Myocarditis Associated with COVID-19 Vaccination
Abstract
:1. Introduction
2. Methods
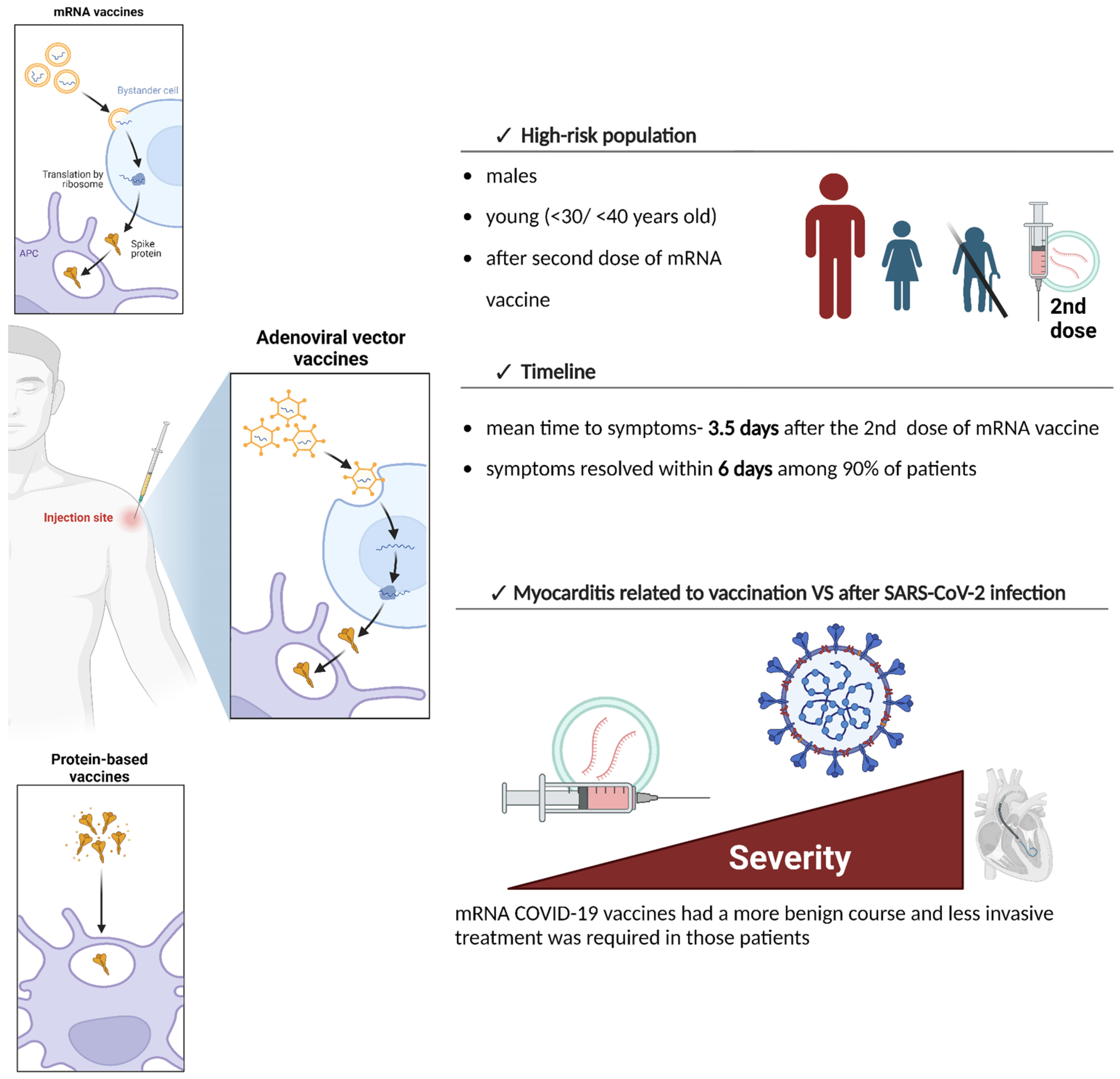
3. Available COVID-19 Vaccines and the Differences in the Risk of Myocarditis
4. Epidemiology and Clinical Presentation of COVID-19 Vaccine-Induced Myocarditis
4.1. Patients with COVID-19 Vaccine-Induced Myocarditis
4.2. Diagnosis of COVID-19 Vaccine-Induced Myocarditis
4.3. Clinical Presentation of COVID-19 Vaccine-Induced Myocarditis
5. Pathological Observations
6. Treatment of Post-Vaccine Myocarditis
7. Risk of Myocarditis After Infection and Vaccination
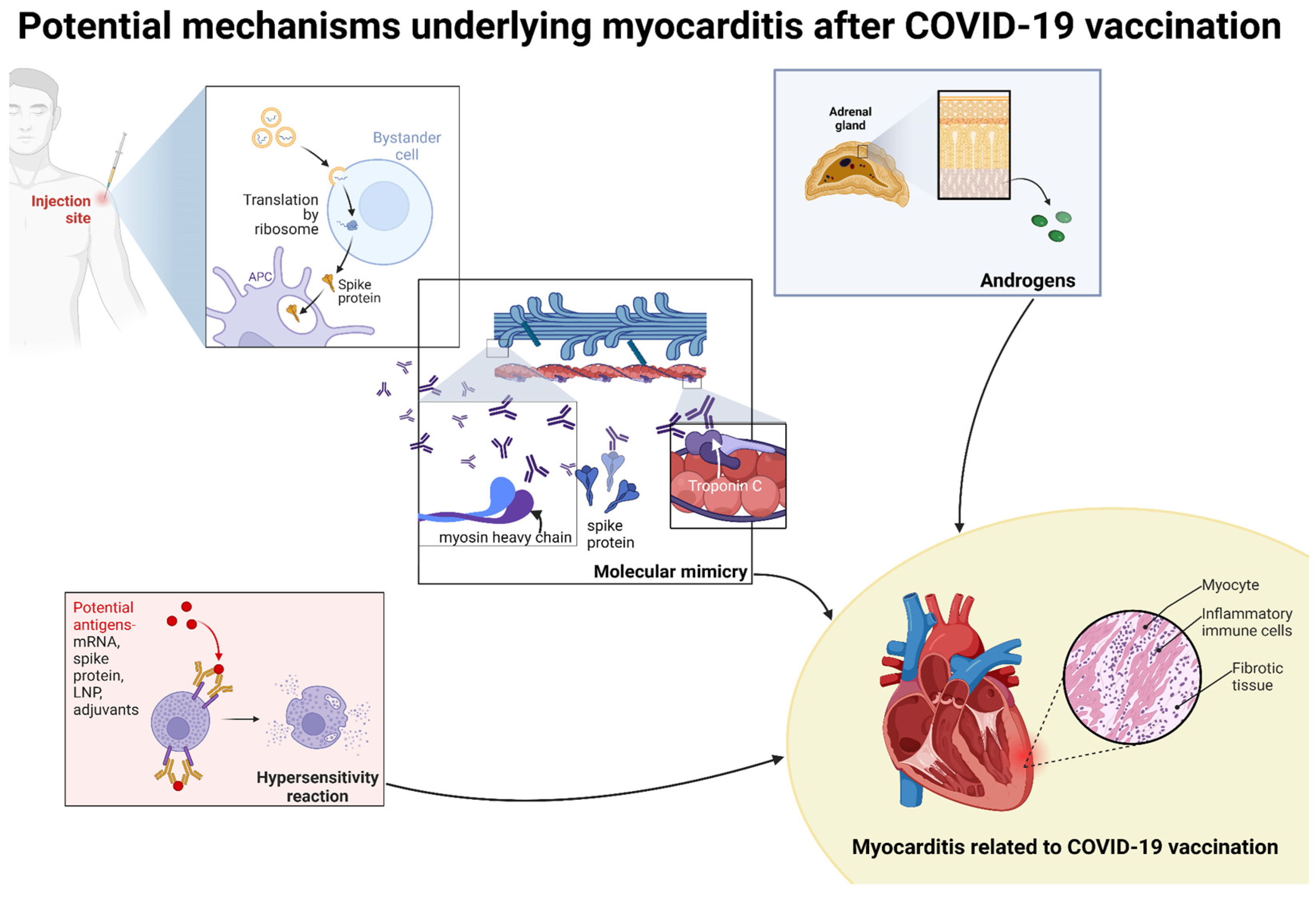
8. Potential Mechanisms of Myocarditis with COVID-19 Vaccines
9. Follow-Up among Patients with Myocarditis After COVID-19 Vaccination
10. Conclusions
11. Limitations of the Study
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ammirati, E.; Moslehi, J.J. Diagnosis and treatment of acute myocarditis. JAMA 2023, 329, 1098. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.S.; Cooper, L.T.; Kerneis, M.; Funck-Brentano, C.; Silvain, J.; Brechot, N.; Hekimian, G.; Ammirati, E.; Ben M’barek, B.; Redheuil, A.; et al. Systematic analysis of drug-associated myocarditis reported in the World Health Organization pharmacovigilance database. Nat. Commun. 2022, 13, 25. [Google Scholar] [CrossRef] [PubMed]
- Brociek, E.; Tymińska, A.; Giordani, A.S.; Caforio, A.L.P.; Wojnicz, R.; Grabowski, M.; Ozierański, K. Myocarditis: Etiology, pathogenesis, and their implications in clinical practice. Biology 2023, 12, 874. [Google Scholar] [CrossRef] [PubMed]
- Chabior, A.; Tymińska, A.; Pawlak, A.; Giordani, A.; Caforio, A.; Grabowski, M.; Ozierański, K. Advances in myocarditis management in the light of the latest research and recent guidelines of the European Society of Cardiology. Cardiol. J. 2024, 31, 342–351. [Google Scholar] [CrossRef]
- Monath, T.P.; Frey, S.E. Possible autoimmune reactions following smallpox vaccination: The biologic false positive test for syphilis. Vaccine 2009, 27, 1645–1650. [Google Scholar] [CrossRef]
- Engler, R.J.M.; Montgomery, J.R.; Spooner, C.E.; Nelson, M.R.; Collins, L.C.; Ryan, M.A.; Chu, C.S.; Atwood, J.E.; Hulten, E.A.; Rutt, A.A.; et al. Myocarditis and pericarditis recovery following smallpox vaccine 2002–2016: A comparative observational cohort study in the military health system. PLoS ONE 2023, 18, e0283988. [Google Scholar] [CrossRef]
