Immunogenicity and Protectivity of Sputnik V Vaccine in hACE2-Transgenic Mice against Homologous and Heterologous SARS-CoV-2 Lineages Including Far-Distanced Omicron BA.5
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Laboratory Animals
2.3. Vaccine
2.4. Immunization and Further Sampling Procedures
2.5. ELISA
2.6. SARS-CoV-2 Preparation
2.7. Neutralization Assay
2.8. Study of Cellular Immune Response
2.9. Animal Challenge
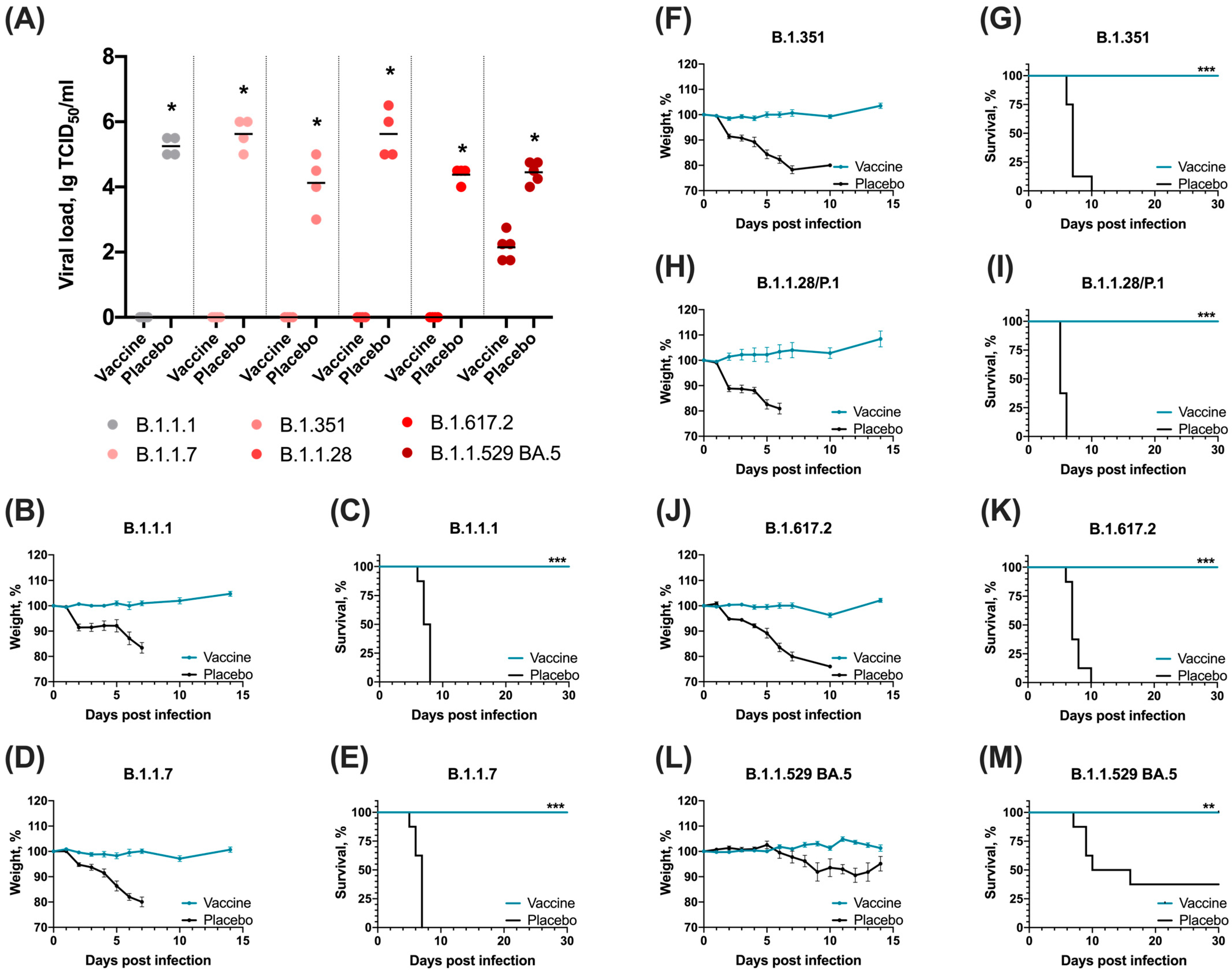
2.10. Determination of Viral Load in Lungs
2.11. Histopathological Analysis of the Lungs
2.12. Statistical Analysis
3. Results
3.1. Sputnik V Prime-Boost Immunization Results in Broad Cross-Reactive Humoral and Cellular Immune Response across Different VOCs
3.2. Sputnik V Prime-Boost Immunization Results in Protection of Lung Pathology Caused by Different VOCs
3.3. Sputnik V Prime-Boost Immunization Results in 100% Survival after Lethal SARS-CoV-2 Challenge with Different VOCs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A

References
- World Health Organization. COVID-19 Public Health Emergency of International Concern (PHEIC) Global Research and Innovation Forum. Available online: https://www.who.int/publications/m/item/covid-19-public-health-emergency-of-international-concern-(pheic)-global-research-and-innovation-forum (accessed on 3 October 2024).
