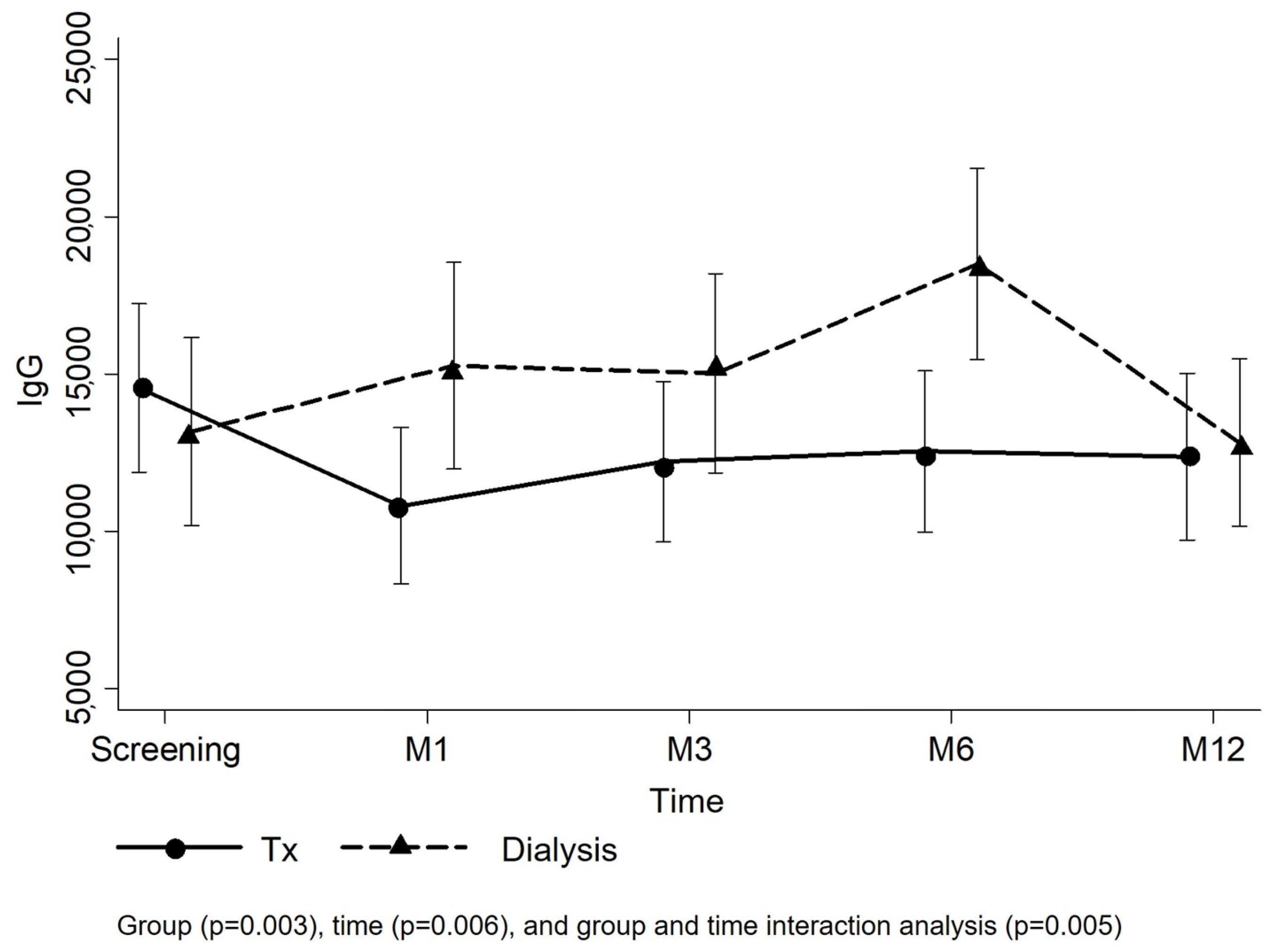
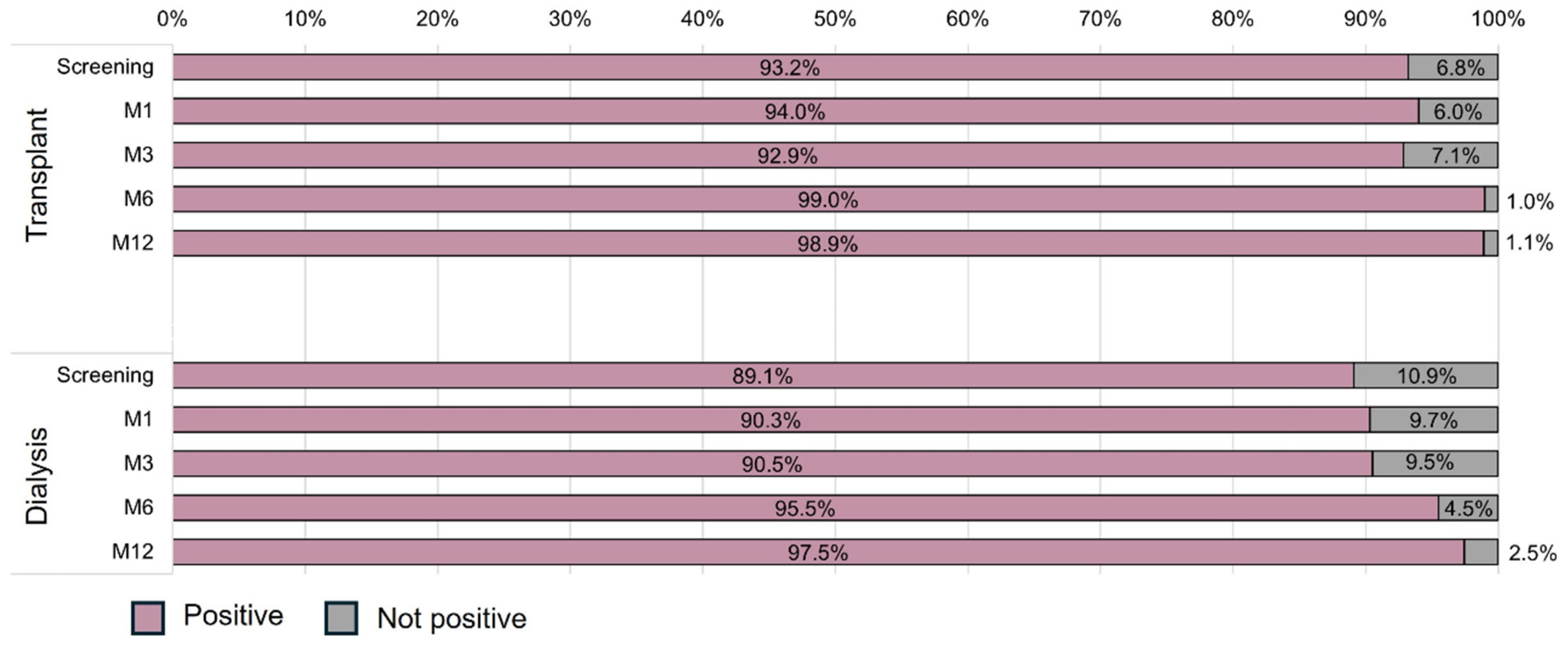
3.2. Primary Endpoint
3.3. Secondary Endpoints
3.4. Neutralizing Antibodies
3.5. Vaccines
3.6. Clinical Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CKD | Chronic Kidney Disease |
| CIT | Cold Ischemia Time |
| COVID-19 | Coronavirus Disease 2019 |
| ECD | Expanded Criteria Donor |
| HLA | Human Leukocyte Antigen |
| KDPI | Kidney Donor Profile Index |
| KDRI | Kidney Donor Risk Index |
| KTR | Kidney Transplant Recipient |
| IgG | Immunoglobulin G |
| M | Month |
| mTORi | Mammalian Target Of Rapamycin Inhibitor |
| PRA | Panel-reactive Antibody |
| rATG | Rabbit Anti-thymocyte Globulin |
| SARS-CoV-2 | Severe Acute Respiratory Syndrome Coronavirus 2 |
References
- Miyah, Y.; Benjelloun, M.; Lairini, S.; Lahrichi, A. COVID-19 Impact on Public Health, Environment, Human Psychology, Global Socioeconomy, and Education. Sci. World J. 2022, 2022, 5578284. [Google Scholar] [CrossRef] [PubMed]
- Azzi, Y.; Bartash, R.; Scalea, J.; Loarte-Campos, P.; Akalin, E. COVID-19 and Solid Organ Transplantation: A Review Article. Transplantation 2021, 105, 37–55. [Google Scholar] [CrossRef] [PubMed]
