Clustering Analysis Identified Distinct Clinical Phenotypes among Hemodialysis Patients in Their Immunological Response to the BNT162b2 mRNA Vaccine against SARS-CoV-2
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Study Design
2.1.1. Study Population
- −
- Male or female patients, over 18 years of age.
- −
- Patients undergoing hemodialysis at the dialysis center of Claude Galien Hospital.
- −
- Patients vaccinated for the first time against COVID-19.
- −
- Patients informed and agreeing to participate to the study.
- −
- Patients affiliated to or benefiting from a social security scheme.
- −
- The non-inclusion criteria were as follows:
- −
- Patients contraindicated to vaccination or not vaccinated.
- −
- Administration of any other vaccine within the previous 3 weeks.
- −
- Protected patients: adults under guardianship, curatorship, or other legal protection, deprived of liberty by judicial or administrative decision.
- −
- Pregnant women.
2.1.2. Study Design
2.2. Determination of IgG Anti-SARS-CoV-2 Asntibodies
2.3. Analysis of Dialysis Charts and Medical Records
2.4. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- El Karoui, K.; De Vriese, A.S. COVID-19 in dialysis: Clinical impact, immune response, prevention and treatment. Kidney Int. 2022, 101, 883–894. [Google Scholar] [CrossRef] [PubMed]
- Barouch, D.H. COVID-19 vaccines—Immunity, Variants, Boosters. N. Engl. J. Med. 2022, 387, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- Ashby, D.R.; Caplin, B.; Corbett, R.W.; Asgari, E.; Kumar, N.; Sarnowski, A.; Hull, R.; Makanjuola, D.; Cole, N.; Chan, J.; et al. Severity of COVID-19 after vaccination among hemodialysis patients: An observational cohort study. Clin. J. Am. Soc. Nephrol. 2022, 17, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Mink, S.; Fraunberger, P. Anti-SARS-CoV-2 antibody testing: Role and indications. J. Clin. Med. 2023, 12, 7575. [Google Scholar] [CrossRef] [PubMed]
- Chan, L.; Fuca, N.; Zeldis, E.; Campbell, K.N.; Shaikh, A. Antibody Response to mRNA-1273 SARS-CoV-2 Vaccine in hemodialysis Patients with and without Prior COVID-19. Clin. J. Am. Soc. Med. 2021, 16, 1258–1260. [Google Scholar] [CrossRef] [PubMed]