- Parmar, K.; Subramanyam, S.; Del Rio-Pertuz, G.; Sethi, P.; Argueta-Sosa, E. Cardiac Adverse Events after Vaccination—A Systematic Review. Vaccines 2022, 10, 700. [Google Scholar] [CrossRef]
- Gao, J.; Feng, L.; Li, Y.; Lowe, S.; Guo, Z.; Bentley, R.; Xie, C.; Wu, B.; Xie, P.; Xia, W.; et al. A systematic review and meta-analysis of the association between SARS-CoV-2 vaccination and myocarditis or pericarditis. Am. J. Prev. Med. 2023, 64, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Ling, R.R.; Ramanathan, K.; Tan, F.L.; Tai, B.C.; Somani, J.; Fisher, D.; MacLaren, G. Myopericarditis following COVID-19 vaccination and non-COVID-19 vaccination: A systematic review and meta-analysis. Lancet Respir. Med. 2022, 10, 679–688. [Google Scholar] [CrossRef]
- Ishisaka, Y.; Watanabe, A.; Aikawa, T.; Kanaoka, K.; Takagi, H.; Wiley, J.; Yasuhara, J.; Kuno, T. Overview of SARS-CoV-2 infection and vaccine associated myocarditis compared to non-COVID-19-associated myocarditis: A systematic review and meta-analysis. Int. J. Cardiol. 2024, 395, 131401. [Google Scholar] [CrossRef]
- Fairweather, D.; Beetler, D.J.; Di Florio, D.N.; Musigk, N.; Heidecker, B.; Cooper, L.T. COVID-19, myocarditis and pericarditis. Circ. Res. 2023, 132, 1302–1319. [Google Scholar] [CrossRef] [PubMed]
- Yaamika, H.; Muralidas, D.; Elumalai, K. Review of adverse events associated with COVID-19 vaccines, highlighting their frequencies and reported cases. J. Taibah Univ. Med. Sci. 2023, 18, 1646–1661. [Google Scholar] [CrossRef] [PubMed]
- Molina, G.; Khawaja, U.; Contreras, R.; Burdowski, J. COVID VACCINE MYOCARDITIS WITH COMPLETE RECOVERY. J. Am. Coll. Cardiol. 2023, 81, 2626. [Google Scholar] [CrossRef]
- Jaiswal, V.; Mukherjee, D.; Ang, S.P.; Kainth, T.; Naz, S.; Shrestha, A.B.; Agrawal, V.; Mitra, S.; Chia, J.E.; Jilma, B.; et al. COVID-19 vaccine-associated myocarditis: Analysis of the suspected cases reported to the EudraVigilance and a systematic review of the published literature. Int. J. Cardiol. Heart Vasc. 2023, 49, 101280. [Google Scholar] [CrossRef]
- De Gier, B.; Van Asten, L.; Boere, T.M.; Van Roon, A.; Van Roekel, C.; Pijpers, J.; van Werkhoven, C.H.; Ende, C.v.D.; Hahné, S.J.; de Melker, H.E.; et al. Effect of COVID-19 vaccination on mortality by COVID-19 and on mortality by other causes, the Netherlands, January 2021–January 2022. Vaccine 2023, 41, 4488–4496. [Google Scholar] [CrossRef]