- De Sandes-Freitas, T.V.; de Andrade, L.G.M.; Moura, L.R.R.; Cristelli, M.P.; Medina-Pestana, J.O.; Lugon, J.R.; Ricardo Sesso For the Brazilian Covid-19 Dialysis Investigators and the Covid-19-KT Brazilian Study Group. Comparison of 30-day case-fatality rate between dialysis and transplant Covid-19 patients: A propensity score matched cohort study. J. Nephrol. 2022, 35, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Pestana, J.M.; Cristelli, M.P.; Tedesco Silva, H., Jr. The Challenges of Risk Aversion in Kidney Transplantation: Lessons From the SARS-CoV-2 Pandemic in Brazil. Transplantation 2024, 108, 813–818. [Google Scholar] [CrossRef]
- Syed-Ahmed, M.; Narayanan, M. Immune Dysfunction and Risk of Infection in Chronic Kidney Disease. Adv. Chronic Kidney Dis. 2019, 26, 8–15. [Google Scholar] [CrossRef]
- Sanders, J.F.; Messchendorp, A.L.; de Vries, R.D.; Baan, C.C.; van Baarle, D.; van Binnendijk, R.; Diavatopoulos, D.A.; Geers, D.; Schmitz, K.S.; GeurtsvanKessel, C.H.; et al. Antibody and T-Cell Responses 6 Months After Coronavirus Disease 2019 Messenger RNA-1273 Vaccination in Patients with Chronic Kidney Disease, on Dialysis, or Living With a Kidney Transplant. Clin. Infect. Dis. 2023, 76, e188–e199. [Google Scholar] [CrossRef]
- Zong, K.; Peng, D.; Yang, H.; Huang, Z.; Luo, Y.; Wang, Y.; Xiang, S.; Li, T.; Mou, T.; Wu, Z. Risk Factors for Weak Antibody Response of SARS-CoV-2 Vaccine in Adult Solid Organ Transplant Recipients: A Systemic Review and Meta-Analysis. Front Immunol. 2022, 13, 888385. [Google Scholar] [CrossRef]
