Lung IL-17A-Producing CD4+ T Cells Correlate with Protection after Intrapulmonary Vaccination with Differentially Adjuvanted Tuberculosis Vaccines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mice and Immunisations
2.2. Blood Sample Collection and Analysis
2.3. Preparation of Tissue Samples for Flow Cytometry
2.4. Staining of Cells for Flow Cytometry
2.5. Flow Cytometric Data Analysis
2.6. Antibody Enzyme-Linked Immunosorbent Assays (ELISAs)
2.7. Statistical Analysis
3. Results
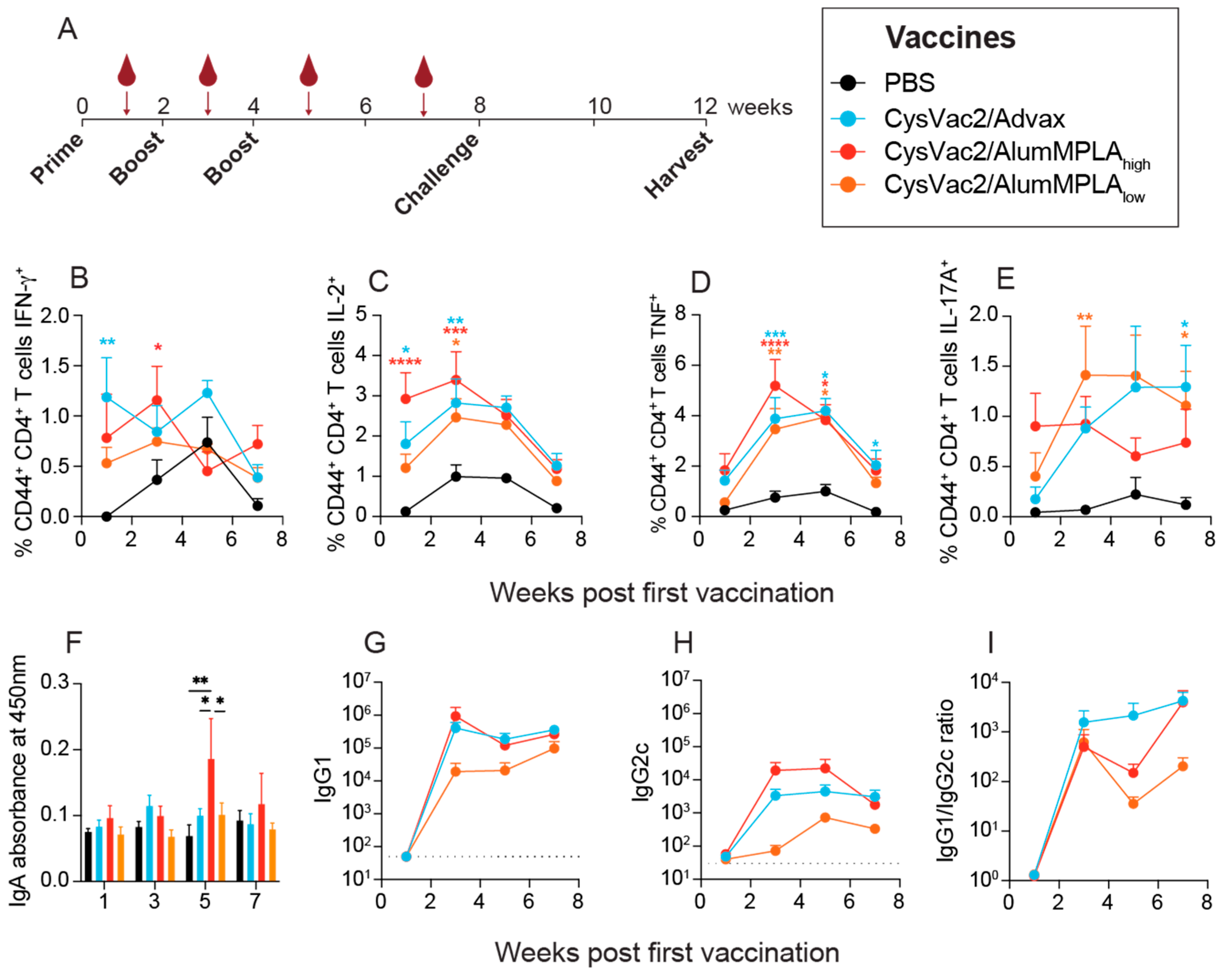
3.1. Intratracheal Vaccination with Differently Adjuvanted CysVac2 Vaccines Induces Similar Circulating Immune Responses
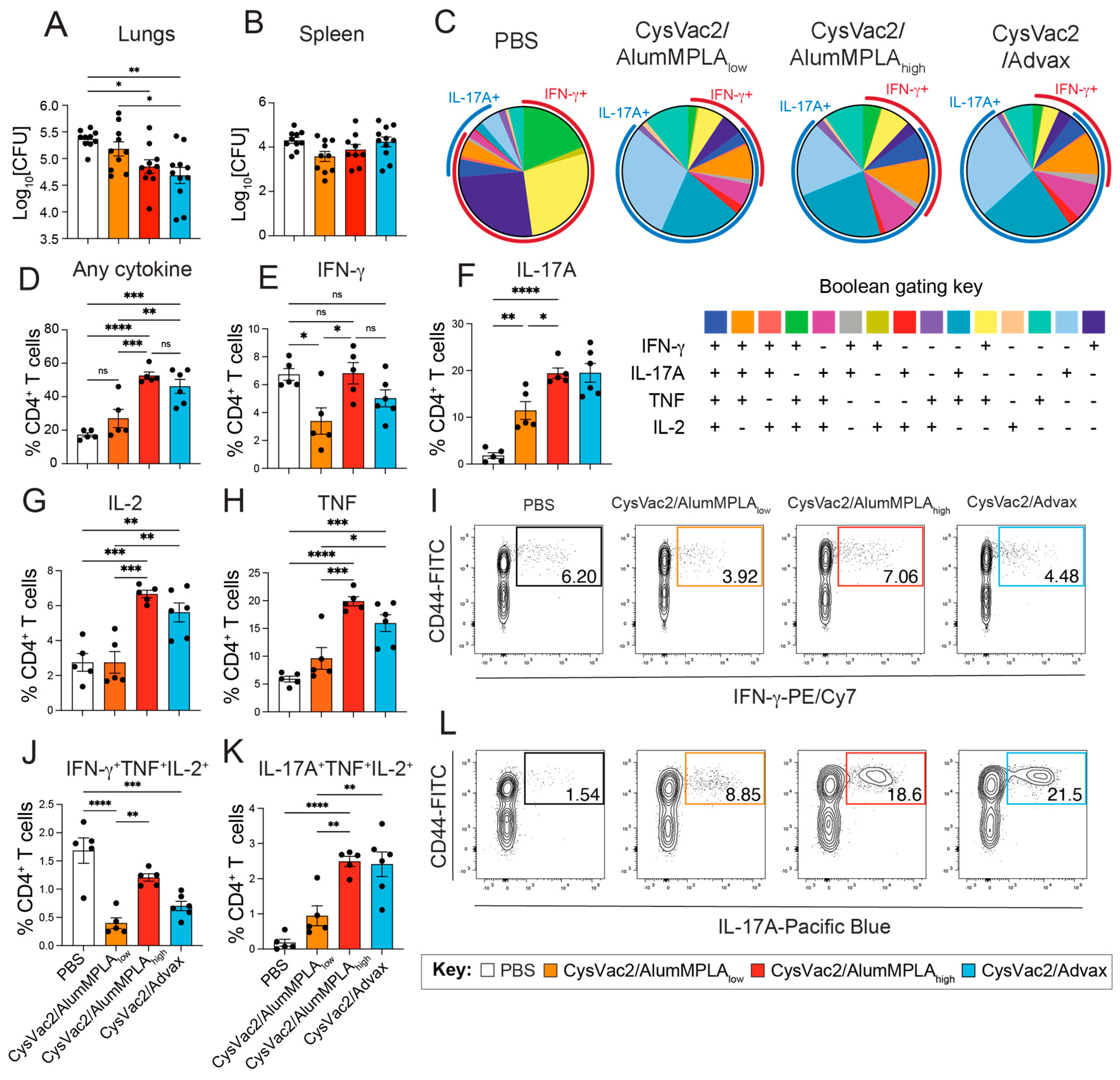
3.2. CysVac2/Advax and CysVac2/AlumMPLA Are Protective in the Lungs When Administered Intratracheally, and Protection Is Associated with IL-17A Expression by Pulmonary CD4+ T Cells
3.3. Intratracheal Vaccination with CysVac2/Advax and CysVac2/AlumMPLA Generates Similar Myeloid Cell Recruitment to the Lungs after M. tuberculosis Challenge
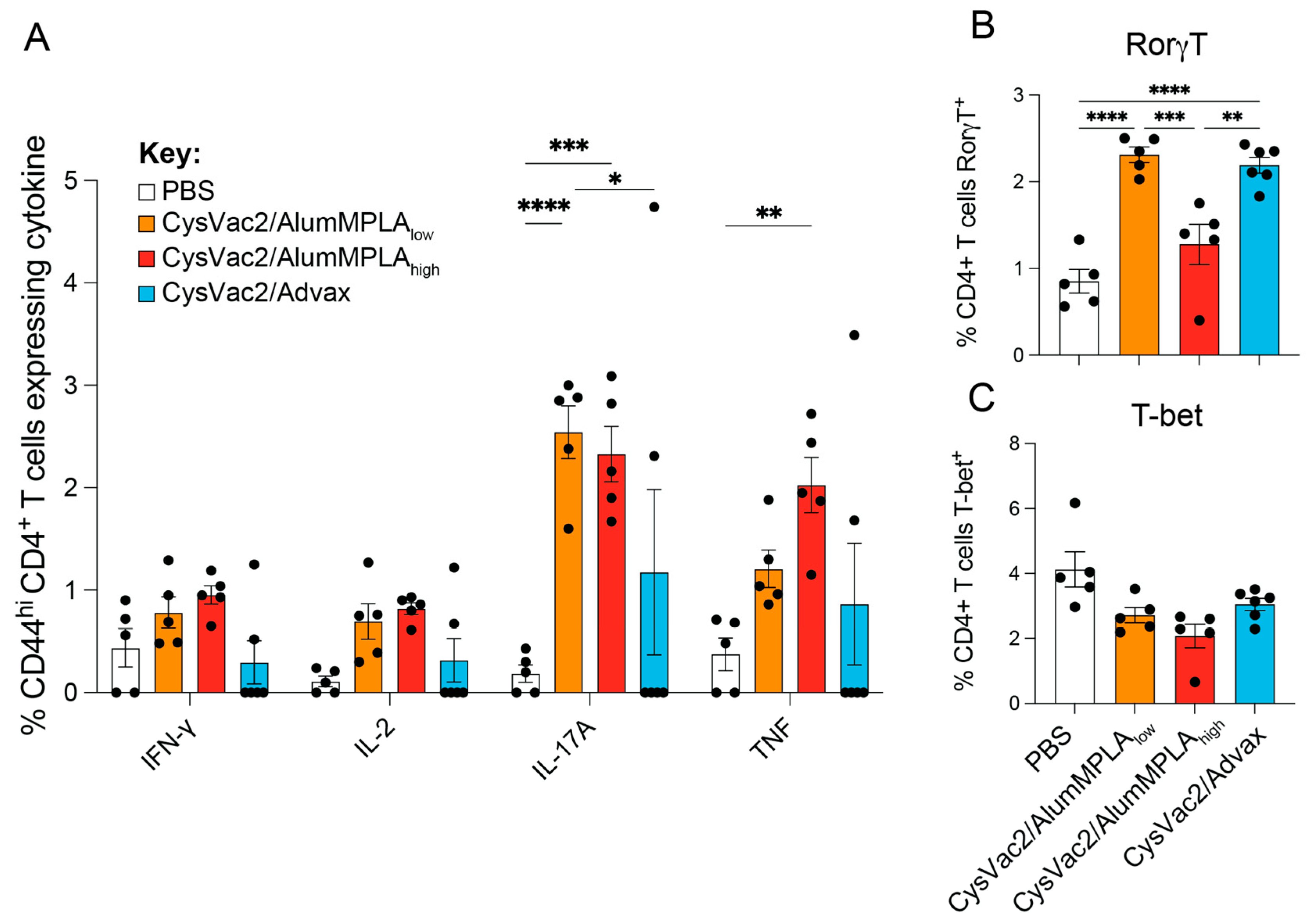
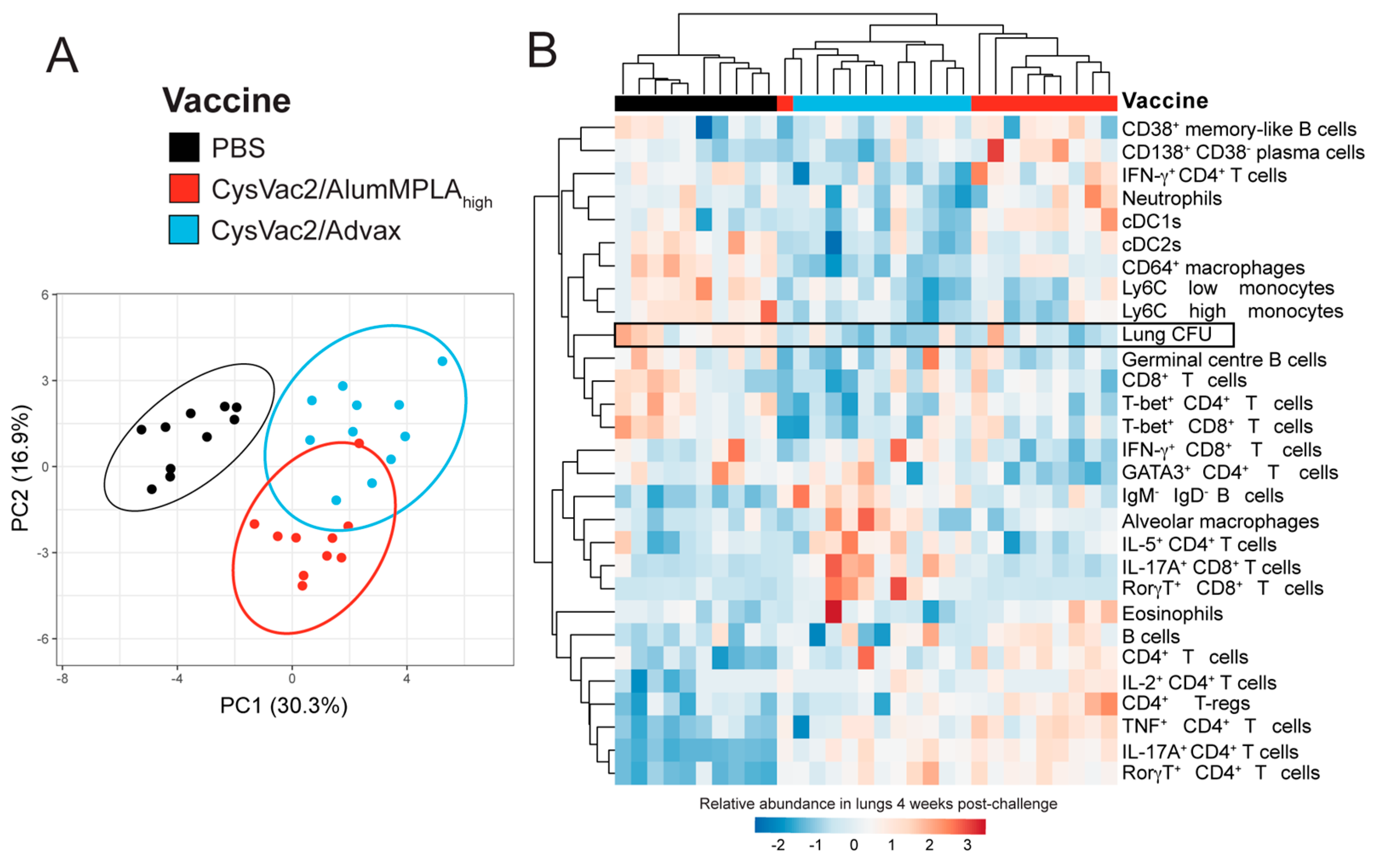
3.4. Intratracheal CysVac2/Advax and CysVac2/AlumMPLAhigh Generate Distinct Immunological Profiles after M. tuberculosis Challenge, but a Shared Th17 Signature
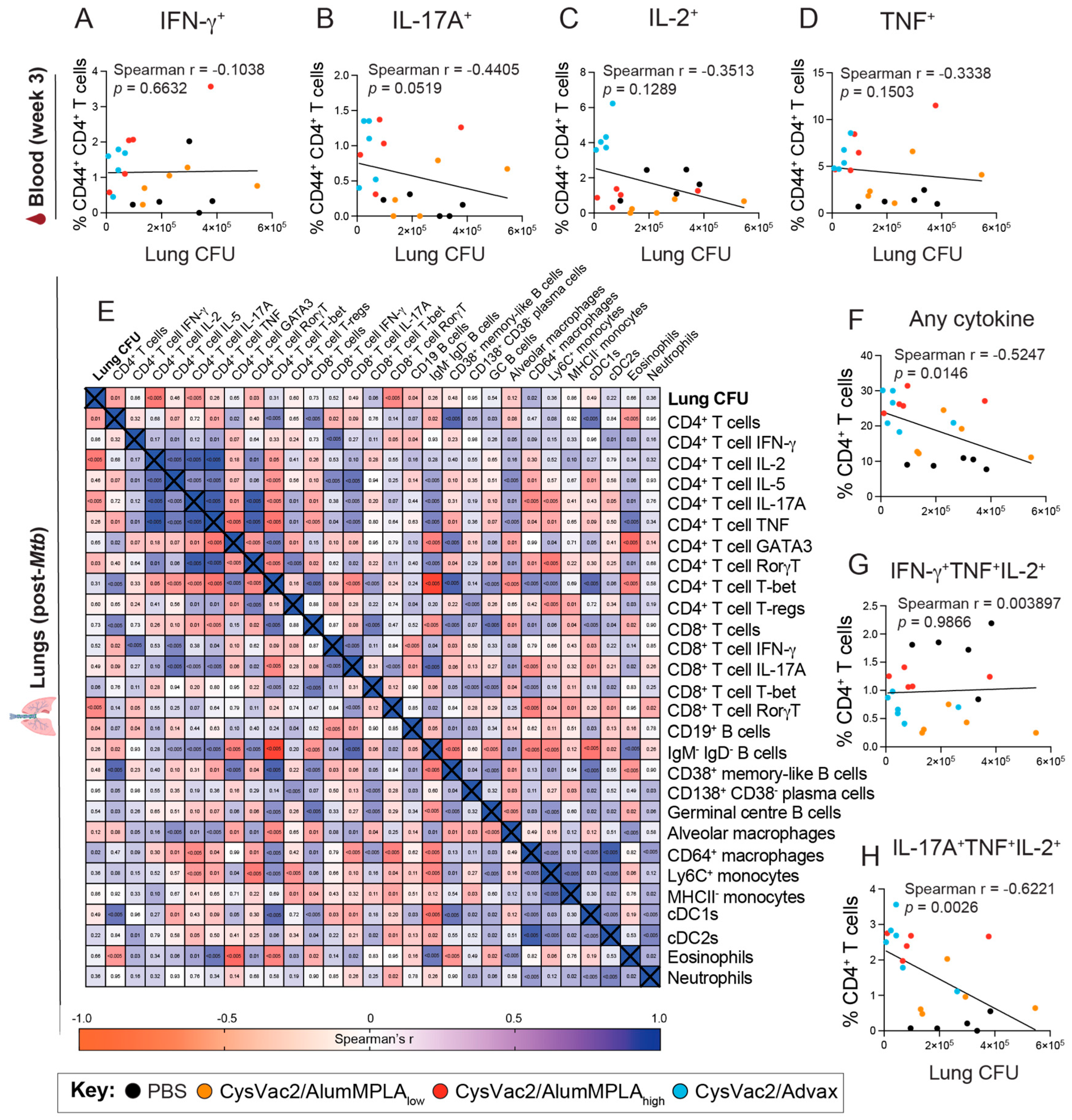