- World Health Organization. WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19—11 March 2020. Available online: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 (accessed on 3 October 2024).
- World Health Organization. WHO COVID-19 Dashboard. Available online: https://data.who.int/dashboards/covid19/cases?n=c (accessed on 3 October 2024).
- Walls, A.C.; Park, Y.J.; Tortorici, M.A.; Wall, A.; McGuire, A.T.; Veesler, D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell 2020, 181, 281–292.e6. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Deng, Y.Q.; Ye, Q.; Cao, L.; Sun, C.Y.; Fan, C.; Huang, W.; Sun, S.; Sun, Y.; Zhu, L.; et al. Structural basis for neutralization of SARS-CoV-2 and SARS-CoV by a potent therapeutic antibody. Science 2020, 369, 1505–1509. [Google Scholar] [CrossRef] [PubMed]
- Huo, J.; Le Bas, A.; Ruza, R.R.; Duyvesteyn, H.M.E.; Mikolajek, H.; Malinauskas, T.; Tan, T.K.; Rijal, P.; Dumoux, M.; Ward, P.N.; et al. Neutralizing nanobodies bind SARS-CoV-2 spike RBD and block interaction with ACE2. Nat. Struct. Mol. Biol. 2020, 27, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wang, Y.; Jin, Y.; Zhu, Y.; Wu, Y.; Li, C.; Kong, Y.; Song, W.; Tian, X.; Zhan, W.; et al. A non-ACE2 competing human single-domain antibody confers broad neutralization against SARS-CoV-2 and circulating variants. Signal Transduct. Target. Ther. 2021, 6, 378. [Google Scholar] [CrossRef] [PubMed]
- Khandker, S.S.; Godman, B.; Jawad, M.I.; Meghla, B.A.; Tisha, T.A.; Khondoker, M.U.; Haq, M.A.; Charan, J.; Talukder, A.A.; Azmuda, N.; et al. A Systematic Review on COVID-19 Vaccine Strategies, Their Effectiveness, and Issues. Vaccines 2021, 9, 1387. [Google Scholar] [CrossRef]
- Francis, A.I.; Ghany, S.; Gilkes, T.; Umakanthan, S. Review of COVID-19 vaccine subtypes, efficacy and geographical distributions. Postgrad. Med. J. 2022, 98, 389–394. [Google Scholar] [CrossRef]
- Magazine, N.; Zhang, T.; Wu, Y.; McGee, M.C.; Veggiani, G.; Huang, W. Mutations and Evolution of the SARS-CoV-2 Spike Protein. Viruses 2022, 14, 640. [Google Scholar] [CrossRef]
- Palyanov, A.Y.; Palyanova, N.V. On the space of SARS-CoV-2 genetic sequence variants. Vavilovskii Zhurnal Genet. Selektsii. 2023, 27, 839–850. [Google Scholar] [CrossRef]
- Laidlaw, B.J.; Ellebedy, A.H. The germinal centre B cell response to SARS-CoV-2. Nat. Rev. Immunol. 2022, 22, 7–18. [Google Scholar] [CrossRef]
- Carabelli, A.M.; Peacock, T.P.; Thorne, L.G.; Harvey, W.T.; Hughes, J.; COVID-19 Genomics UK Consortium; Peacock, S.J.; Barclay, W.S.; de Silva, T.I.; Towers, G.J.; et al. SARS-CoV-2 variant biology: Immune escape, transmission and fitness. Nat. Rev. Microbiol. 2023, 21, 162–177. [Google Scholar] [CrossRef]
- Ding, K.; Jiang, W.; Xiong, C.; Lei, M. Turning point: A new global COVID-19 wave or a signal of the beginning of the end of the global COVID-19 pandemic? Immun. Inflamm. Dis. 2022, 10, e606. [Google Scholar] [CrossRef] [PubMed]