- L’Huillier, A.G.; Ferreira, V.H.; Hirzel, C.; Nellimarla, S.; Ku, T.; Natori, Y.; Humar, A.; Kumar, D. T-cell responses following Natural Influenza Infection or Vaccination in Solid Organ Transplant Recipients. Sci. Rep. 2020, 10, 10104. [Google Scholar] [CrossRef]
- Loinaz, C.; de Juanes, J.R.; Gonzalez, E.M.; Lopez, A.; Lumbreras, C.; Gomez, R.; Gonzalez-Pinto, I.; Jiménez, C.; Garcia, I.; Fuertes, A. Hepatitis B vaccination results in 140 liver transplant recipients. Hepatogastroenterology 1997, 44, 235–238. [Google Scholar]
- Dendle, C.; Stuart, R.L.; Mulley, W.R.; Holdsworth, S.R. Pneumococcal vaccination in adult solid organ transplant recipients: A review of current evidence. Vaccine 2018, 36, 6253–6261. [Google Scholar] [CrossRef]
- Zinszer, K.; Charland, K.; Pierce, L.; Saucier, A.; McKinnon, B.; Hamelin, M.E.; Cheriet, I.; Da Torre, M.B.; Carbonneau, J.; Nguyen, C.T.; et al. Pre-Omicron seroprevalence, seroconversion, and seroreversion of infection-induced SARS-CoV-2 antibodies among a cohort of children and teenagers in Montreal, Canada. Int. J. Infect. Dis. 2023, 131, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Abbott. AdviseDx SARS-CoV-2 IgG II Instructions for Use. 2021. Available online: https://www.fda.gov/media/146371/download (accessed on 10 December 2021).