- Shiri, T.; Evans, M.; Talarico, C.A.; Morgan, A.R.; Mussad, M.; Buck, P.O.; McEwan, P.; Strain, W.D. Vaccinating Adolescents and Children Significantly Reduces COVID-19 Morbidity and Mortality across All Ages: A Population-Based Modeling Study Using the UK as an Example. Vaccines 2021, 9, 1180. [Google Scholar] [CrossRef]
- Iacobucci, G. COVID-19: Vaccines have saved at least 1.4 million lives in Europe, WHO reports. BMJ 2024, 384, q125. [Google Scholar] [CrossRef]
- Johns Hopkins Coronavirus Resource Center. Vaccine Research & Development—Johns Hopkins Coronavirus Resource Center. Available online: https://coronavirus.jhu.edu/vaccines/timeline (accessed on 4 May 2024).
- Tanveer, S.; Rowhani-Farid, A.; Hong, K.; Jefferson, T.; Doshi, P. Transparency of COVID-19 vaccine trials: Decisions without data. BMJ Evid. Based Med. 2021, 27, 199–205. [Google Scholar] [CrossRef]
- European Medicines Agency (EMA). COVID-19 Vaccines: Development, Evaluation, Approval and Monitoring|European Medicines Agency (EMA). Available online: https://www.ema.europa.eu/en/human-regulatory-overview/public-health-threats/coronavirus-disease-covid-19/covid-19-public-health-emergency-international-concern-2020-23/covid-19-vaccines-development-evaluation-approval-and-monitoring (accessed on 4 May 2024).
- Bakos, T.; Mészáros, T.; Kozma, G.T.; Berényi, P.; Facskó, R.; Farkas, H.; Dézsi, L.; Heirman, C.; de Koker, S.; Schiffelers, R.; et al. MRNA-LNP COVID-19 vaccine lipids induce complement activation and production of proinflammatory cytokines: Mechanisms, effects of complement inhibitors, and relevance to adverse reactions. Int. J. Mol. Sci. 2024, 25, 3595. [Google Scholar] [CrossRef]
- European Medicines Agency. Authorised COVID-19 Vaccines. Published 1BC. Available online: https://www.ema.europa.eu/en/human-regulatory-overview/public-health-threats/coronavirus-disease-covid-19/covid-19-medicines (accessed on 7 July 2024).
- Our World in Data. COVID-19 Vaccine Doses Administered by Manufacturer. Available online: https://ourworldindata.org/grapher/covid-vaccine-doses-by-manufacturer (accessed on 7 July 2024).
- European Medicines Agency. Vaxzevria (Previously COVID-19 Vaccine AstraZeneca). Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/vaxzevria-previously-covid-19-vaccine-astrazeneca (accessed on 7 July 2024).
- European Medicines Agency. Jcovden (Previously COVID-19 Vaccine Janssen). Published 3 November 2021. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/jcovden-previously-covid-19-vaccine-janssen (accessed on 7 July 2024).