3.5. Pulmonary CD4+ T Cells Expressing IL-17A Correlate with Protection against M. tuberculosis but IFN-γ-Expressing CD4+ T Cells Do Not
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kinsella, R.L.; Zhu, D.X.; Harrison, G.A.; Mayer Bridwell, A.E.; Prusa, J.; Chavez, S.M.; Stallings, C.L. Perspectives and Advances in the Understanding of Tuberculosis. Annu. Rev. Pathol. Mech. Dis. 2021, 16, 377–408. [Google Scholar] [CrossRef] [PubMed]
- Schrager, L.K.; Vekemens, J.; Drager, N.; Lewinsohn, D.M.; Olesen, O.F. The status of tuberculosis vaccine development. Lancet Infect. Dis. 2020, 20, e28–e37. [Google Scholar] [CrossRef] [PubMed]
- Tait, D.R.; Hatherill, M.; Van Der Meeren, O.; Ginsberg, A.M.; Van Brakel, E.; Salaun, B.; Scriba, T.J.; Akite, E.J.; Ayles, H.M.; Bollaerts, A.; et al. Final Analysis of a Trial of M72/AS01E Vaccine to Prevent Tuberculosis. N. Engl. J. Med. 2019, 381, 2429–2439. [Google Scholar] [CrossRef] [PubMed]
- Ottenhoff, T.H.; Kumararatne, D.; Casanova, J.L. Novel human immunodeficiencies reveal the essential role of type-I cytokines in immunity to intracellular bacteria. Immunol. Today 1998, 19, 491–494. [Google Scholar] [CrossRef] [PubMed]
- Macleod, M. Learning lessons from MVA85A, a failed booster vaccine for BCG. BMJ-Br. Med. J. 2018, 360, k66. [Google Scholar] [CrossRef] [PubMed]