- Tan, C.W.; Chia, W.N.; Qin, X.; Liu, P.; Chen, M.I.; Tiu, C.; Hu, Z.; Chen, V.C.-W.; Young, B.E.; Sia, W.R.; et al. A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2-spike protein-protein interaction. Nat. Biotechnol. 2020, 38, 1073–1078. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira-Silva, T.; Andrews, J.R.; Boaventura, V.S.; Ranzani, O.T.; de Araujo Oliveira, V.; Paixao, E.S.; Bertoldo Júnior, J.; Machado, T.M.; Hitchings, M.D.T.; Dorion, M.; et al. Effectiveness of CoronaVac, ChAdOx1 nCoV-19, BNT162b2, and Ad26.COV2.S among individuals with previous SARS-CoV-2 infection in Brazil: A test-negative, case-control study. Lancet Infect. Dis. 2022, 22, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Medina-Pestana, J.; Covas, D.T.; Viana, L.A.; Dreige, Y.C.; Nakamura, M.R.; Lucena, E.F.; Júnior, J.B.; Machado, T.M.; Hitchings, M.D.T.; Dorion, M.; et al. Inactivated Whole-virus Vaccine Triggers Low Response Against SARS-CoV-2 Infection Among Renal Transplant Patients: Prospective Phase 4 Study Results. Transplantation 2022, 106, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Skrondal, A.; Rabe-Hesketh, S. Generalized Latent Variable Modeling: Multilevel, Longitudinal and Structural Equation Models; Boca Chapman and Hall/CRC: Raton, FL, USA, 2004. [Google Scholar]
- Gelman, A.; Hill, J. Data Analysis Using Regression and Multilevel/Hierarchical Models; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Van Praet, J.; Reynders, M.; De Bacquer, D.; Viaene, L.; Schoutteten, M.K.; Caluwe, R.; Doubel, P.; Heylen, L.; De Bel, A.V.; Van Vlem, B.; et al. Predictors and Dynamics of the Humoral and Cellular Immune Response to SARS-CoV-2 mRNA Vaccines in Hemodialysis Patients: A Multicenter Observational Study. J. Am. Soc. Nephrol. 2021, 32, 3208–3220. [Google Scholar] [CrossRef]
- Markov, P.V.; Ghafari, M.; Beer, M.; Lythgoe, K.; Simmonds, P.; Stilianakis, N.I.; Katzourakis, A. The evolution of SARS-CoV-2. Nat. Rev. Microbiol. 2023, 21, 361–379. [Google Scholar] [CrossRef]
- Feikin, D.R.; Higdon, M.M.; Abu-Raddad, L.J.; Andrews, N.; Araos, R.; Goldberg, Y.; Groome, M.J.; Huppert, A.; O’Brien, K.L.; Smith, P.G.; et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: Results of a systematic review and meta-regression. Lancet 2022, 399, 924–944. [Google Scholar] [CrossRef]
- Notarte, K.I.; Guerrero-Arguero, I.; Velasco, J.V.; Ver, A.T.; Santos de Oliveira, M.H.; Catahay, J.A.; Khan, M.M.S.R.; Pastrana, A.; Juszczyk, G.; Torrelles, J.B.; et al. Characterization of the significant decline in humoral immune response six months post-SARS-CoV-2 mRNA vaccination: A systematic review. J. Med. Virol. 2022, 94, 2939–2961. [Google Scholar] [CrossRef]