- Guinault, D.; Bernier, M.; Delarche, A.; Neuville, S.; Milioto, O.; Molinari, N.; Dahan, P. Immunogenicity of the BNT162b2 vaccine in patients undergoing maintenance hemodialysis is associated with medical conditions. Nephron 2022, 146, 553–558. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Gandra, S.; Reske, K.A.; Olsen, M.A.; Bommarito, S.; Miller, C.; Hock, K.G.; Ballman, C.A.; Su, C.; Le Dang, N. Predictors of humoral response to SARS-CoV-2 mRNA vaccine BNT162b2 in patients receiving maintenance dialysis. Antimicrob. Steward. Healthc. Epidemiol. 2022, 2, e48. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.; Park, S.; Kim, E.K.; Koyanagi, A.; Jacob, L.; Yon, D.K.; Lee, S.W.; Kim, M.S.; Radua, J.; Elena, D.; et al. Immunogenicity of COVID-19 vaccines in patients with diverse health conditions: A comprehensive systematic review. J. Med. Virol. 2022, 94, 4144–4155. [Google Scholar] [CrossRef] [PubMed]
- Pellegrino, R.; Pellino, G.; Selvaggi, L.; Selvaggi, F.; Federico, A.; Romano, M.; Gravina, A.G. BNT162b2 mRNA COVID-19 vaccine is safe in a setting of patients on biologic therapy with inflammatory bowel diseases: A monocentric real-life study. Expert Rev. Clin. Pharmacol. 2022, 15, 1243–1252. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, A.P.; Richards, G.; De la Iglesia, B.; Rayward-Smith, V.J. Clustering Rules: A Comparison of Partitioning and Hierarchical Clustering Algorithms. J. Math. Model. Algorithms 2006, 5, 475–504. [Google Scholar] [CrossRef]
- Gower, J.C. A general coefficient of similarity and some of its properties. Biometrics 1971, 27, 857–871. [Google Scholar] [CrossRef]
- Rousseeuw, P.J. Silhouettes: A graphical aid to the interpretation and validation of cluster analysis. J. Comput. Appl. Math. 1987, 20, 53–65. [Google Scholar] [CrossRef]
- Rossi, M.; Pessolano, G.; Gambaro, G. What has vaccination against COVID-19 in CKD taught us? J. Nephrol. 2023, 36, 1257–1266. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Eguchi, J.; Watanabe, M.; Nakayama, M.; Wada, J. Reduced immunogenicity of COVID-19 vaccine in obese patients with type 2 diabetes: A cross-sectional study. Acta Med. Okayama 2024, 78, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Vena, W.; Pigni, S.; Betella, N.; Navarra, A.; Mirani, M.; Mazziotti, G.; Lania, A.G. Covid-19 vaccines and blood glucose control: Friend or Foe? Hum. Vaccines Immunother. 2024, 20, 236306. [Google Scholar] [CrossRef] [PubMed]