- Counoupas, C.; Triccas, J.A.; Britton, W.J. Deciphering protective immunity against tuberculosis: Implications for vaccine development. Expert Rev. Vaccines 2019, 18, 353–364. [Google Scholar] [CrossRef]
- Tameris, M.D.D.; Hatherill, M.F.C.P.; Landry, B.S.M.P.H.; Scriba, T.J.P.; Snowden, M.A.M.P.H.; Lockhart, S.D.M.; Shea, J.E.P.; McClain, J.B.M.D.; Hussey, G.D.P.; Hanekom, W.A.P.; et al. Safety and efficacy of MVA85A, a new tuberculosis vaccine, in infants previously vaccinated with BCG: A randomised, placebo-controlled phase 2b trial. Lancet 2013, 381, 1021–1028. [Google Scholar] [CrossRef] [PubMed]
- Counoupas, C.; Triccas, J.A. The generation of T-cell memory to protect against tuberculosis. Immunol. Cell Biol. 2019, 97, 656–663. [Google Scholar] [CrossRef]
- Wolf, A.J.; Desvignes, L.; Linas, B.; Banaiee, N.; Tamura, T.; Takatsu, K.; Ernst, J.D. Initiation of the adaptive immune response to Mycobacterium tuberculosis depends on antigen production in the local lymph node, not the lungs. J. Exp. Med. 2007, 205, 105–115. [Google Scholar] [CrossRef]
- Jeyanathan, M.; Yao, Y.; Afkhami, S.; Smaill, F.; Xing, Z. New Tuberculosis Vaccine Strategies: Taking Aim at Un-Natural Immunity. Trends Immunol. 2018, 39, 419–433. [Google Scholar] [CrossRef]