- Andrews, N.; Stowe, J.; Kirsebom, F.; Toffa, S.; Rickeard, T.; Gallagher, E.; Gower, C.; Kall, M.; Groves, N.; O’Connell, A.-M.; et al. COVID-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant. N. Engl. J. Med. 2022, 386, 1532–1546. [Google Scholar] [CrossRef]
- Gazit, S.; Shlezinger, R.; Perez, G.; Lotan, R.; Peretz, A.; Ben-Tov, A.; Herzel, E.; Alapi, H.; Cohen, D.; Muhsen, K.; et al. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Naturally Acquired Immunity versus Vaccine-induced Immunity, Reinfections versus Breakthrough Infections: A Retrospective Cohort Study. Clin. Infect. Dis. 2022, 75, e545–e551. [Google Scholar] [CrossRef]
- Ozkaya, E.; Yazici, M.; Baran, I.; Cetin, N.S.; Tosun, I.; Buruk, C.K.; Kaklıkkaya, N.; Aydın, F. Neutralization of Wild-Type and Alpha SARS-CoV-2 Variant by CoronaVac(R) Vaccine and Natural Infection- Induced Antibodies. Curr. Microbiol. 2023, 80, 162. [Google Scholar] [CrossRef] [PubMed]
- Barin, B.; Kasap, U.; Selcuk, F.; Volkan, E.; Uluckan, O. Comparison of SARS-CoV-2 anti-spike receptor binding domain IgG antibody responses after CoronaVac, BNT162b2, ChAdOx1 COVID-19 vaccines, and a single booster dose: A prospective, longitudinal population-based study. Lancet Microbe 2022, 3, e274–e283. [Google Scholar] [CrossRef] [PubMed]
- Seija, M.; Rammauro, F.; Santiago, J.; Orihuela, N.; Zulberti, C.; Machado, D.; Recalde, C.; Noboa, J.; Frantchez, V.; Astesiano, R.; et al. Comparison of antibody response to SARS-CoV-2 after two doses of inactivated virus and BNT162b2 mRNA vaccines in kidney transplant. Clin. Kidney J. 2022, 15, 527–533. [Google Scholar] [CrossRef]
- Medina-Pestana, J.; Almeida Viana, L.; Nakamura, M.R.; Lucena, E.F.; Granato, C.F.H.; Dreige, Y.C.; Amorim, L.V.P.; Chow, C.Y.Z.; Demarchi Foresto, R.; Roberto Requião-Moura, L.; et al. Immunogenicity After a Heterologous BNT262b2 Versus Homologous Booster in Kidney Transplant Recipients Receiving 2 Doses of CoronaVac Vaccine: A Prospective Cohort Study. Transplantation 2022, 106, 2076–2084. [Google Scholar] [CrossRef] [PubMed]

| Total N = 221 | Transplant N = 113 | Dialysis N = 108 | p | |
|---|---|---|---|---|
| Age, years (IQR) | 49.9 (38.0–59.5) | 45.4 (35.7–58.2) | 54.0 (43.3–62.5) | <0.001 |
| Male sex, N (%) | 124 (56.1) | 62 (54.9) | 62 (57.4) | 0.704 |
| Race, N (%) | 0.708 | |||
| White | 138 (62.4) | 71 (62.8) | 67 (62.0) | |
| Black | 21 (9.5) | 9 (8.0) | 12 (11.1) | |
| Other | 62 (28.1) | 33 (29.2) | 29 (26.9) | |
| Hypertension, N (%) | 186 (84.2) | 93 (82.3) | 93 (86.1) | 0.438 |