- Fall, A.; Eldesouki, R.E.; Sachithanandham, J.; Morris, C.P.; Norton, J.M.; Gaston, D.C.; Forman, M.; Abdullah, O.; Gallagher, N.; Li, M.; et al. The displacement of the SARS-CoV-2 variant Delta with Omicron: An investigation of hospital admissions and upper respiratory viral loads. EBioMedicine 2022, 79, 104008. [Google Scholar] [CrossRef] [PubMed]
- Tegally, H.; Moir, M.; Everatt, J.; Giovanetti, M.; Scheepers, C.; Wilkinson, E.; Subramoney, K.; Makatini, Z.; Moyo, S.; Amoako, D.G.; et al. Emergence of SARS-CoV-2 Omicron lineages BA.4 and BA.5 in South Africa. Nat. Med. 2022, 28, 1785–1790. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Yisimayi, A.; Jian, F.; Song, W.; Xiao, T.; Wang, L.; Du, S.; Wang, J.; Li, Q.; Chen, X.; et al. BA.2.12.1, BA.4 and BA.5 escape antibodies elicited by Omicron infection. Nature 2022, 608, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Karuppanan, K.; Subramaniam, G. Omicron (BA.1) and sub-variants (BA.1.1, BA.2, and BA.3) of SARS-CoV-2 spike infectivity and pathogenicity: A comparative sequence and structural-based computational assessment. J. Med. Virol. 2022, 94, 4780–4791. [Google Scholar] [CrossRef]
- Alkhatib, M.; Salpini, R.; Carioti, L.; Ambrosio, F.A.; D’Anna, S.; Duca, L.; Costa, G.; Bellocchi, M.C.; Piermatteo, L.; Artese, A.; et al. Update on SARS-CoV-2 Omicron Variant of Concern and Its Peculiar Mutational Profile. Microbiol. Spectr. 2022, 10, e0273221. [Google Scholar] [CrossRef]
- Kandeel, A.; Moatasim, Y.; Fahim, M.; Bahaaeldin, H.; El-Shesheny, R.; Roshdy, W.H.; Kamel, M.N.; Shawky, S.; Gomaa, M.; Naguib, A.; et al. Comparison of SARS-Cov-2 omicron variant with the previously identified SARS-Cov-2 variants in Egypt, 2020–2022: Insight into SARS-Cov-2 genome evolution and its impact on epidemiology, clinical picture, disease severity, and mortality. BMC Infect. Dis. 2023, 23, 542. [Google Scholar] [CrossRef]
- Selvavinayagam, S.T.; Yong, Y.K.; Joseph, N.; Hemashree, K.; Tan, H.Y.; Zhang, Y.; Rajeshkumar, M.; Kumaresan, A.; Kalpana, R.; Kalaivani, V.; et al. Low SARS-CoV-2 viral load among vaccinated individuals infected with Delta B.1.617.2 and Omicron BA.1.1.529 but not with Omicron BA.1.1 and BA.2 variants. Front. Public Health 2022, 10, 1018399. [Google Scholar] [CrossRef]
- Tuekprakhon, A.; Nutalai, R.; Dijokaite-Guraliuc, A.; Zhou, D.; Ginn, H.M.; Selvaraj, M.; Liu, C.; Mentzer, A.J.; Supasa, P.; Duyvesteyn, H.M.E.; et al. Antibody escape of SARS-CoV-2 Omicron BA.4 and BA.5 from vaccine and BA.1 serum. Cell 2022, 185, 2422–2433.e13. [Google Scholar] [CrossRef]
- Fan, Y.; Li, X.; Zhang, L.; Wan, S.; Zhang, L.; Zhou, F. SARS-CoV-2 Omicron variant: Recent progress and future perspectives. Signal Transduct. Target Ther. 2022, 7, 141. [Google Scholar] [CrossRef]
- Xia, S.; Wang, L.; Zhu, Y.; Lu, L.; Jiang, S. Origin, virological features, immune evasion and intervention of SARS-CoV-2 Omicron sublineages. Signal Transduct. Target Ther. 2022, 7, 241. [Google Scholar] [CrossRef] [PubMed]
- GOST 33044-2014. Interstate Standard. Principles of Good Laboratory Practice. Available online: https://protect.gost.ru/document.aspx?control=7&baseC=6&page=6&month=2&year=2023&search=&RegNum=1&DocOnPageCount=15&id=238531 (accessed on 3 October 2024).