- Counoupas, C.; Ferrell, K.C.; Ashhurst, A.; Bhattacharyya, N.D.; Nagalingam, G.; Stewart, E.L.; Feng, C.G.; Petrovsky, N.; Britton, W.J.; Triccas, J.A. Mucosal delivery of a multistage subunit vaccine promotes development of lung-resident memory T cells and affords interleukin-17-dependent protection against pulmonary tuberculosis. Npj Vaccines 2020, 5, 105. [Google Scholar] [CrossRef]
- Flórido, M.; Muflihah, H.; Lin, L.C.W.; Xia, Y.; Sierro, F.; Palendira, M.; Feng, C.G.; Bertolino, P.; Stambas, J.; Triccas, J.A.; et al. Pulmonary immunization with a recombinant influenza A virus vaccine induces lung-resident CD4+ memory T cells that are associated with protection against tuberculosis. Mucosal Immunol. 2018, 11, 1743–1752. [Google Scholar] [CrossRef] [PubMed]
- Darrah, P.A.; Zeppa, J.J.; Maiello, P.; Hackney, J.A.; Wadsworth, M.H.; Hughes, T.K.; Pokkali, S.; Swanson, P.A.; Grant, N.L.; Rodgers, M.A.; et al. Prevention of tuberculosis in macaques after intravenous BCG immunization. Nature 2020, 577, 95–102. [Google Scholar] [CrossRef]
- Darrah, P.A.; Zeppa, J.J.; Wang, C.; Irvine, E.B.; Bucsan, A.N.; Rodgers, M.A.; Pokkali, S.; Hackney, J.A.; Kamath, M.; White, A.G.; et al. Airway T cells are a correlate of i.v. Bacille Calmette-Guerin-mediated protection against tuberculosis in rhesus macaques. Cell Host Microbe 2023, 31, 962–977.e968. [Google Scholar] [CrossRef]