- Li, X.; Burn, E.; Duarte-Salles, T.; Yin, C.; Reich, C.; Delmestri, A.; Verhamme, K.; Rijnbeek, P.; A Suchard, M.; Li, K.; et al. Comparative risk of thrombosis with thrombocytopenia syndrome or thromboembolic events associated with different COVID-19 vaccines: International network cohort study from five European countries and the US. BMJ 2022, 379, e071594. [Google Scholar] [CrossRef]
- Ahmed, S.K.; Mohamed, M.G.; Essa, R.A.; Rashad, E.A.A.; Ibrahim, P.K.; Khdir, A.A.; Wsu, Z.H. Global reports of myocarditis following COVID-19 vaccination: A systematic review and meta-analysis. Diabetes Metab. Syndr. 2022, 16, 102513. [Google Scholar] [CrossRef] [PubMed]
- Karlstad, Ø.; Hovi, P.; Husby, A.; Härkänen, T.; Selmer, R.M.; Pihlström, N.; Hansen, J.V.; Nohynek, H.; Gunnes, N.; Sundström, A.; et al. SARS-CoV-2 vaccination and myocarditis in a Nordic cohort study of 23 million residents. JAMA Cardiol. 2022, 7, 600. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Bimervax. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/bimervax (accessed on 7 July 2024).
- Borralleras, C.; Sanz, J.C.; Arrazola, P.; Hijón, C.C.; Eiros, J.M.; Fernández-Prada, M.; de Miguel, Á.G.; Masip, G.M.; Moraga-Llop, F.; Rodríguez, D.O.; et al. The PHH-1V HIPRA vaccine: A new tool in the vaccination strategy against COVID-19. Rev. Española Quimioter. 2023, 36, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Kang, L.Y.; Liu, J.; Liu, M. Immunogenicity, effectiveness, and safety of COVID-19 vaccines among children and adolescents aged 2–18 years: An updated systematic review and meta-analysis. World J. Pediatr. 2023, 19, 1041–1054. [Google Scholar] [CrossRef]
- Oster, M.E.; Shay, D.K.; Su, J.R.; Gee, J.; Creech, C.B.; Broder, K.R.; Edwards, K.; Soslow, J.H.; Dendy, J.M.; Schlaudecker, E.; et al. Myocarditis cases reported after mRNA-Based COVID-19 vaccination in the US from December 2020 to August 2021. JAMA 2022, 327, 331. [Google Scholar] [CrossRef]
- Guo, B.Q.; Li, H.B.; Yang, L.Q. Incidence of myopericarditis after mRNA COVID-19 vaccination: A meta-analysis with focus on adolescents aged 12–17 years. Vaccine 2023, 41, 4067–4080. [Google Scholar] [CrossRef]
- Das, B.B.; Kohli, U.; Ramachandran, P.; Nguyen, H.H.; Greil, G.; Hussain, T.; Tandon, A.; Kane, C.; Avula, S.; Duru, C.; et al. Myopericarditis after messenger RNA Coronavirus Disease 2019 Vaccination in Adolescents 12 to 18 Years of Age. J. Pediatr. 2021, 238, 26–32.e1. [Google Scholar] [CrossRef]
- Aggarwal, S.; Dhaliwal, J.S.; Kumar, N.; Sakthivel, H.; Ahmed, R.; Verma, R.; Ramphul, K. An epidemiological study of multisystem inflammatory syndrome in children (MIS-C) and young adults among COVID-19-positive patients—Data from National Inpatient Sample database. Reumatologia 2024, 62, 214–216. [Google Scholar] [CrossRef]
- Ludwikowska, K.M.; Moksud, N.; Tracewski, P.; Sokolski, M.; Szenborn, L. Cardiac Involvement in Patients with Multisystem Inflammatory Syndrome in Children (MIS-C) in Poland. Biomedicines 2023, 11, 1251. [Google Scholar] [CrossRef]
- Sık, G.; Inamlık, A.; Akçay, N.; Kesici, S.; Aygun, F.; Kendırlı, T.; Atay, G.; Sandal, O.; Varol, F.; Ozkaya, P.Y.; et al. Mortality risk factors among critically ill children with MIS-C in PICUs: A multicenter study. Pediatr. Res. 2023, 94, 730–737. [Google Scholar] [CrossRef]
- Nygaard, U.; Holm, M.; Hartling, U.B.; Glenthøj, J.; Schmidt, L.S.; Nordly, S.B.; Matthesen, A.T.; von Linstow, M.-L.; Espenhain, L. Incidence and clinical phenotype of multisystem inflammatory syndrome in children after infection with the SARS-CoV-2 delta variant by vaccination status: A Danish nationwide prospective cohort study. Lancet Child Adolesc. Health 2022, 6, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Copland, E.; Patone, M.; Saatci, D.; Handunnetthi, L.; Hirst, J.; Hunt, D.P.J.; Mills, N.L.; Moss, P.; Sheikh, A.; Coupland, C.A.C.; et al. Safety outcomes following COVID-19 vaccination and infection in 5.1 million children in England. Nat. Commun. 2024, 15, 3822. [Google Scholar] [CrossRef] [PubMed]
- Thevathasan, T.; Kenny, M.A.; Gaul, A.L.; Paul, J.; Krause, F.J.; Lech, S.; Stadler, G.; Meyer, A.; Schreiber, F.; Fairweather, D.; et al. Sex and Age Characteristics in Acute or Chronic Myocarditis. JACC Adv. 2024, 3, 100857. [Google Scholar] [CrossRef]
- Fairweather, D.; Beetler, D.J.; Musigk, N.; Heidecker, B.; Lyle, M.A.; Cooper, L.T.; Bruno, K.A. Sex and gender differences in myocarditis and dilated cardiomyopathy: An update. Front. Cardiovasc. Med. 2023, 10, 1129348. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.W.Y.; Liu, R.B.; Huang, C.Y.; Li, H.Y.; Zhang, Z.X.; Li, X.Z.; Liu, J.-L.; Zhang, C.; Xiong, X.; Niu, Y.-M. Global, regional, and national burdens of myocarditis, 1990–2019: Systematic analysis from GBD 2019. BMC Public Health 2023, 23, 714. [Google Scholar] [CrossRef] [PubMed]
- Current State of Knowledge on Aetiology, Diagnosis, Management, and Therapy of Myocarditis. Available online: https://www.escardio.org/Working-groups/Working-Group-on-Myocardial-and-Pericardial-Diseases/Publications/Paper-of-the-Month/Current-state-of-knowledge-on-aetiology-diagnosis-management-and-therapy-of-m (accessed on 5 May 2024).