| Diabetes mellitus, N (%) | 53 (24.0) | 24 (21.2) | 29 (26.9) | 0.329 |
| CKD ethiology, N (%) | 0.291 | |||
| Undetermined | 72 (32.6) | 41 (36.6) | 31 (28.7) | |
| Glomerulonephitis | 59 (26.7) | 32 (28.3) | 27 (25.0) | |
| Diabetes Mellitus | 40 (18.1) | 21 (18.6) | 19 (18.1) | |
| Polycystic Kidney Disease | 20 (9.0) | 8 (7.1) | 12 (11.1) | |
| Hypertension | 18 (8.1) | 5 (4.4) | 13 (12.0) | |
| Urologic | 12 (5.4) | 6 (5.3) | 6 (5.6) | |
| Dialysis vintage, months (IQR) | 33.6 (15.1–76.9) | 25.1 (12.9–47.4) | 51.9 (17.7–98.8) | <0.001 |
| Dialysis modality, N (%) | 0.019 | |||
| Hemodialysis | 183 (82.8) | 90 (79.6) | 93 (86.1) | |
| Peritoneal Dialysis | 30 (13.6) | 15 (13.3) | 15 (13.9) | |
| Preemptive | 8 (3.6) | 8 (7.1) | - | |
| HLA Mismatch (ABDR), N (IQR) | - | 2 (1–3) | - | - |
| PRA > 0, N (%) | - | 21 (18.6) | - | - |
| Immunosuppression, N (%) | - | |||
| Pred + TAC + MPS | - | 66 (58.4) | - | |
| Pred + TAC + AZA | - | 45 (39.8) | - | |
| Pred + TAC + mTORi | - | 2 (1.8) | - |
| IgG Status | Transplant | Dialysis | p | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Screening | M1 | M3 | M6 | M12 | Screening | M1 | M3 | M6 | M12 | Transplant | Dialysis | |
| Entire population | ||||||||||||
| Seroreversion, N (%) | 0/107 (0.0) | 1/102 (1.0) | 0/101 (0.0) | 0/101 (0.0) | 0/97 (0.0) | 0/102 (0.0) | 1/95 (1.1) | 1/93 (1.1) | 1/91 (1.1) | 0/82 (0.0) | ||
| Adjusted population | 0.406 | 0.663 | ||||||||||
| Seroreversion, N (%) | 0/94 (0.0) | 1/94 (1.1) | 0/94 (0.0) | 0/94 (0.0) | 0/94 (0.0) | 0/78 (0.0) | 1/78 (1.3) | 1/78 (1.3) | 1/78 (1.3) | 0/78 (0.0) | ||
| Model 1 | Model 2 * | |||
|---|---|---|---|---|
| Coefficient (CI 95%) | p | Coefficient (CI 95%) | p | |
| Transplant–Screening (ref. Dialysis) | 1390 (−2372 to 5152) | 0.469 | 2432 (−1348 to 6213) | 0.207 |
| Time (All patients; ref. Screening) | 0.005 | 0.006 | ||
| M1 | 2125 (−908 to 5158) | 0.170 | 2.102 (−932 to 5136) | 0.174 |
| M3 | 1981 (−1073 to 5035) | 0.204 | 1900 (−1156 to 4955) | 0.223 |
| M6 | 5072 (1998 to 8147) | 0.001 | 4980 (1905 to 8056) | 0.002 |
| M12 | −474 (−3652 to 2704) | 0.770 | −530 (−3708 to 2649) | 0.744 |
| Group + time interaction | 0.005 | 0.006 | ||
| Transplant-M1 | −5998 (−10,221 to −1.775) | 0.005 | −5951 (−10,175 to −1726) | 0.006 |
| Transplant-M3 | −4288 (−8533 to −42) | 0.048 | −4200 (−8448 to 47) | 0.053 |
| Transplant-M6 | −7201 (−11,462 to −2941) | 0.001 | −7100 (−11,362 to −2839) | 0.001 |
| Transplant-M12 | −1690 (−6051 to 2670) | 0.447 | −1629 (−5991 to 2732) | 0.464 |
| Model 1 | Model 2 * | |||
|---|---|---|---|---|
| OR (CI 95%) | p | OR (CI 95%) | p | |