- Jeyanathan, M.; Fritz, D.K.; Afkhami, S.; Aguirre, E.; Howie, K.J.; Zganiacz, A.; Dvorkin-Gheva, A.; Thompson, M.R.; Silver, R.F.; Cusack, R.P.; et al. Aerosol delivery, but not intramuscular injection, of adenovirus-vectored tuberculosis vaccine induces respiratory-mucosal immunity in humans. JCI Insight 2022, 7, e155655. [Google Scholar] [CrossRef] [PubMed]
- Rodo, M.J.; Rozot, V.; Nemes, E.; Dintwe, O.; Hatherill, M.; Little, F.; Scriba, T.J. A comparison of antigen-specific T cell responses induced by six novel tuberculosis vaccine candidates. PLoS Pathog. 2019, 15, e1007643. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.; Aguilo, N.; Marinova, D.; Gonzalo-Asensio, J. Update on TB Vaccine Pipeline. Appl. Sci. 2020, 10, 2632. [Google Scholar] [CrossRef]
- Burny, W.; Callegaro, A.; Bechtold, V.; Clement, F.; Delhaye, S.; Fissette, L.; Janssens, M.; Leroux-Roels, G.; Marchant, A.; van den Berg, R.A.; et al. Different Adjuvants Induce Common Innate Pathways That Are Associated with Enhanced Adaptive Responses against a Model Antigen in Humans. Front. Immunol. 2017, 8, 943. [Google Scholar] [CrossRef]