- Ferreira, V.M.; Schulz-Menger, J.; Holmvang, G.; Kramer, C.M.; Carbone, I.; Sechtem, U.; Kindermann, I.; Gutberlet, M.; Cooper, L.T.; Liu, P.; et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation. J. Am. Coll. Cardiol. 2018, 72, 3158–3176. [Google Scholar] [CrossRef]
- Doeblin, P.; Kelle, S. Going after COVID-19 myocarditis. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 852–854. [Google Scholar] [CrossRef]
- Heidecker, B.; Dagan, N.; Balicer, R.; Eriksson, U.; Rosano, G.; Coats, A.; Tschöpe, C.; Kelle, S.; Poland, G.A.; Frustaci, A.; et al. Myocarditis following COVID-19 vaccine: Incidence, presentation, diagnosis, pathophysiology, therapy, and outcomes put into perspective. A clinical consensus document supported by the Heart Failure Association of the European Society of Cardiology (ESC) and the ESC Working Group on Myocardial and Pericardial Diseases. Eur. J. Heart Fail. 2022, 24, 2000–2018. [Google Scholar] [CrossRef]
- Sokolska, J.M.; Kurcz, J.; Kosmala, W. Every rose has its thorns—Acute myocarditis following COVID-19 vaccination. Kardiol. Pol. 2021, 79, 1153–1154. [Google Scholar] [CrossRef]
- Chen, J.; Wu, T.; Zhang, C.; Zhang, Y.; Liu, Z.; Wang, Y. Clinically suspected lethal viral myocarditis combined with encephalitis: A COVID-19 vaccine complication. ESC Heart Fail. 2022, 10, 1422–1425. [Google Scholar] [CrossRef]
- Marsukjai, A.; Theerasuwipakorn, N.; Tumkosit, M.; Chattranukulchai, P.; Srichomkwun, P.; Prechawat, S. Concomitant myocarditis and painless thyroiditis after AstraZeneca coronavirus disease 2019 vaccination: A case report. J. Med. Case Rep 2022, 16, 212. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, B.; Kamat, I.; Hotez, P.J. Myocarditis with COVID-19 mRNA vaccines. Circulation 2021, 144, 471–484. [Google Scholar] [CrossRef] [PubMed]
- JCPSP|Journal of College of Physicians and Surgeons Pakistan. Available online: https://www.jcpsp.pk/article-detail/pcardiac-troponini-a-biomarker-for-predicting-covidinduced-myocardial-damage-prognosisorp (accessed on 7 July 2024).
- Manno, E.C.; Amodio, D.; Cotugno, N.; Rossetti, C.; Giancotta, C.; Santilli, V.; Zangari, P.; Rotulo, G.A.; Villani, A.; Giglioni, E.; et al. Higher Troponin Levels on Admission are associated With Persistent Cardiac Magnetic Resonance Lesions in Children Developing Myocarditis After mRNA-Based COVID-19 Vaccination. Pediatr. Infect. Dis. J. 2022, 42, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Pueyo, P.P.; Ruberte, E.G.; Ordás, J.G.; Blanco, L.M.; Figal, D.P.; Moreira, J.M.L.; Barrado, J.J.G.; Calle, D.G.; Bonet, L.A.; Salinas, G.L.A.; et al. Vaccine–carditis study: Spanish multicenter registry of inflammatory heart disease after COVID-19 vaccination. Clin. Res. Cardiol. 2023, 113, 223–234. [Google Scholar] [CrossRef]
- Kato, S.; Horita, N.; Utsunomiya, D. Imaging characteristics of myocarditis after mRNA-based COVID-19 vaccination: A meta-analysis. ESC Heart Fail. 2022, 10, 748–750. [Google Scholar] [CrossRef] [PubMed]
- Samimisedeh, P.; Afshar, E.J.; Tayebi, A.; Rastad, H. Post-acute midterm follow-up cardiac MRI findings and clinical outcomes in patients with COVID-19 vaccine-associated myocarditis: A comprehensive systematic review and meta-analysis. Infect. Dis. 2023, 56, 193–205. [Google Scholar] [CrossRef]
- Salah, H.M.; Mehta, J.L. COVID-19 vaccine and myocarditis. Am. J. Cardiol. 2021, 157, 146–148. [Google Scholar] [CrossRef]
- Chou, O.H.I.; Mui, J.; Chung, C.T.; Radford, D.; Ranjithkumar, S.; Evbayekha, E.; Nam, R.; Pay, L.; Satti, D.I.; Garcia-Zamora, S.; et al. COVID-19 vaccination and carditis in children and adolescents: A systematic review and meta-analysis. Clin. Res. Cardiol. 2022, 111, 1161–1173. [Google Scholar] [CrossRef]
- Hulscher, N.; Hodkinson, R.; Makis, W.; McCullough, P.A. Autopsy findings in cases of fatal COVID-19 vaccine-induced myocarditis. ESC Heart Fail. 2024. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Mall, G.; Westphal, J.G.; Weingärtner, O.; Möbius-Winkler, S.; Schulze, P.C. Acute myocarditis after COVID-19 vaccination with mRNA-1273 in a patient with former SARS-CoV-2 infection. ESC Heart Fail. 2021, 8, 4710–4714. [Google Scholar] [CrossRef]
- Nagasaka, T.; Koitabashi, N.; Ishibashi, Y.; Aihara, K.; Takama, N.; Ohyama, Y.; Yokoyama, T.; Kaneko, Y. Acute myocarditis associated with COVID-19 vaccination: A case report. J. Cardiol. Cases 2021, 25, 285–288. [Google Scholar] [CrossRef] [PubMed]