- Roederer, M. Interpretation of cellular proliferation data: Avoid the panglossian. Cytom. A 2011, 79, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Tukhvatulin, A.I.; Gordeychuk, I.V.; Dolzhikova, I.V.; Dzharullaeva, A.S.; Krasina, M.E.; Bayurova, E.O.; Grousova, D.M.; Kovyrshina, A.V.; Kondrashova, A.S.; Avdoshina, D.V.; et al. Immunogenicity and protectivity of intranasally delivered vector-based heterologous prime-boost COVID-19 vaccine Sputnik V in mice and non-human primates. Emerg. Microbes Infect. 2022, 11, 2229–2247. [Google Scholar] [CrossRef] [PubMed]
- Lapa, D.; Grousova, D.M.; Matusali, G.; Meschi, S.; Colavita, F.; Bettini, A.; Gramigna, G.; Francalancia, M.; Garbuglia, A.R.; Girardi, E.; et al. Retention of Neutralizing Response against SARS-CoV-2 Omicron Variant in Sputnik V-Vaccinated Individuals. Vaccines 2022, 10, 817. [Google Scholar] [CrossRef] [PubMed]
- Gushchin, V.A.; Dolzhikova, I.V.; Shchetinin, A.M.; Odintsova, A.S.; Siniavin, A.E.; Nikiforova, M.A.; Pochtovyi, A.A.; Shidlovskaya, E.V.; Kuznetsova, N.A.; Burgasova, O.A.; et al. Neutralizing Activity of Sera from Sputnik V-Vaccinated People against Variants of Concern (VOC: B.1.1.7, B.1.351, P.1, B.1.617.2, B.1.617.3) and Moscow Endemic SARS-CoV-2 Variants. Vaccines 2021, 9, 779. [Google Scholar] [CrossRef]
- Hachmann, N.P.; Miller, J.; Collier, A.Y.; Ventura, J.D.; Yu, J.; Rowe, M.; Bondzie, E.A.; Powers, O.; Surve, N.; Hall, K.; et al. Neutralization Escape by SARS-CoV-2 Omicron Subvariants BA.2.12.1, BA.4, and BA.5. N. Engl. J. Med. 2022, 387, 86–88. [Google Scholar] [CrossRef]
- Bormann, M.; Brochhagen, L.; Alt, M.; Otte, M.; Thümmler, L.; van de Sand, L.; Kraiselburd, I.; Thomas, A.; Gosch, J.; Braß, P.; et al. Immune responses in COVID-19 patients during breakthrough infection with SARS-CoV-2 variants Delta, Omicron-BA.1 and Omicron-BA.5. Front. Immunol. 2023, 14, 1150667. [Google Scholar] [CrossRef]
- Rice, A.; Verma, M.; Shin, A.; Zakin, L.; Sieling, P.; Tanaka, S.; Adisetiyo, H.; Taft, J.; Patel, R.; Buta, S.; et al. A Next Generation Bivalent Human Ad5 COVID-19 Vaccine Delivering Both Spike and Nucleocapsid Antigens Elicits Th1 Dominant CD4+, CD8+ T-cell and Neutralizing Antibody Responses. bioRxiv 2020. [Google Scholar] [CrossRef]
- Shoushtari, M.; Roohvand, F.; Salehi-Vaziri, M.; Arashkia, A.; Bakhshi, H.; Azadmanesh, K. Adenovirus vector-based vaccines as forefront approaches in fighting the battle against flaviviruses. Hum. Vaccin. Immunother. 2022, 18, 2079323. [Google Scholar] [CrossRef]
- Romagnani, S. Th1/Th2 cells. Inflamm. Bowel Dis. 1999, 5, 285–294. [Google Scholar] [CrossRef]
- Wang, Q.; Guo, Y.; Iketani, S.; Nair, M.S.; Li, Z.; Mohri, H.; Wang, M.; Yu, J.; Bowen, A.D.; Chang, J.Y.; et al. Antibody evasion by SARS-CoV-2 Omicron subvariants BA.2.12.1, BA.4 and BA.5. Nature 2022, 608, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.L.; Zhang, J.W.; Wang, P.T.; Zhang, Z.Y. Immune response and evasion mechanisms of major SARS-CoV-2 variants. Iscience 2022, 25, 105044. [Google Scholar] [CrossRef] [PubMed]
- GISAID. Tracking of hCoV-19 Variants. Available online: https://gisaid.org/hcov19-variants (accessed on 3 October 2024).