- Steiger, S.; Rossaint, J.; Zarbock, A.; Anders, H.J. Secondary immunodeficiency related to kidney disease (SIDKD)-Definition, unmet need, and mechanisms. J. Am. Soc. Nephrol. 2022, 33, 259–278. [Google Scholar] [CrossRef] [PubMed]
- Yau, K.; Chan, C.T.; Abe, K.T.; Jiang, Y.; Atiquzzaman, M.; Mullin, S.I.; Shadowitz, E.; Liu, L.; Kostadinovic, E.; Sukovic, T.; et al. Difference in mRNA-1273 (Moderna) and BNT162b2 (Pfizer-BioNTech) SARS-CoV-2 vaccine immunogenicity among patients undergoing dialysis. CMAJ 2022, 28, E297–E305. [Google Scholar] [CrossRef] [PubMed]
- Yau, K.; Tam, P.; Chan, C.T.; Hu, Q.; Qi, F.; Abe, K.T.; Kurtesi, A.; Jiang, Y.; Estrada-Codecido, J.; Brown, T.; et al. BNT162b2 versus mRNA-1273 third dose COVID-19 vaccine in patients with CKD and maintenance dialysis patients. Clin. J. Am. Soc. Nephrol. 2024, 19, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Debelle, F.; Phuong Nguyen, V.T.; Boitquin, L.; Guillen-Anaya, M.A.; Gankam, F.; Declèves, A.E.; CoviDial Study Group. Monitoring strategy of COVID-19 vaccination in dialysis patients based on a multiplex immunodot method: The CoviDial Study. Semin. Dial. 2024, 37, 145–152. [Google Scholar] [CrossRef] [PubMed]
| Total n = 117 | |
|---|---|
| IgG anti-spike after the second dose (BAU/mL) | 690.9 (149.8–3755.4) |
| Age (years) | 68.0 (57.0–78.0) |
Sex
| 71 (60.7%) 46 (39.3%) |
| Dialysis vintage (months) | 41.0 (14.0–73.3) |
| 0.3–265.1 |
Type of dialysis technique
| 35 (29.9%) 28 (23.9%) 20 (17.1%) 19 (16.2%) 15 (12.8%) |
Diabetes Mellitus
| 51 (43.6%) 66 (56.4%) |
| Modified Charlson’s comorbidity index | 7 (5–8) |
| C-reactive protein (mg/L) | 3.2 (1.8–8.2) |
| Albumin (g/L) | 36.0 (33.0–38.0) |
| Prealbumin (g/L) | 0.30 (0.26–0.37) |
| Darbepoetin dose in the yearbefore vaccination (µg/week) | 36.0 (23.0–67.0) |
| Parenteral iron sucrose dose in the year before vaccination (mg/month) | 266.0 (158.0–333.0) |
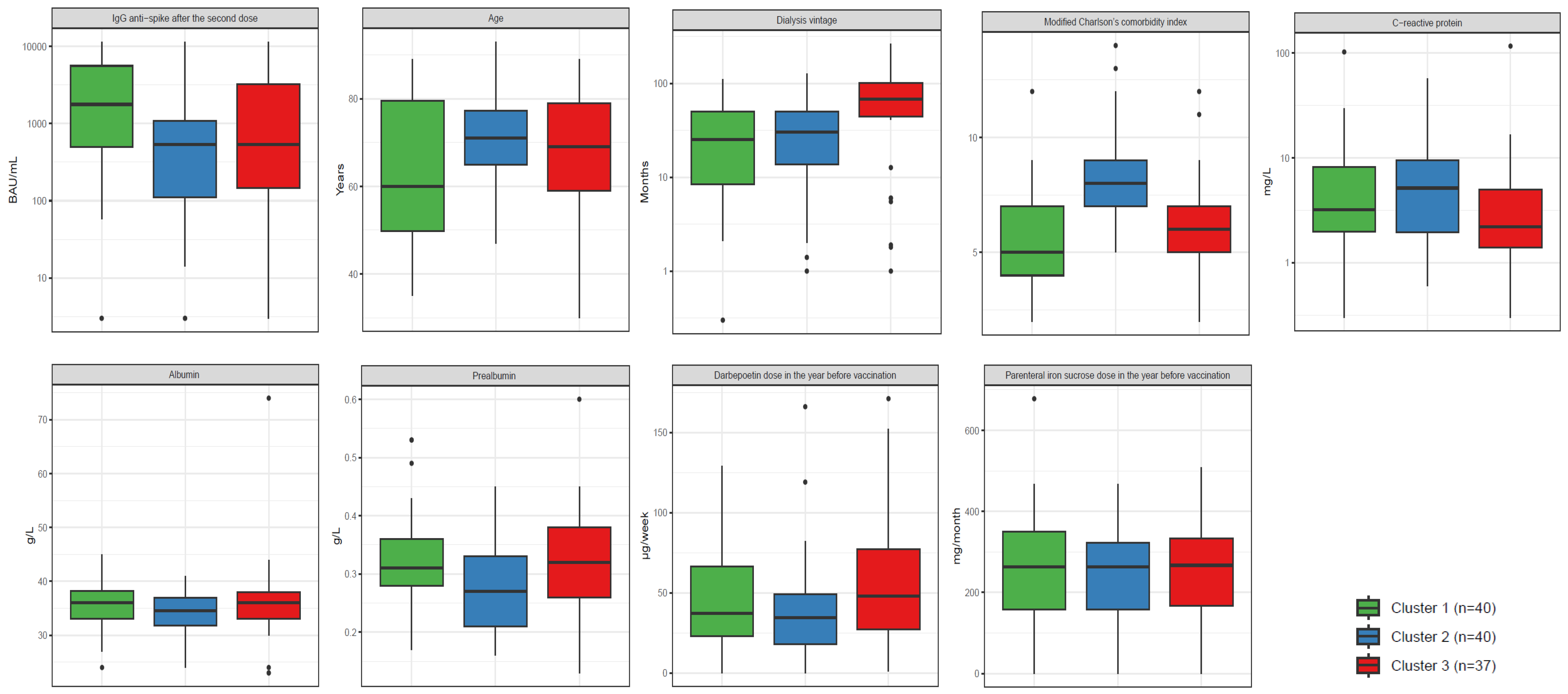
| Cluster 1 n = 40 | Cluster 2 n = 40 | Cluster 3 n = 37 | p-Value * | |
|---|---|---|---|---|
| IgG anti-spike after the second dose (BAU/mL) | 1772.6 (503.5–5586.9) | 537.1 (111.6–1081.2) | 538.3 (145.2–3259.8) | 0.0090 |
| Age (years) | 60.0 (49.8–79.5) | 71.0 (65.0–77.3) | 69.0 (59.0–79.0) | 0.0560 |
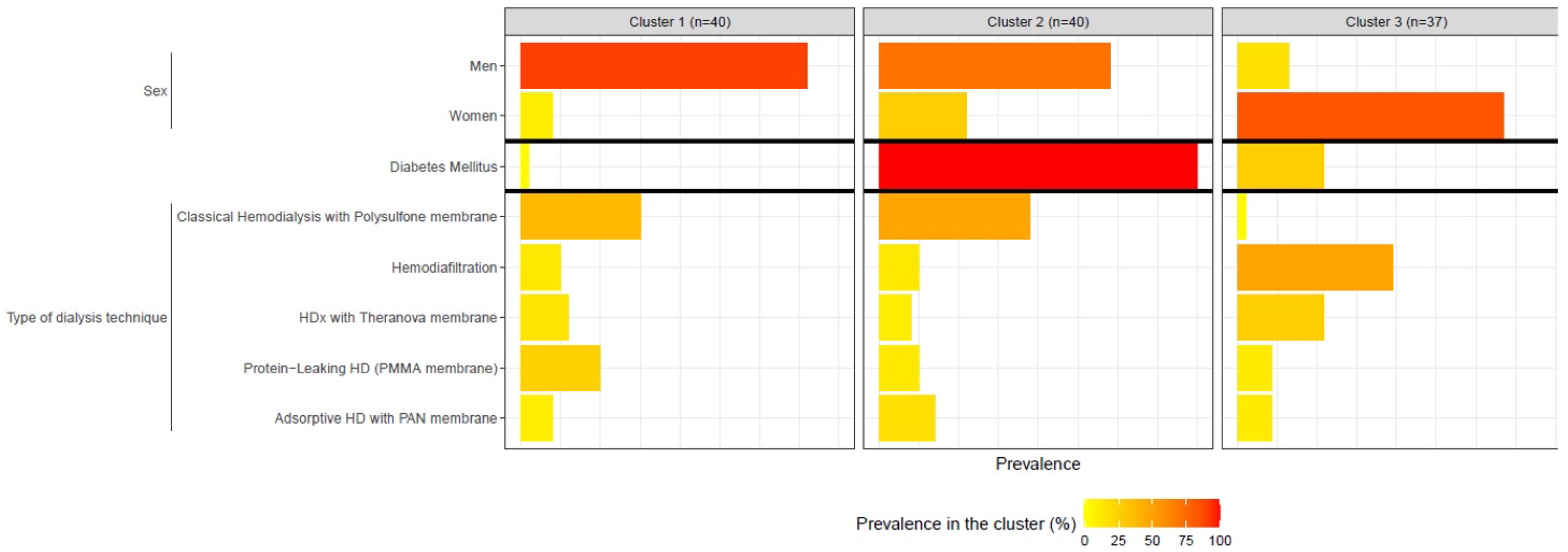
Sex
| 36 (90.0%) 4 (10.0%) | 29 (72.5%) 11 (27.5%) | 6 (16.2%) 31 (83.8%) | <0.0001 |
| Dialysis vintage (months) | 25.4 (8.5–50.6) | 30.4 (13.8–50.6) | 68.2 (44.6–102.0) | 0.0003 |