- Schwab, C.; Domke, L.M.; Hartmann, L.; Stenzinger, A.; Longerich, T.; Schirmacher, P. Autopsy-based histopathological characterization of myocarditis after anti-SARS-CoV-2-vaccination. Clin. Res. Cardiol. 2022, 112, 431–440. [Google Scholar] [CrossRef] [PubMed]
- De Boer, H.H.; Crawford, N.W.; Parsons, S. Commentary on: “Autopsy-based histopathological characterization of myocarditis after anti-SARS-CoV-2-vaccination” by C. Schwab et al. Clin. Res. Cardiol. 2023, 113, 353–355. [Google Scholar] [CrossRef] [PubMed]
- Kiblboeck, D.; Klingel, K.; Genger, M.; Traxler, S.; Braunsteiner, N.; Steinwender, C.; Kellermair, J. Myocarditis following mRNA COVID-19 vaccination: Call for endomyocardial biopsy. ESC Heart Fail. 2022, 9, 1996–2002. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.H.; Na, J.Y.; Yang, J.H.; Moon, S.H.; Kim, S.H.; Jung, J.J.; Cha, H.-J.; Ahn, J.-H.; Park, Y.-W.; Cho, S.-Y.; et al. Fulminant Giant Cell Myocarditis following Heterologous Vaccination of ChAdOx1 nCoV-19 and Pfizer-BioNTech COVID-19. Medicina 2022, 58, 449. [Google Scholar] [CrossRef]
- Ameratunga, R.; Woon, S.T.; Sheppard, M.N.; Garland, J.; Ondruschka, B.; Wong, C.X.; Stewart, R.A.H.; Tatley, M.; Stables, S.R.; Tse, R.D. First Identified Case of Fatal Fulminant Necrotizing Eosinophilic Myocarditis Following the Initial Dose of the Pfizer-BioNTech mRNA COVID-19 Vaccine (BNT162b2, Comirnaty): An Extremely Rare Idiosyncratic Hypersensitivity Reaction. J. Clin. Immunol. 2022, 42, 441–447. [Google Scholar] [CrossRef]
- Hirsch, V.G.; Schallhorn, S.; Zwadlo, C.; Diekmann, J.; Länger, F.; Jonigk, D.D.; Kempf, T.; Schultheiss, H.; Bauersachs, J. Giant cell myocarditis after first dose of BNT162b2—A case report. Eur. J. Heart Fail. 2022, 24, 1319–1322. [Google Scholar] [CrossRef]
- Guglin, M.E.; Etuk, A.; Shah, C.; Ilonze, O.J. Fulminant myocarditis and cardiogenic Shock Following COVID-19 Infection versus COVID-19 Vaccination: A Systematic literature review. J. Clin. Med. 2023, 12, 1849. [Google Scholar] [CrossRef]
- Saputra, P.B.T.; Kurniawan, R.B.; Trilistyoati, D.; Farabi, M.J.A.; Susilo, H.; Alsagaff, M.Y.; Oktaviono, Y.H.; Sutanto, H.; Gusnanto, A.; Wungu, C.D.K. Myocarditis and coronavirus disease 2019 vaccination: A systematic review and meta-summary of cases. Biomol. Biomed. 2023, 23, 546–567. [Google Scholar] [CrossRef]
- Kimura, M.; Hashimoto, T.; Noda, E.; Ishikawa, Y.; Ishikita, A.; Fujino, T.; Matsushima, S.; Ide, T.; Kinugawa, S.; Nagaoka, K.; et al. Fulminant necrotizing eosinophilic myocarditis after COVID-19 vaccination survived with mechanical circulatory support. ESC Heart Fail. 2022, 9, 2732–2737. [Google Scholar] [CrossRef]
- Janga, C.; Patel, T.; Hennawi, H.A.; Atam, S.; Shah, S.; Naeem, I.; Memon, R.; Haas, D. Delayed presentation of biopsy-proven eosinophilic myocarditis following COVID-19 mRNA vaccine. Glob. Cardiol. Sci. Pract. 2023, 2023, e202310. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, K.; Matsuo, T.; Ishimatsu, T.; Fukae, A.; Hamamoto, T.; Oku, K.; Ito, M. A case of BNT162b2 COVID-19 vaccine-associated fulminant myocarditis in a very elderly woman. Clin. Case Rep. 2022, 10, e6161. [Google Scholar] [CrossRef] [PubMed]
- Kawano, H.; Motokawa, T.; Kurohama, H.; Okano, S.; Akashi, R.; Yonekura, T.; Ikeda, S.; Izumikawa, K.; Maemura, K. Fulminant Myocarditis 24 Days after Coronavirus Disease Messenger Ribonucleic Acid Vaccination. Intern. Med. 2022, 61, 2319–2325. [Google Scholar] [CrossRef]
- Munjal, J.S.; Flores, S.M.; Yousuf, H.; Gupta, V.; Munjal, R.S.; Anamika, F.; Mendpara, V.; Shah, P.; Jain, R. COVID- 19 vaccine-induced myocarditis. J. Community Hosp. Intern. Med. Perspect. 2023, 13, 9. [Google Scholar] [CrossRef]
- Khan, M.Z.; Janus, S.; Franklin, S.; Figueredo, V.; Baqi, A.; Alvarez, R. COVID-19 Vaccination-Induced cardiomyopathy requiring permanent left ventricular assist device. Cureus 2022, 14, e24477. [Google Scholar] [CrossRef]
- Naghashzadeh, F.; Shafaghi, S.; Dorudinia, A.; Naji, S.A.; Marjani, M.; Amin, A.; Mohamadifar, A.; Noorali, S.; Kashani, B.S. Myocarditis following rAd26 and rAd5 vector-based COVID-19 vaccine: Case report. ESC Heart Fail. 2022, 9, 1483–1486. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Kim, K.H. COVID-19 Vaccination-Related myocarditis: What we learned from our experience and what we need to do in the future. Korean Circ. J. 2024, 54, 295–310. [Google Scholar] [CrossRef]