- Counoupas, C.; Pinto, R.; Nagalingam, G.; Britton, W.J.; Petrovsky, N.; Triccas, J.A. Delta inulin-based adjuvants promote the generation of polyfunctional CD4(+) T cell responses and protection against Mycobacterium tuberculosis infection. Sci. Rep. 2017, 7, 8582. [Google Scholar] [CrossRef]
- Gordon, D.L.; Sajkov, D.; Honda-Okubo, Y.; Wilks, S.H.; Aban, M.; Barr, I.G.; Petrovsky, N. Human Phase 1 trial of low-dose inactivated seasonal influenza vaccine formulated with Advax delta inulin adjuvant. Vaccine 2016, 34, 3780–3786. [Google Scholar] [CrossRef]
- Saade, F.; Honda-Okubo, Y.; Trec, S.; Petrovsky, N. A novel hepatitis B vaccine containing Advax™, a polysaccharide adjuvant derived from delta inulin, induces robust humoral and cellular immunity with minimal reactogenicity in preclinical testing. Vaccine 2013, 31, 1999–2007. [Google Scholar] [CrossRef]
- Counoupas, C.; Pinto, R.; Nagalingam, G.; Hill-Cawthorne, G.A.; Feng, C.G.; Britton, W.J.; Triccas, J.A. Mycobacterium tuberculosis components expressed during chronic infection of the lung contribute to long-term control of pulmonary tuberculosis in mice. Npj Vaccines 2016, 1, 16012. [Google Scholar] [CrossRef]
- Jaadi, Z. A Step-by-Step Explanation of Principal Component Analysis (PCA). 2021. Available online: https://builtin.com/data-science/step-step-explanation-principal-component-analysis (accessed on 1 September 2021).