- Shao, W.; Chen, X.; Zheng, C.; Liu, H.; Wang, G.; Zhang, B.; Li, Z.; Zhang, W. Effectiveness of COVID-19 vaccines against SARS-CoV-2 variants of concern in real-world: A literature review and meta-analysis. Emerg. Microbes Infect. 2022, 11, 2383–2392. [Google Scholar] [CrossRef] [PubMed]


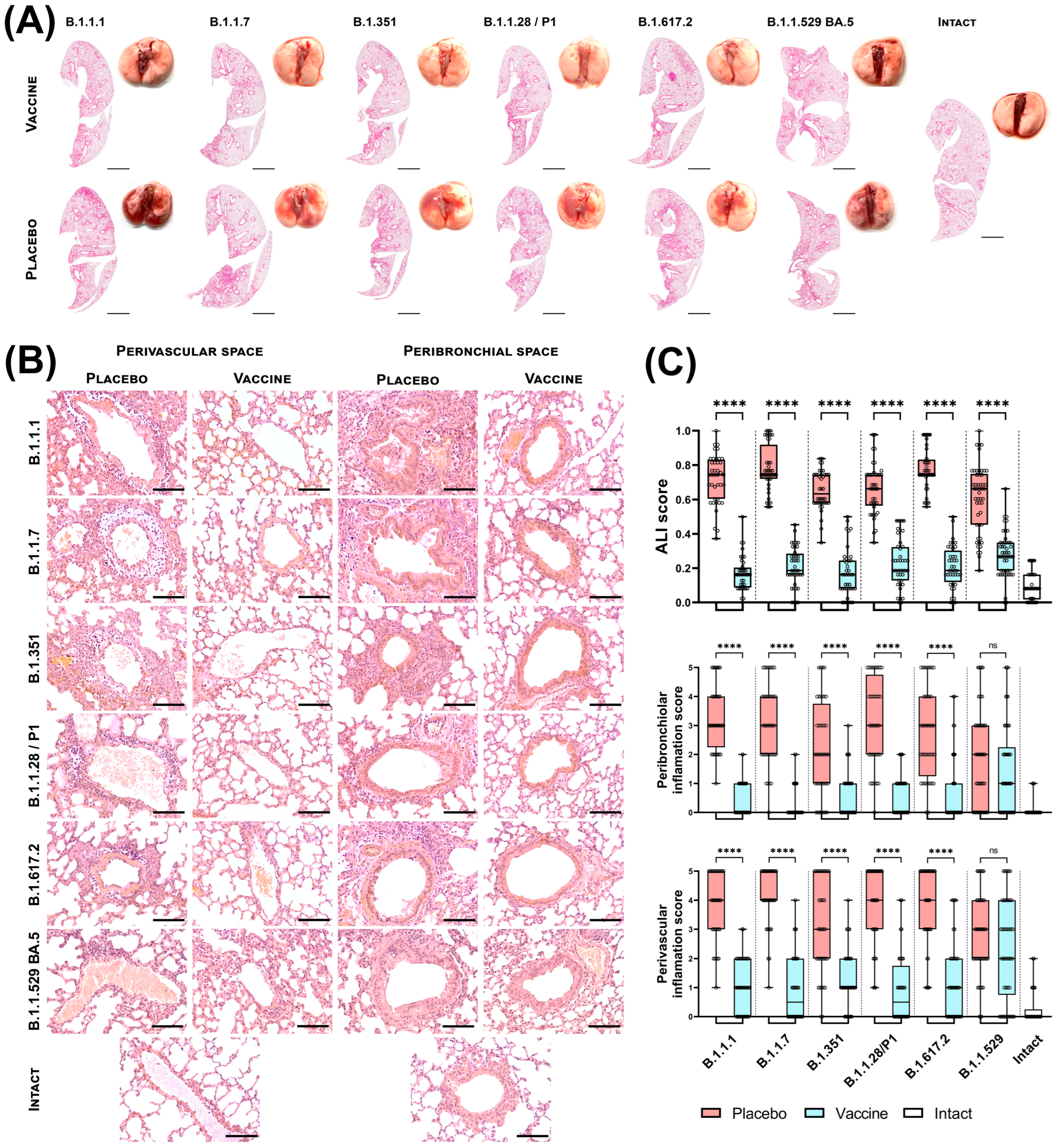
| Parameter | Score | |||
|---|---|---|---|---|
| 0 | 1 | 2 | ||
| A | Neutrophils in the alveolar space | - | 1–5 | >5 |
| B | Neutrophils in the interstitial space | - | 1–5 | >5 |
| C | Proteinaceous debris filling the airspaces | - | 1 | >1 |
| D | Alveolar septal thickening | <2× | 2×–4× | >4× |
| Score | The Degree of Manifestation of Inflammation |
|---|---|
| 0 | Absolute absence of inflammatory infiltrate |
| 1 | The presence of single leukocytes |
| 2 | The presence of 1–2 groups of leukocytes |
| 3 | Infiltration of half of the bronchiole/vessel perimeter |
| 4 | Infiltration of most of the bronchiole/vessel perimeter |
| 5 | Infiltration of the entire perimeter of the bronchiole/vessel with radial strands of leukocytes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dolzhikova, I.V.; Tukhvatulin, A.I.; Grousova, D.M.; Zorkov, I.D.; Komyakova, M.E.; Ilyukhina, A.A.; Kovyrshina, A.V.; Shelkov, A.Y.; Botikov, A.G.; Samokhvalova, E.G.; et al. Immunogenicity and Protectivity of Sputnik V Vaccine in hACE2-Transgenic Mice against Homologous and Heterologous SARS-CoV-2 Lineages Including Far-Distanced Omicron BA.5. Vaccines 2024, 12, 1152. https://doi.org/10.3390/vaccines12101152