| Transplant (ref. Dialysis) | 2.40 (0.40 to 14.46) | 0.339 | 4.35 (0.60 to 31.35) | 0.145 |
| Time (All patients; ref. screening) | 0.022 | 0.027 | ||
| M1 | 1.35 (0.35 to 5.13) | 0.662 | 1.34 (0.36 to 5.06) | 0.665 |
| M3 | 1.37 (0.36 to 5.18) | 0.647 | 1.34 (0.35 to 5.05) | 0.667 |
| M6 | 7.30 (1.35 to 39.66) | 0.021 | 6.98 (1.29 to 37.91) | 0.024 |
| M12 | 23.73 (2.71 to 207.43) | 0.004 | 22.38 (2.56 to 195.35) | 0.005 |
| Group + time interaction | 0.832 | 0.812 | ||
| Transplant-M1 | 0.68 (0.09 to 5.07) | 0.703 | 0.67 (0.09 to 5.08) | 0.700 |
| Transplant-M3 | 0.71 (0.10 to 5.25) | 0.734 | 0.71 (0.10 to 5.37) | 0.744 |
| Transplant-M6 | 5.67 (0.15 to 209.70) | 0.346 | 6.23 (0.16 to 237.37) | 0.325 |
| Transplant-M12 | 1.73 (0.04 to 80.79) | 0.779 | 1.93 (0.04 to 91.76) | 0.739 |
| Total N = 221 | Transplant N = 113 | Dialysis N = 108 | p | |
|---|---|---|---|---|
| COVID-19, N (%) | 53 (24.0) | 37 (32.7) | 16 (14.8) | 0.002 |
| Death, N (%) | 12 (5.4) | 6 (5.3) | 6 (5.6) | 0.936 |
| Cause of death, N (%) | 0.241 | |||
| Infections other than COVID-19 | 5 (41.7) | 3 (50.0) | 2 (33.3) | |
| Cardiovascular | 4 (33.3) | 1 (16.7) | 3 (50.0) | |
| COVID-19 | 2 (16.7) | 2 (33.3) | 0 (0.0) | |
| Other | 1 (8.3) | 0 (0.0) | 1 (16.7) | |
| Hospitalization, N (%) | 85 (38.5) | 58 (51.3) | 27 (25.0) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Foresto, R.D.; Souza, R.M.; dos Anjos, G.R.; Nakamura, M.R.; Goulart, H.d.S.; Sampaio, R.; França, D.P.; Marques, E.F.; Lucena, E.F.; Cristelli, M.P.; et al. The Influence of Initial Immunosuppression on the Kinetics of Humoral Response after SARS-CoV-2 Vaccination in Patients Undergoing Kidney Transplantation. Vaccines 2024, 12, 1135. https://doi.org/10.3390/vaccines12101135
Foresto RD, Souza RM, dos Anjos GR, Nakamura MR, Goulart HdS, Sampaio R, França DP, Marques EF, Lucena EF, Cristelli MP, et al. The Influence of Initial Immunosuppression on the Kinetics of Humoral Response after SARS-CoV-2 Vaccination in Patients Undergoing Kidney Transplantation. Vaccines. 2024; 12(10):1135. https://doi.org/10.3390/vaccines12101135
Chicago/Turabian StyleForesto, Renato Demarchi, Roberto Matias Souza, Gustavo Rodrigues dos Anjos, Mônica Rika Nakamura, Haryanne de Souza Goulart, Rayra Sampaio, Daniela Pereira França, Emanuelle Ferreira Marques, Elisabeth França Lucena, Marina Pontello Cristelli, and et al. 2024. "The Influence of Initial Immunosuppression on the Kinetics of Humoral Response after SARS-CoV-2 Vaccination in Patients Undergoing Kidney Transplantation" Vaccines 12, no. 10: 1135. https://doi.org/10.3390/vaccines12101135