- Metsalu, T.; Vilo, J. ClustVis: A web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap. Nucleic Acids Res. 2015, 43, W566–W570. [Google Scholar] [CrossRef]
- Stewart, E.L.; Counoupas, C.; Johansen, M.D.; Nguyen, D.H.; Miemczyk, S.; Hansbro, N.G.; Ferrell, K.C.; Ashhurst, A.; Alca, S.; Ashley, C.; et al. Mucosal immunization with a delta-inulin adjuvanted recombinant spike vaccine elicits lung-resident immune memory and protects mice against SARS-CoV-2. Mucosal Immunol. 2022, 15, 1405–1415. [Google Scholar] [CrossRef]
- Achkar, J.M.; Casadevall, A. Antibody-mediated immunity against tuberculosis: Implications for vaccine development. Cell Host Microbe 2013, 13, 250–262. [Google Scholar] [CrossRef]
- Evans, C.M.; Jenner, R.G. Transcription factor interplay in T helper cell differentiation. Brief Funct. Genom. 2013, 12, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Sia, J.K.; Georgieva, M.; Rengarajan, J. Innate Immune Defenses in Human Tuberculosis: An Overview of the Interactions between Mycobacterium tuberculosis and Innate Immune Cells. J. Immunol. Res. 2015, 2015, 747543. [Google Scholar] [CrossRef] [PubMed]
- Bohrer, A.C.; Castro, E.; Hu, Z.; Queiroz, A.T.L.; Tocheny, C.E.; Assmann, M.; Sakai, S.; Nelson, C.; Baker, P.J.; Ma, H.; et al. Eosinophils are part of the granulocyte response in tuberculosis and promote host resistance in mice. J. Exp. Med. 2021, 218, e20210469. [Google Scholar] [CrossRef] [PubMed]
- Dijkman, K.; Sombroek, C.C.; Vervenne, R.A.W.; Hofman, S.O.; Boot, C.; Remarque, E.J.; Kocken, C.H.M.; Ottenhoff, T.H.M.; Kondova, I.; Khayum, M.A.; et al. Prevention of tuberculosis infection and disease by local BCG in repeatedly exposed rhesus macaques. Nat. Med. 2019, 25, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Martonik, D.; Parfieniuk-Kowerda, A.; Rogalska, M.; Flisiak, R. The Role of Th17 Response in COVID-19. Cells 2021, 10, 1550. [Google Scholar] [CrossRef] [PubMed]
- Enriquez, A.B.; Izzo, A.; Miller, S.M.; Stewart, E.L.; Mahon, R.N.; Frank, D.J.; Evans, J.T.; Rengarajan, J.; Triccas, J.A. Advancing Adjuvants for Mycobacterium tuberculosis Therapeutics. Front. Immunol. 2021, 12, 740117. [Google Scholar] [CrossRef] [PubMed]
- Christensen, D.; Mortensen, R.; Rosenkrands, I.; Dietrich, J.; Andersen, P. Vaccine-induced Th17 cells are established as resident memory cells in the lung and promote local IgA responses. Mucosal Immunol. 2017, 10, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Mitsdoerffer, M.; Lee, Y.; Jäger, A.; Kim, H.-J.; Korn, T.; Kolls, J.K.; Cantor, H.; Bettelli, E.; Kuchroo, V.K. Proinflammatory T helper type 17 cells are effective B-cell helpers. Proc. Natl. Acad. Sci. USA 2010, 107, 14292–14297. [Google Scholar] [CrossRef] [PubMed]
- Tomar, J.; Patil, H.P.; Bracho, G.; Tonnis, W.F.; Frijlink, H.W.; Petrovsky, N.; Vanbever, R.; Huckriede, A.; Hinrichs, W.L.J. Advax augments B and T cell responses upon influenza vaccination via the respiratory tract and enables complete protection of mice against lethal influenza virus challenge. J. Control. Release Off. J. Control. Release Soc. 2018, 288, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Covián, C.; Fernández-Fierro, A.; Retamal-Díaz, A.; Díaz, F.E.; Vasquez, A.E.; Lay, M.K.; Riedel, C.A.; González, P.A.; Bueno, S.M.; Kalergis, A.M. BCG-Induced Cross-Protection and Development of Trained Immunity: Implication for Vaccine Design. Front. Immunol. 2019, 10, 6570–6578. [Google Scholar] [CrossRef] [PubMed]
- Aguilo, N.; Alvarez-Arguedas, S.; Uranga, S.; Marinova, D.; Monzón, M.; Badiola, J.; Martin, C. Pulmonary but Not Subcutaneous Delivery of BCG Vaccine Confers Protection to Tuberculosis-Susceptible Mice by an Interleukin 17–Dependent Mechanism. J. Infect. Dis. 2016, 213, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Moliva, J.I.; Hossfeld, A.P.; Sidiki, S.; Canan, C.H.; Dwivedi, V.; Beamer, G.; Turner, J.; Torrelles, J.B. Selective delipidation of Mycobacterium bovis BCG enables direct pulmonary vaccination and enhances protection against Mycobacterium tuberculosis. Mucosal Immunol. 2019, 12, 805–815. [Google Scholar] [CrossRef]
- Orr, M.T.; Beebe, E.A.; Hudson, T.E.; Argilla, D.; Huang, P.-W.D.; Reese, V.A.; Fox, C.B.; Reed, S.G.; Coler, R.N. Mucosal delivery switches the response to an adjuvanted tuberculosis vaccine from systemic TH1 to tissue-resident TH17 responses without impacting the protective efficacy. Vaccine 2015, 33, 6570–6578. [Google Scholar] [CrossRef]
- Clemmensen, H.S.; Knudsen, N.P.H.; Billeskov, R.; Rosenkrands, I.; Jungersen, G.; Aagaard, C.; Andersen, P.; Mortensen, R. Rescuing ESAT-6 Specific CD4 T Cells From Terminal Differentiation Is Critical for Long-Term Control of Murine Mtb Infection. Front. Immunol. 2020, 11, 585359. [Google Scholar] [CrossRef]