- Voleti, N.; Reddy, S.P.; Ssentongo, P. Myocarditis in SARS-CoV-2 infection vs. COVID-19 vaccination: A systematic review and meta-analysis. Front. Cardiovasc. Med. 2022, 9, 951314. [Google Scholar] [CrossRef]
- Alami, A.; Krewski, D.; Farhat, N.; Mattison, D.; Wilson, K.; Gravel, C.A.; Farrell, P.J.; Crispo, J.A.G.; Haddad, N.; Perez-Lloret, S.; et al. Risk of myocarditis and pericarditis in mRNA COVID-19-vaccinated and unvaccinated populations: A systematic review and meta-analysis. BMJ Open 2023, 13, e065687. [Google Scholar] [CrossRef]
- Patone, M.; Mei, X.W.; Handunnetthi, L.; Dixon, S.; Zaccardi, F.; Shankar-Hari, M.; Watkinson, P.; Khunti, K.; Harnden, A.; Coupland, C.A.; et al. Risk of myocarditis after sequential doses of COVID-19 vaccine and SARS-CoV-2 infection by age and sex. Circulation 2022, 146, 743–754. [Google Scholar] [CrossRef]
- Patone, M.; Mei, X.W.; Handunnetthi, L.; Dixon, S.; Zaccardi, F.; Shankar-Hari, M.; Watkinson, P.; Khunti, K.; Harnden, A.; Coupland, C.A.C.; et al. Risks of myocarditis, pericarditis, and cardiac arrhythmias associated with COVID-19 vaccination or SARS-CoV-2 infection. Nat. Med. 2021, 28, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Buergin, N.; Lopez-Ayala, P.; Hirsiger, J.R.; Mueller, P.; Median, D.; Glarner, N.; Rumora, K.; Herrmann, T.; Koechlin, L.; Haaf, P.; et al. Sex-specific differences in myocardial injury incidence after COVID-19 mRNA-1273 booster vaccination. Eur. J. Heart Fail. 2023, 25, 1871–1881. [Google Scholar] [CrossRef] [PubMed]
- Mills, N.L.; Patone, M.; Hippisley-Cox, J. Response by Mills et al. Regarding Article, “Risk of Myocarditis After Sequential Doses of COVID-19 Vaccine and SARS-CoV-2 Infection by Age and Sex”. Circulation 2023, 147, e655–e656. [Google Scholar] [CrossRef]
- Buoninfante, A.; Andeweg, A.; Genov, G.; Cavaleri, M. Myocarditis associated with COVID-19 vaccination. NPJ Vaccines 2024, 9, 122. [Google Scholar] [CrossRef]
- Sadeghi, S.; Kalantari, Y.; Shokri, S.; Fallahpour, M.; Nafissi, N.; Goodarzi, A.; Valizadeh, R. Immunologic response, Efficacy, and Safety of Vaccines against COVID-19 Infection in Healthy and immunosuppressed Children and Adolescents Aged 2—21 years old: A Systematic Review and Meta-analysis. J. Clin. Virol. 2022, 153, 105196. [Google Scholar] [CrossRef]
- Tsang, H.W.; Kwan, M.Y.W.; Chua, G.T.; Tsao, S.S.L.; Wong, J.S.C.; Tung, K.T.S.; Chan, G.C.F.; To, K.K.W.; Wong, I.C.K.; Leung, W.H.; et al. The central role of natural killer cells in mediating acute myocarditis after mRNA COVID-19 vaccination. Med 2024, 5, 335–347.e3. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, B. Shedding light on mechanisms of myocarditis with COVID-19 mRNA vaccines. Circulation 2023, 147, 877–880. [Google Scholar] [CrossRef]
- Vojdani, A.; Kharrazian, D. Potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases. Clin. Immunol. 2020, 217, 108480. [Google Scholar] [CrossRef]
- Yonker, L.M.; Swank, Z.; Bartsch, Y.C.; Burns, M.D.; Kane, A.; Boribong, B.P.; Davis, J.P.; Loiselle, M.; Novak, T.; Senussi, Y.; et al. Circulating spike protein detected in Post–COVID-19 mRNA vaccine myocarditis. Circulation 2023, 147, 867–876. [Google Scholar] [CrossRef]
- Miyazaki, K.; Fujii, T.; Mori, K.; Tamimoto, R.; Nagamatsu, H.; Murakami, T.; Cho, Y.; Goto, S.; Mori, H. Sustained Myocarditis following Messenger RNA Vaccination against Coronavirus Disease 2019: Relation to Neutralizing Antibody and Amelioration by Low-Dose Booster Vaccination. J. Clin. Med. 2023, 12, 1421. [Google Scholar] [CrossRef]
- Yang, B.C.; Castells, M.C. Utilizing biologics in drug desensitization. Curr. Allergy Asthma Rep. 2022, 23, 1–11. [Google Scholar] [CrossRef]
- Arutyunov, G.P.; Tarlovskaya, E.I.; Arutyunov, A.G.; Lopatin, Y.M. Impact of heart failure on all-cause mortality in COVID-19: Findings from the Eurasian International Registry. ESC Heart Fail. 2022, 10, 1013–1024. [Google Scholar] [CrossRef] [PubMed]
- Talib, N.; Fronza, M.; Marschner, C.A.; Thavendiranathan, P.; Karur, G.R.; Hanneman, K. Cardiovascular Magnetic Resonance Imaging and Clinical Follow-Up in Patients with Clinically Suspected Myocarditis after COVID-19 Vaccination. J. Cardiovasc. Magn. Reson. 2024, 26, 101036. [Google Scholar] [CrossRef]