| 0.3–110.2 | 1–127.8 | 1–265.1 | |
Type of dialysis technique
| 15 (37.5%) 5 (12.5%) 6 (15.0%) 10 (25.0%) 4 (10.0%) | 19 (47.5%) 5 (12.5%) 4 (10.0%) 5 (12.5%) 7 (17.5%) | 1 (2.7%) 18 (48.6%) 10 (27.0%) 4 (10.8%) 4 (10.8%) | <0.0001 |
Diabetes Mellitus
| 1 (2.5%) 39 (97.5%) | 40 (100%) 0 (0.0%) | 10 (27.0%) 27 (73%) | <0.0001 |
| Modified Charlson’s comorbidity index | 5 (4–7) | 8 (7–9) | 6 (5–7) | <0.0001 |
| C-reactive protein (mg/L) | 3.2 (2.0–8.1) | 5.2 (2.0–9.5) | 2.2 (1.4–5.0) | 0.0520 |
| Albumin (g/L) | 36.0 (33.0–38.3) | 34.5 (31.8–37.0) | 36.0 (33.0–38.0) | 0.2600 |
| Prealbumin (g/L) | 0.31 (0.28–0.36) | 0.27 (0.21–0.33) | 0.32 (0.26–0.38) | 0.0390 |
| Darbepoetin dose in the year before vaccination (µg/week) | 37.0 (22.8–66.3) | 34.5 (18.0–49.3) | 48.0 (27.0–77.0) | 0.1100 |
| Parenteral iron sucrose dose in the year before vaccination (mg/month) | 263.5 (158.5–350.0) | 262.5 (158.0–323.3) | 267.0 (167.0–333.0) | 0.9800 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rostoker, G.; Rouanet, S.; Griuncelli, M.; Loridon, C.; Boulahia, G.; Gagnon, L. Clustering Analysis Identified Distinct Clinical Phenotypes among Hemodialysis Patients in Their Immunological Response to the BNT162b2 mRNA Vaccine against SARS-CoV-2. Vaccines 2024, 12, 1150. https://doi.org/10.3390/vaccines12101150
Rostoker G, Rouanet S, Griuncelli M, Loridon C, Boulahia G, Gagnon L. Clustering Analysis Identified Distinct Clinical Phenotypes among Hemodialysis Patients in Their Immunological Response to the BNT162b2 mRNA Vaccine against SARS-CoV-2. Vaccines. 2024; 12(10):1150. https://doi.org/10.3390/vaccines12101150
Chicago/Turabian StyleRostoker, Guy, Stéphanie Rouanet, Mireille Griuncelli, Christelle Loridon, Ghada Boulahia, and Luc Gagnon. 2024. "Clustering Analysis Identified Distinct Clinical Phenotypes among Hemodialysis Patients in Their Immunological Response to the BNT162b2 mRNA Vaccine against SARS-CoV-2" Vaccines 12, no. 10: 1150. https://doi.org/10.3390/vaccines12101150
APA StyleRostoker, G., Rouanet, S., Griuncelli, M., Loridon, C., Boulahia, G., & Gagnon, L. (2024). Clustering Analysis Identified Distinct Clinical Phenotypes among Hemodialysis Patients in Their Immunological Response to the BNT162b2 mRNA Vaccine against SARS-CoV-2. Vaccines, 12(10), 1150. https://doi.org/10.3390/vaccines12101150