- Sallin, M.A.; Sakai, S.; Kauffman, K.D.; Young, H.A.; Zhu, J.F.; Barber, D.L. Th1 Differentiation Drives the Accumulation of Intravascular, Non-protective CD4 T Cells during Tuberculosis. Cell Rep. 2017, 18, 3091–3104. [Google Scholar] [CrossRef]
- Bell, G.K.; Eaton, S.M.; Rangel-Moreno, J.; Cilley, G.E.; Cooper, A.M.; Pearl, J.E.; Swain, S.L.; Randall, T.D.; Locksley, R.M.; Shen, F.; et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat. Immunol. 2007, 8, 369–377. [Google Scholar] [CrossRef]
- Lückel, C.; Picard, F.S.R.; Huber, M. Tc17 biology and function: Novel concepts. Eur. J. Immunol. 2020, 50, 1257–1267. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Kjer-Nielsen, L.; Shi, M.; D’Souza, C.; Pediongco, T.J.; Cao, H.; Kostenko, L.; Lim, X.Y.; Eckle, S.B.G.; Meehan, B.S.; et al. IL-23 costimulates antigen-specific MAIT cell activation and enables vaccination against bacterial infection. Sci. Immunol. 2019, 4, eaaw0402. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Singh, V.K.; Hunter, R.L.; Jagannath, C. Macrophage heterogeneity and plasticity in tuberculosis. J. Leukoc. Biol. 2019, 106, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Balboa, L.; Romero, M.M.; Basile, J.I.; Sabio y García, C.A.; Schierloh, P.; Yokobori, N.; Geffner, L.; Musella, R.M.; Castagnino, J.; Abbate, E.; et al. Paradoxical role of CD16+CCR2+CCR5+ monocytes in tuberculosis: Efficient APC in pleural effusion but also mark disease severity in blood. J. Leukoc. Biol. 2011, 90, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Wolf, A.J.; Linas, B.; Trevejo-Nunez, G.J.; Kincaid, E.; Tamura, T.; Takatsu, K.; Ernst, J.D. Mycobacterium tuberculosis Infects Dendritic Cells with High Frequency and Impairs Their Function In Vivo. J. Immunol. 2007, 179, 2509–2519. [Google Scholar] [CrossRef] [PubMed]
- Billeskov, R.; Lindenstrøm, T.; Woodworth, J.; Vilaplana, C.; Cardona, P.-J.; Cassidy, J.P.; Mortensen, R.; Agger, E.M.; Andersen, P. High Antigen Dose Is Detrimental to Post-Exposure Vaccine Protection against Tuberculosis. Front. Immunol. 2018, 8, 1973. [Google Scholar] [CrossRef]
- Quan, D.H.; Counoupas, C.; Nagalingam, G.; Pinto, R.; Petrovsky, N.; Britton, W.J.; Triccas, J.A. Advax adjuvant formulations promote protective immunity against aerosol Mycobacterium tuberculosis in the absence of deleterious inflammation and reactogenicity. bioRxiv 2020. [Google Scholar] [CrossRef]
- Lindenstrøm, T.; Moguche, A.; Damborg, M.; Agger, E.M.; Urdahl, K.; Andersen, P. T Cells Primed by Live Mycobacteria Versus a Tuberculosis Subunit Vaccine Exhibit Distinct Functional Properties. EBioMedicine 2018, 27, 27–39. [Google Scholar] [CrossRef]
- Silva-Sanchez, A.; Randall, T.D. Role of iBALT in Respiratory Immunity. In Inducible Lymphoid Organs; Current Topics in Microbiology and Immunology; Springer: Cham, Switzerland, 2020; Volume 426, pp. 21–43. [Google Scholar] [CrossRef]
- Ferrell, K.C.; Stewart, E.L.; Counoupas, C.; Ashhurst, T.M.; Britton, W.J.; Petrovsky, N.; Triccas, J.A. Intrapulmonary vaccination with delta-inulin adjuvant stimulates non-polarised chemotactic signalling and diverse cellular interaction. Mucosal Immunol. 2021, 14, 762–773. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stewart, E.L.; Counoupas, C.; Quan, D.H.; Wang, T.; Petrovsky, N.; Britton, W.J.; Triccas, J.A. Lung IL-17A-Producing CD4+ T Cells Correlate with Protection after Intrapulmonary Vaccination with Differentially Adjuvanted Tuberculosis Vaccines. Vaccines 2024, 12, 128. https://doi.org/10.3390/vaccines12020128
Stewart EL, Counoupas C, Quan DH, Wang T, Petrovsky N, Britton WJ, Triccas JA. Lung IL-17A-Producing CD4+ T Cells Correlate with Protection after Intrapulmonary Vaccination with Differentially Adjuvanted Tuberculosis Vaccines. Vaccines. 2024; 12(2):128. https://doi.org/10.3390/vaccines12020128
Chicago/Turabian StyleStewart, Erica L., Claudio Counoupas, Diana H. Quan, Trixie Wang, Nikolai Petrovsky, Warwick J. Britton, and James A. Triccas. 2024. "Lung IL-17A-Producing CD4+ T Cells Correlate with Protection after Intrapulmonary Vaccination with Differentially Adjuvanted Tuberculosis Vaccines" Vaccines 12, no. 2: 128. https://doi.org/10.3390/vaccines12020128
APA StyleStewart, E. L., Counoupas, C., Quan, D. H., Wang, T., Petrovsky, N., Britton, W. J., & Triccas, J. A. (2024). Lung IL-17A-Producing CD4+ T Cells Correlate with Protection after Intrapulmonary Vaccination with Differentially Adjuvanted Tuberculosis Vaccines. Vaccines, 12(2), 128. https://doi.org/10.3390/vaccines12020128