- Kracalik, I.; Oster, M.E.; Broder, K.R.; Cortese, M.M.; Glover, M.; Shields, K.; Creech, C.B.; Romanson, B.; Novosad, S.; Soslow, J.; et al. Outcomes at least 90 days since onset of myocarditis after mRNA COVID-19 vaccination in adolescents and young adults in the USA: A follow-up surveillance study. Lancet Child Adolesc. Health 2022, 6, 788–798. [Google Scholar] [CrossRef] [PubMed]
- Husby, A.; Gulseth, H.L.; Hovi, P.; Hansen, J.V.; Pihlström, N.; Gunnes, N.; Härkänen, T.; Dahl, J.; Karlstad, Ø.; Heliö, T.; et al. Clinical outcomes of myocarditis after SARS-CoV-2 mRNA vaccination in four Nordic countries: Population based cohort study. BMJ Med. 2023, 2, e000373. [Google Scholar] [CrossRef] [PubMed]
- Schroth, D.; Garg, R.; Bocova, X.; Hansmann, J.; Haass, M.; Yan, A.; Fernando, C.; Chacko, B.; Oikonomou, A.; White, J.; et al. Predictors of persistent symptoms after mRNA SARS-CoV-2 vaccine-related myocarditis (myovacc registry). Front. Cardiovasc. Med. 2023, 10, 1204232. [Google Scholar] [CrossRef] [PubMed]
- Palazzuoli, A.; Metra, M.; Collins, S.P.; Adamo, M.; Ambrosy, A.P.; Antohi, L.E.; Ben Gal, T.; Farmakis, D.; Gustafsson, F.; Hill, L.; et al. Heart failure during the COVID-19 pandemic: Clinical, diagnostic, management, and organizational dilemmas. ESC Heart Fail. 2022, 9, 3713–3736. [Google Scholar] [CrossRef]
- Morishita, T.; Takada, D.; Shin, J.; Higuchi, T.; Kunisawa, S.; Fushimi, K.; Imanaka, Y. Effects of the COVID-19 pandemic on heart failure hospitalizations in Japan: Interrupted time series analysis. ESC Heart Fail. 2021, 9, 31–38. [Google Scholar] [CrossRef]
- Kubica, J.; Ostrowska, M.; Stolarek, W.; Kasprzak, M.; Grzelakowska, K.; Kryś, J.; Kubica, A.; Adamski, P.; Podhajski, P.; Navarese, E.P.; et al. Impact of COVID-19 pandemic on acute heart failure admissions and mortality: A multicentre study (COV-HF-SIRIO 6 study). ESC Heart Fail. 2021, 9, 721–728. [Google Scholar] [CrossRef]
- Sokolski, M.; Trenson, S.; Sokolska, J.M.; D’Amario, D.; Meyer, P.; Poku, N.K.; Biering-Sørensen, T.; Lassen, M.C.H.; Skaarup, K.G.; Barge-Caballero, E.; et al. Heart failure in COVID-19: The multicentre, multinational PCHF-COVICAV registry. ESC Heart Fail. 2021, 8, 4955–4967. [Google Scholar] [CrossRef]
- Sokolski, M.; Reszka, K.; Suchocki, T.; Adamik, B.; Doroszko, A.; Drobnik, J.; Gorka-Dynysiewicz, J.; Jedrzejczyk, M.; Kaliszewski, K.; Kilis-Pstrusinska, K.; et al. History of heart failure in patients hospitalized due to COVID-19: Relevant factor of In-Hospital complications and All-Cause mortality up to six months. J. Clin. Med. 2022, 11, 241. [Google Scholar] [CrossRef] [PubMed]
- Bruno, R.R.; Wernly, B.; Wolff, G.; Fjølner, J.; Artigas, A.; Pinto, B.B.; Schefold, J.C.; Kindgen-Milles, D.; Baldia, P.H.; Kelm, M.; et al. Association of chronic heart failure with mortality in old intensive care patients suffering from COVID-19. ESC Heart Fail. 2022, 9, 1756–1765. [Google Scholar] [CrossRef] [PubMed]
| Vaccine Name | Vaccine Type | Risk of Myocarditis |
|---|---|---|
| BNT162b2 (Corminaty, Pfizer) | mRNA | 4–7 excess cases per 100,000 doses [27,28] |
| mRNA-1273 (Spikevax, Moderna) | 9–28 excess cases per 100,000 doses [27,28] | |
| Ad26.COV2-S (Jcovden) | Viral Vector | Lower risk compared to mRNA vaccines [23,24] |
| ChAdOx1-S (Vaxzevria) | Lower risk compared to mRNA vaccines [23,24] | |
| PHH-1V HIPRA (Bimervax) | Protein-based | Myocarditis observed; limited data [27,28] |
| NVX-CoV-2373 (NOVAXOVID) | Myocarditis observed; limited data [27,28] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Florek, K.; Sokolski, M. Myocarditis Associated with COVID-19 Vaccination. Vaccines 2024, 12, 1193. https://doi.org/10.3390/vaccines12101193
Florek K, Sokolski M. Myocarditis Associated with COVID-19 Vaccination. Vaccines. 2024; 12(10):1193. https://doi.org/10.3390/vaccines12101193
Chicago/Turabian StyleFlorek, Kamila, and Mateusz Sokolski. 2024. "Myocarditis Associated with COVID-19 Vaccination" Vaccines 12, no. 10: 1193. https://doi.org/10.3390/vaccines12101193
APA StyleFlorek, K., & Sokolski, M. (2024). Myocarditis Associated with COVID-19 Vaccination. Vaccines, 12(10), 1193. https://doi.org/10.3390/vaccines12101193


_Kwok.png)