Dolzhikova IV, Tukhvatulin AI, Grousova DM, Zorkov ID, Komyakova ME, Ilyukhina AA, Kovyrshina AV, Shelkov AY, Botikov AG, Samokhvalova EG, et al. Immunogenicity and Protectivity of Sputnik V Vaccine in hACE2-Transgenic Mice against Homologous and Heterologous SARS-CoV-2 Lineages Including Far-Distanced Omicron BA.5. Vaccines. 2024; 12(10):1152. https://doi.org/10.3390/vaccines12101152
Chicago/Turabian StyleDolzhikova, Inna V., Amir I. Tukhvatulin, Daria M. Grousova, Ilya D. Zorkov, Marina E. Komyakova, Anna A. Ilyukhina, Anna V. Kovyrshina, Artem Y. Shelkov, Andrey G. Botikov, Ekaterina G. Samokhvalova, and et al. 2024. "Immunogenicity and Protectivity of Sputnik V Vaccine in hACE2-Transgenic Mice against Homologous and Heterologous SARS-CoV-2 Lineages Including Far-Distanced Omicron BA.5" Vaccines 12, no. 10: 1152. https://doi.org/10.3390/vaccines12101152
APA StyleDolzhikova, I. V., Tukhvatulin, A. I., Grousova, D. M., Zorkov, I. D., Komyakova, M. E., Ilyukhina, A. A., Kovyrshina, A. V., Shelkov, A. Y., Botikov, A. G., Samokhvalova, E. G., Reshetnikov, D. A., Siniavin, A. E., Savina, D. M., Shcheblyakov, D. V., Izhaeva, F. M., Dzharullaeva, A. S., Erokhova, A. S., Popova, O., Ozharovskaya, T. A., ... Gintsburg, A. L. (2024). Immunogenicity and Protectivity of Sputnik V Vaccine in hACE2-Transgenic Mice against Homologous and Heterologous SARS-CoV-2 Lineages Including Far-Distanced Omicron BA.5. Vaccines, 12(10), 1152. https://doi.org/10.3390/vaccines12101152








